Abstract
Background
Waltheria indica is a multipurpose medicinal plant with abundance of phytochemical compounds. Antifertility effect of Waltheria indica Linn. root and leaves have been reported. However, the fraction responsible for this antifertility effect needs to be isolated for possible male contraceptive purpose. Therefore, this research was designed to isolate the antifertility fraction of Waltheria indica Linn. root (WILR) in an in vivo model using male Wistar rats. Crude ethanol extract of WILR was sequentially dissolved in hexane, dichloromethane, and ethyl acetate. Rats (n = 5) were administered with 200, 500, or 1000 μg/kg of hexane, dichloromethane, and ethyl acetate soluble extracts of WILR, while control received distilled water, daily for 15 days to determine the soluble extract with most antifertility effect. Thereafter, fractions were separated from dichloromethane soluble WILR extract by column and thin-layer chromatography. Rats (7 groups, n = 5) were administered with each of the fractions (DF1 to DF7; at 1000 μg/kg) to determine the fraction with the highest antifertility. Rats were thereafter sacrificed, and sperm parameters, reproductive hormones, testicular cholesterol, and protein were determined according to standard procedure. Histology of the testis was also done. Data were analyzed using ANOVA at p ≤ 0.05.
Results
Dichloromethane soluble fraction (500 μg/kg) significantly decreased sperm concentration (137.00 ± 9.85 to 107.00 ± 13.08 × 106 cells/mL), levels of testosterone (2.90 ± 0.65 to 1.50 ± 0.37 ng/mL), and FSH (0.08 ± 0.08 to 0.99 ± 0.08 IU/L). The dichloromethane soluble fraction also caused the loss of testicular interstitium and spermatogenic cells. DF5 significantly reduced sperm motility (92.00 ± 2.74 to 76.00 ± 5.48%) and LH (2.86 ± 0.52 to 1.47 ± 0.18 IU/L). DF5 also significantly increased levels of prolactin (1.22 ± 0.10 to 1.88 ± 0.48 ng/mL), testicular total protein (7.36 ± 0.35 to 8.54 ± 1.06 g/dL), and testicular cholesterol (34.17 ± 3.65 to 55.76 ± 6.08 mg/mL).
Conclusion
The results indicate that the DF5 is the bioactive fraction of WILR responsible for its antifertility effect. The possible antifertility mechanisms are through the reduction in sperm parameters, reproductive hormones, and histological changes in the testis.
Similar content being viewed by others
Background
Waltheria indica Linn. plant belongs to the family Sterculiaceae (Cacao family). It is a perennial shrub that grows in the tropical and subtropical environment. Waltheria indica Linn. plant has been reported for use in the management of many diseases in traditional medicine. Some of these diseases are animal and human trypanosomosis, malaria, bacterial infection, anemia, and fungal infection [1]. These diseases are of economic and public health significance in sub-Sahara Africa [2]. This makes Waltheria indica Linn. plant an essential ethnomedicinal plant in Africa.
Waltheria indica is a medicinal plant with abundance of phytochemical compounds. These compounds are responsible for its medicinal properties. Ethnobotanical use of decoction of root of Waltheria indica plant as contraceptive agent [3] and antifertility effect of Waltheria indica leave has been reported [4]. In addition, we demonstrated the antifertility effect of crude ethanol extract of Waltheria indica Linn. root [5]. However, the fraction responsible for this antifertility effect needs to be isolated. Therefore, this research was designed to study and isolate the antifertility fraction of Waltheria indica Linn. root in male Wistar rats.
Methods
Collection, preparation of plant materials and crude extract
Waltheria indica Linn. plants were obtained from Ibadan, Oyo State, Nigeria, and the voucher number (UIH-22371) for the plant was obtained from a university herbarium. The details of the collection and preparation of crude extract of Waltheria indica Linn. root was reported by [6].
Sequential extraction of crude ethanol extract of Waltheria indica Linn. root
The crude ethanol extract of Waltheria indica Linn. root (100 g) was sequentially extracted with hexane, dichloromethane, and ethyl acetate in order of increasing polarity.
Column chromatographic separation of dichloromethane (DCM) fraction
The glass column was packed with silica gel using n-hexane. The Waltheria indica Linn. root extract absorbed with silica gel was packed into the column layer, de-aerated, and then allowed to settle. Filter paper discs were used to separate the layers [7].
The mobile phase consisted of 3 solvents: hexane (nonpolar), ethyl acetate (mid-polar), and methanol (polar). The solvents were mixed in various proportions to achieve a “gentle gradient” in terms of separation. The various proportions of solvents were pushed through the bed. The fractions obtained were pooled together using thin-layer chromatography (TLC).
Separation of column fractions by TLC
A line of about 1.5 cm from the bottom of the silica-coated plate was drawn. The samples obtained from the column chromatographic separations were spotted (using a capillary tube filled with the fraction) on this line, equidistant from each other. The plates were placed in a TLC chamber saturated with ethyl acetate: methanol as mobile phase. The plate was thereafter removed from the chamber when the solvent had risen towards the end of the plate. The position of the solvent front was marked.
The TLC plate was thereafter examined under ultraviolet light. Fractions with similar retardation factor (Rf) were pooled together.
Experimental animals and dosing protocol
Ninety healthy adult male Wistar rats were used in this experiment. The rats were obtained from the experimental animal house of the Faculty of Veterinary Medicine, Nigeria. The rats were fed with pelletized grower poultry feeds (Vital feeds®, Grand cereals Limited, Jos, Nigeria) and water was provided ad libitum. All experimental protocols were carried out according to internationally approved principles for the handling of experimental animal, use, and care. The ethical approval was obtained from University of Ibadan Animal Care and Use Research Ethics Committee with reference number UI-ACUREC/APP/2016/002. Thirty rats were randomly divided into ten groups (n = 5). Three doses (200, 500, and 1000 μg/Kg BW) each of the solvent fractions (hexane, dichloromethane, and ethyl acetate) were administered to each group, while the control was administered with distilled water. Thereafter, the chromatographic fractions (DF1–DF7) were also administered to rats (n = 5) at 1000 μg/Kg BW, while the control (n = 5) was given distilled water. The administration was done using oral gavage for 15 consecutive days. The dosage was chosen based on the LD50 of Waltheria indica Linn. root [5] and the trypanocidal dose of Waltheria indica Linn. root [8].
Determination of sperm parameters and serum levels of testosterone, follicle stimulating hormone, luteinizing hormone, and prolactin
Sperm collection
Rats were anesthetized with combination of xylazine (10 mg/kg) and ketamine (90 mg/kg). The rats were thereafter sacrificed by cervical dislocation and the epididymis excised. Sperms were squeezed from the caudal epididymis of anesthetized adult male Wistar rats.
Sperm parameters
Epididymal sperm concentration, motility, and live–dead ratio were determined according to methods of [8,9,10], respectively, while the sperm morphology was determined as described by [11]. The detailed procedure was reported by [5].
Hormonal assay
Serum levels of testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin were assayed using ELISA kits (Calbiotech Inc., USA) following the kit manual.
Preparation of the testicular homogenates and assay of testicular protein and cholesterol
The testes were homogenized in cold 0.25 M sucrose solution (1:5 w/v) using a tissue homogenizer (Bio-Gen PRO200®, Oxford, USA). The homogenates were spun at 5000 rpm for 30 min using a centrifuge (Axiom Medical Ltd, UK). The supernatants obtained were then used for the determination of the protein and cholesterol using Randox assay kits (Randox Diagnostics, Crumlin, UK).
Histopathology of the testis
Briefly, fixed testes in 10% buffered formaldehyde were dehydrated through ascending concentrations of ethanol (70, 90, and 95%). They were cleared in xylene, impregnated, and embedded in molten paraffin wax (melting point 56 °C). The embedded tissue in paraffin wax was sectioned using a semi-automatic microtome (Kedee®, China) at a preset thickness of 4 μm. The satisfactory sections were picked up with microscope glass slides that had been coated on one side with glycerin egg albumin (to prevent detachment from slides during staining procedure). The slides carrying the sections were then labeled with a diamond pencil. They were then arranged in a slide carrier and then put in an oven (DHG-9023A oven, Lab science, England) to dry. After staining with hematoxylin and eosin (H&E), the slides were passed through ascending concentration of alcohol (20–100%) for dehydration and then cleaned with xylene. A thin glass-covered slip was placed on the covering–mounting medium, and underlying tissue sections were allowed to dry. The slides were then examined under the microscope (Olympus®, Germany) for histopathological changes and photographed with camera (AmScope®, Japan) mounted on the microscope [12].
Statistical analysis
The data obtained were expressed as mean ± standard deviation (Mean ± SD). The data were subjected to one-way analysis of variance (ANOVA), and differences between the control and treatment groups were determined by Dunnett’s multiple comparison test using GraphPad Prism® (Version 5.0, San Diego, CA). P values ≤ 5% were regarded as significant.
Results
Sequential extraction and chromatographic separation of Waltheria indica Linn. root
The sequential extraction of crude ethanol extract of Waltheria indica Linn. root with hexane, dichloromethane, and ethyl acetate yielded 18.47, 1.82, and 4.3 g of hexane, dichloromethane, and ethyl acetate soluble extracts, respectively. The column and thin-layer chromatographic analysis of the dichloromethane soluble extract of Waltheria indica Linn. root yielded seven fractions (labeled as DF1 to DF7).
Reproductive effect of dichloromethane, hexane, and ethyl acetate soluble extracts of Waltheria indica Linn. root in male Wistar rats
The sperm parameters of male Wistar rats following the administration of dichloromethane, hexane, and ethyl acetate soluble extracts of Waltheria indica Linn. root are as follows.
Dichloromethane extract caused a significant decrease in sperm concentration for all the treated groups, 200 μg/Kg (p < 0.05), 500 μg/Kg (p < 0.01), and 1000 μg/Kg (p < 0.001), when compared with the group administered with distilled water (Table 1).
All the doses of hexane extract administered caused significant reduction (p < 0.001) in sperm concentration. The sperm motility was also significantly (p < 0.001) reduced at the highest dose (Table 2).
The effect of ethyl acetate extract of WILR on sperm parameters are shown in Table 3. There was a significant decrease in sperm concentration of male Wistar when administered with 500 μg/Kg (p < 0.05) and 1000 μg/Kg (p < 0.01) of ethyl acetate fraction of Waltheria indica Linn. root. The percentage of motile spermatozoa was also significantly reduced (p < 0.05) at 500 and 1000 μg/Kg BW doses. The was no significant change in the sperm live–dead ratio.
Effect of dichloromethane, hexane, and ethyl acetate fractions of Waltheria indica Linn. root on serum levels of testosterone, FSH, LH, and prolactin of male Wistar rats
Dichloromethane soluble extract caused a significant (p < 0.05) decrease in serum level of testosterone at 500 and 1000 μg/Kg BW when compared with the control. There was also a significant (p < 0.05) decrease in serum level of FSH at 500 μg/Kg BW. The serum level of prolactin showed significant (p < 0.001) increase at the highest dose (1000 μg/Kg) (Table 4).
The result of the effect of hexane extract on reproductive hormones of male Wistar rats is shown in Table 5. Rats administered with ethyl acetate soluble extract showed a significant reduction (p < 0.05) at the 500 and 1000 μg/Kg BW in testosterone level (Table 6).
Reproductive effects of chromatographic fractions of Waltheria indica Linn. root in male Wistar rats
The sperm parameters of rats administered with chromatographic fractions of WILR are as follows: fractions 5 (DF5) and 7 (DF7) caused significant (p < 0.001) decrease in sperm concentration and motility. The DF6 also caused a significant reduction in sperm concentration (p < 0.001) and motility (p < 0.05). Also, there was a significant decrease in sperm concentration when fractions DF1 (p < 0.01), DF3 (p < 0.01), and DF4 (p < 0.001) were administered to male Wistar rats (Table 7).
In the sperm morphology of rats following administration of chromatographic fractions of WILR, the chromatographic fraction four (DF4) caused a significant (p < 0.05) increase in the percentage of the total abnormal sperm cell. The percentage of sperm cells without head (headless tail) significantly (p < 0.01) increased in rats administered with fractions DF5 and DF7. Moreover, the percentage of bent tail showed a significant increase for fractions DF4 (p < 0.01) and DF7 (p < 0.05). There was also a significant increase in the percentage of the curved tail for the fractions DF3 and DF4 (p < 0.01) and DF5 and DF7 (p < 0.001) (Table 8).
For serum levels of testosterone, FSH, LH, and prolactin of rats following administration of chromatographic fractions of WILR, chromatographic fractions 5 and 7 (DF5 and DF7) caused a significant (p < 0.001) reduction in serum testosterone when compared with control group. There was also significant reduction in serum level of FSH when fractions DF3 (p < 0.01), DF4 (p < 0.001), DF5 (p < 0.001), DF6 (p < 0.05), and DF7 (p < 0.01) were administered to rats. Fraction DF2 also showed a significant (p < 0.01) reduction serum level of LH. In contrast, there was a significant increase in serum level of prolactin for the fractions DF2 (p < 0.01), DF5 (p < 0.001) and DF7 (p < 0.05).
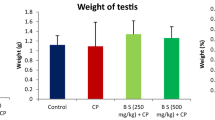
For the testicular protein and cholesterol of experimental rats, Table 9 shows the testicular protein and cholesterol of male Wistar rats following the administration of chromatographic fractions of Waltheria indica Linn. root. There was a significant increase in testicular protein (p < 0.05) and cholesterol (p < 0.01) of rats when fraction DF5 was administered to male Wistar rats.
Result of histopathology of the testes
The result of the histopathology of the testes of Wistar is shown in Figs. 1, 2 and 3. The histopathological changes observed following the administration of dichloromethane, hexane, and ethyl acetate soluble fractions of Waltheria indica Linn. root to adult male Wistar rats were loss of interstitium, reduction in germinal epithelial height, erosion of germ cell layers, and reduction of spermatozoa in the lumen of the seminiferous tubule.
Photomicrographs of the testis of male Wistar rats exposed to different doses of dichloromethane soluble fraction of WILR. Control (C): normal testis showing the seminiferous tubules (star) and interstitium (black arrow). 200 μg/kg (DA): loss of interstitium (blue arrow) and germ cells. 500 and 1000 μg/kg (DB and DC): loss of interstitium (blue arrow) and germ cells. H&E × 100
Photomicrographs of the testis of male Wistar rats exposed to different doses of ethyl acetate soluble fraction of WILR. Control (C): normal testis showing the seminiferous tubules (star) and interstitium (blue arrow). 200 μg/kg (EA): loss of interstitium (blue arrow) and germ cells. 500 μg/kg (EB): loss of interstitium (blue arrow) and germ cells. 1000 μg/kg (EC): loss of interstitium (blue arrow) and erosion of germinal epithelium. H&E × 100
Photomicrographs of the testis of male Wistar rats exposed to different doses of hexane soluble fraction of WILR. Control (C): normal testis showing the seminiferous tubules (star) and interstitium (black arrow). 200 μg/kg (HA): loss of interstitium (arrow) and germ cells. 500 μg/kg (HB): loss of interstitium (arrow) and erosion of germinal epithelium. 1000 μg/kg (HC): loss of interstitium (black arrow). H&E × 100
Discussion
The male reproductive system is a complex and well-organized process that is often subjected to the deleterious effects of common therapeutic agents [13]. A common example is the adverse effect of many antimalarial drugs [14] and antimicrobial agents [13]. Waltheria indica Linn. plant has been reported to have anti-protozoan and antibacterial effects [1]. It has however been reported that most plants with anti-protozoan and antibacterial effects have an antifertility effect in male animals [15]. This antifertility effect of medicinal plants was attributed to the toxic principle in this plant that disrupts the male reproductive processes aside from its anti-protozoan and antibacterial effect. The difference in the quantity of hexane, dichloromethane, and ethyl acetate soluble fractions obtained in this study may be attributed to the difference in the polarities of the solvent [16]. Different phytochemical compound dissolves better at different polarities. Sperm parameters such as sperm concentration, motility, morphology, and live–dead ratio are indices of male fertility [17]. Reductions in these parameters indicate the antifertility effect of Waltheria indica Linn. root. The significant reduction in sperm concentration by the hexane, dichloromethane, and ethyl acetate solvent extract showed the antifertility effect of these fractions. The dichloromethane showed a significant reduction in FSH and testosterone as well as histopathological changes (Fig. 1) in testes. This shows that the dichloromethane soluble extract has the most antifertility effect compared to hexane and ethyl acetate soluble extract of Waltheria indica Linn. root. Spermatogenesis is regulated by the hypothalamic pituitary testicular (HPT) axis through the production of gonadotropin-releasing hormone (GnRH) from the hypothalamus. The GnRH stimulates the hypophysis to secrete gonadotropins (FSH and LH). The LH stimulates the Leydig cell located within the interstitium of the seminiferous tubule to produce testosterone, while the FSH acts on the Sertoli cell to support the developing germ cells. The loss of the interstitial cells including the Leydig cell observed in the study (Figs. 1, 2 and 3) may be responsible for the low serum level of testosterone. The significant decrease in sperm motility of rats administered with chromatographic fractions 5 and 7 (DF5 and DF7) may be due to tail and mid piece abnormalities (Table 8). More so, the significant reduction in sperm concentration can be attributed to the disturbance in the HPT axis which is evident by the significant reduction in LH, FSH, and testosterone. Testosterone is formed from the cholesterol (androgenesis) by the Leydig cell. A high level of intratesticular testosterone is required for normal spermatogenesis [18]. Testosterone binds with androgen receptors which are located on the peritubular cells, cytoplasm but not on germ cells. This androgen receptor binding with testosterone is critical for male fertility. Testosterone maintains the blood–testis barrier, meiotic stages of spermatogenesis, and spermiation. The significant increase in testicular cholesterol is indicative of defective androgenesis (formation of testosterone from cholesterol) which subsequently led to the reduced serum level of testosterone (Table 10). In addition to increased testicular cholesterol, the significant increase in testicular proteins may be indicative of reduced spermatogenesis. Spermatogenesis is a metabolic process requiring the utilization of protein [19]. Germ cells have specific metabolic needs for their development into spermatozoa. The Sertoli cell utilizes a number of substrate including glycogen and proteins to fulfill the germ cell metabolic requirement. The significant accumulation of testicular protein may indicate reduced utilization of this substrate by germ cell. A high serum level of prolactin is indicative of male infertility [20]. Prolactin which is produced by adenohypophyseal lactotrophs has been shown to inhibit gonadotropin secretion and spermatogenesis [21]. The significant increase in serum prolactin caused by DF5 further supports the antifertility of Waltheria indica Linn. root. It thus appears that the bioactive principle responsible for the antifertility effect of Waltheria indica Linn. root resides in chromatographic fraction 5 (DF5).
Conclusion
The dichloromethane soluble fraction of Waltheria indica Linn. root caused the most significant antifertility effect among the three soluble fractions (i. e., dichloromethane, hexane, and ethyl acetate). More so, the DF5 is the bioactive fraction of Waltheria indica Linn. root responsible for its antifertility effect. However, further work is required to determine the effect of this bioactive fraction on the fertility index of male rats (contraceptive) as well as the mechanism of its antifertility effect.
Availability of data and materials
All data and materials are available upon request.
Abbreviations
- WILR:
-
Waltheria indica Linn. Root
- DF:
-
Dichloromethane fraction
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- DCM:
-
Dichloromethane
- TLC:
-
Thin-layer chromatography
- Rf:
-
Retardation factor
- UI-ACUREC:
-
University of Ibadan Animal Care and Use for Research Committee
- BW:
-
Body weight
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- M:
-
Molarity
- rpm:
-
Revolution per minute
- H&E:
-
Hematoxylin and eosin
- GnRH:
-
Gonadotropin-releasing hormone
References
Zongo F, Ribuot C, Boumendjel A, Guissou IJ (2013) Botany, traditional uses, phytochemistry and pharmacology of Waltheria indica Linn. L. (syn. Waltheria americana). J Ethnophar 148:14–26
Corbel V, Henry M (2011) Prevention and control of malaria and sleeping sickness in Africa: Where are we and where are we going? Parasit Vectors 4(37):1–4
Morton JF (1981) Atlas of medicinal plants of middle America: Bahamas to Yucatan. Springfield
Basiru A, Olayemi FO (2014) Effects of aqueous extract of Waltheria indica leaves on reproductive indices of male albino rats. Afr J Biotech 13(32):3307–3312. https://doi.org/10.5897/AJB2014.13928
Basiru A, Akorede GJ, Soetan K, Olayemi FO (2019) Adverse reproductive effects of ethanolic root extract of Waltheria indica in male Wistar rats. J Complem Integ Med 16(4):1–6
Abbot D, Andrews RS (1970) An introduction to chromatography. Longman Press, London
Sylvian C, Lise B, Lucie P, Chiara A, Laurence M, Samad NE, Matthias H, Remo P, Soumana K, Marcel K, Muriel C, Philippe C (2014) Antitrypanosomal quinoline alkaloids from the roots of Waltheria indica Linn. J Nat Prod 77(10):2304–2311
Pant N, Srivastava SP (2003) Testicular and spermatotoxic effect of quinaphos in rats. J Appl Tox 23(4):271–274. https://doi.org/10.1002/jat.919
Zemjanis R (1977) Collection and evaluation of semen. In: Zemjanis R (ed) Diagnostic and therapeutic techniques in animal reproduction. The Williams and Wilkins Company, Baltimore, pp 139–180
Wells ME, Awa OA (1970) New technique for assessing acrosomal characteristics of spermatozoa. J Diary Sci 53(2):227–232. https://doi.org/10.3168/jds.S0022-0302(70)86184-7
Hammer CE (1970) The semen. In: Hafez ESE (ed) Reproduction and breeding techniques for laboratory animals. Lea and Febiger, Philadelphia, pp 16–22
Musumeci G (2014) Past, present and future: overview on histology and histopathology. J Hist Histop 1:1–5
Durairajanayagam D, Rengan AK, Sharma RK, Agarwal A (2016) Sperm biology from production to ejaculation in “unexplained infertility: pathophysiology, evaluation and treatment”. Springer, London, pp 29–43
Akinsomisoye SO, Raji Y (2011) Long-term administration of artesunate induces reproductive toxicity in male rats. J Reprod Infertil 12(4):249–260
Olayemi FO (2010) A review on some causes of male infertility. Afri J Biotech 9(20):2834–2842
Paini SW, Tarsisius DWB, Fenny AK, Evelyn LW (2014) Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia less leaves extracts. Inter J Pharmacog Phytochem Res 6(4):850–855
Gill K, Jakubik J, Rosiak-Gill A, Kups M, Lukaszuk M, Kurpisz M, Fraczek M, Piasecka M (2019) Utility and predictive value of human standard semen parameters and sperm DNA dispersion for fertility potential. Inter J Enviro Res Pub Health 16(11):2004. https://doi.org/10.3390/ijerph16112004
Zirkin BR, Santulli R, Awoniyi CA, Ewing LL (1989) Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocri 124(6):3043–3049. https://doi.org/10.1210/endo-124-6-3043
Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF (2012) Metabolic regulation is important for spermatogenesis. Nat Rev Uro 9(6):330–338. https://doi.org/10.1038/nrurol.2012.77
Dabbous Z, Atkin SL (2018) Hyperprolactinaemia in male infertility: clinical case scenarios. Arab J Uro 16(1):44–52. https://doi.org/10.1016/j.aju.2017.10.002
Gill-Sharma MK (2009) Prolactin and male fertility: the long and short feedback regulation. Inter J Endo 687259:1–13
Acknowledgements
The authors are thankful to the Faculty of Veterinary Medicine, University of Ilorin, for providing the facilities to conduct this research work. We also want to thank Mr. Ale Tosin of the Department of Pharmaceutical Chemistry, University of Ibadan, Nigeria for his assistance on the chromatographic analysis.
Plant authentication
Authentication of the plant parts was done at the University of Ibadan Herbarium where a voucher specimen of the plant was deposited. The voucher number UIH-22371 was assigned to the plant.
Funding
NA.
Author information
Authors and Affiliations
Contributions
BA drafted the work, designed it, and analyzed the results. OFO and SKO revised the proposal draft and study design and did substantial contribution in manuscript preparation. AOM made substantial contribution in manuscript preparation and revision of the study. AA analyzed part of the results. The authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The rats were kept at the experimental animal house of the Faculty of Veterinary Medicine, University of Ilorin, Nigeria. The rats were fed with Pelletized grower poultry feeds (Vital feeds®, Grand cereals Limited, Jos, Nigeria) and water was provided ad libitum. All experimental protocols were carried out according to internationally approved principles for the handling of experimental animal, use and care. The ethical approval obtained for this work was obtained from University of Ibadan Animal Care and Use Research Ethics Committee with reference number UI-ACUREC/APP/2016/002.
Consent for publication
N/A.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afisu, B., Abdulfatah, A., Mistura, A.O. et al. In vivo bioactivity-guided isolation of antifertility fraction of Waltheria indica Linn. root in male Wistar rats. Futur J Pharm Sci 7, 80 (2021). https://doi.org/10.1186/s43094-021-00228-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00228-0