Abstract
Background
Tuberculosis is evidently a major health threat among human populations worldwide. The current study presents the synthesis of new 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one analogs (4a–o) as potential Mycobacterium tuberculosis DNA gyrase inhibitors. DNA gyrase regulates DNA topology in MTB and has been a target of choice for antibacterial therapy. With this in mind, the synthesized derivatives (4a–o) were subjected to in vitro antitubercular evaluation by the MABA method and were tested for MTB DNA gyrase inhibition by supercoiling assay.
Results
All the synthesized compounds displayed inhibition of MTB within the MIC range of 1.56–12.5 μM. Further, out of the selected compounds that underwent DNA gyrase inhibition, compound 4o proved to be a potent lead molecule by displaying 82% of enzyme inhibition at 1 μM. All the synthesized derivatives also underwent molecular docking studies to comprehend their hypothetical binding interactions with Mycobacterium smegmatis GyrB.
Conclusion
All the results suggested that most of the synthesized derivatives inhibited Mycobacterium tuberculosis, and some 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one analogs could act as leads for the development of antitubercular agents.
Similar content being viewed by others
Background
Tuberculosis (TB) has single-handedly been successful in causing major health threats among human populations worldwide. TB is primarily caused by Mycobacterium tuberculosis (MTB) and has been one of the prime killers among communicable diseases caused by a sole infectious agent in humans, in their most productive age of 15–49 years. This lethal disease has been steadily devouring millions of individuals every year causing ill health and death throughout the globe. According to the Global TB Report 2019 by the World Health Organization, 10 million people developed TB, and 1.2 million deaths were recorded in the year 2018 [1,2,3]. The South-East Asia Region (SEAR) accounts for 41% of the global burden for TB incidence. India alone has been a host to about one fourth (23%) of the global TB bases [4]. Thus, the Indian and the global scenario of the devastating ailment of TB has reached an alarming stage and needs to be efficiently curbed in order to ensure a better quality of life for all human mankind.
The current therapy of TB dates back to a few decades which is at least 40 years old and has resulted in the rapid emergence of drug-resistant MTB strains. The first-line and second-line drugs used to treat TB today are seemingly unsuccessful in reining in this fast-spreading epidemic [5, 6]. Hence, it is of utmost importance to urgently discover alternative drug therapy with a newer mechanism of action which will aid in controlling the spread of TB. DNA gyrase, which is a type II DNA topoisomerase, has been an efficient antitubercular therapy target. It is one of the two DNA topoisomerases found in MTB. DNA topoisomerases are divided into two classes according to their mechanism of action, type I and type II (which cause transient single- and double-stranded breaks in DNA, respectively). They are classified as types IA, IB, and IIA, IIB. Each species has at least one enzyme from each type based on their functions. The MTB genome encodes one of each of type I and type II (DNA gyrase) topoisomerases. This is an important feature in the drug discovery process as it provides a susceptible target for enzyme inhibition. The function of DNA gyrase is to induce negative supercoiling of DNA, reducing the strain during the unwinding of DNA. DNA gyrase possesses two GyrA and GyrB subunits, making it a heterotetrameric (A2B2) structure. GyrA subunit contains tyrosine at the active sire required for cleavage and religation of DNA, while the GyrB subunit aids in the ATP hydrolysis, providing energy for the DNA replication process. There are various studies wherein synthesized quinoline derivatives have successfully acted as antitubercular agents by inhibiting DNA gyrase [7,8,9]. Fluoroquinolones, used as antitubercular drugs, are also known to target the GyrA subunit. However, resistance developed by MTB to these drugs has affected their efficacy, suggesting that the GyrB domain may prove to be an area of opportunity for newer antitubercular drug discovery [10,11,12]. Another biologically important 4-thiazolidinone scaffold has continually been investigated for the development of newer medicinal agents. Various 4-thiazolidinone derivatives have prominently displayed their ability to inhibit MTB [13,14,15,16].
Hence, in this context, and in our ongoing conquest of finding newer drugs for curbing TB [16,17,18,19], the present study was planned to design and synthesize new quinoline analogs via molecular hybridization, as antitubercular agents targeting MTB DNA gyrase.
Method
Chemistry
General procedure for synthesis of 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one analogs (4a–o)
The synthesis of target compounds (4a–o) was achieved via a synthetic procedure as depicted in Scheme 1. The synthesized hydrazone intermediates (3a–o) (0.015 mol) prepared via known procedure [20] were further refluxed with thioglycolic acid (0.015 mol) and a catalytic amount of zinc chloride in N,N-dimethylformamide (20 mL) for 48 h. After cooling, the mixture was poured onto crushed ice. The precipitate formed was filtered, subsequently washed with water, and recrystallized from ethanol to yield the title compounds (4a–o).
Biological evaluation
In vitro antitubercular activity
The antimycobacterial activity was assessed against Mycobacterium tuberculosis H37Rv (ATCC 27294) using the microplate Alamar Blue assay (MABA) method [16, 21].
MTB DNA supercoiling assay
DNA supercoiling assay was carried out using MTB DNA gyrase via a previously established protocol [22, 23].
Molecular docking studies
The various poses of interactions between the synthesized compounds and receptor protein binding sites were studied by performing molecular docking according to the previously reported process [16].
In silico ADME predictive study
Various ADME parameters of all the synthesized derivatives were calculated by using the QikProp module of the Schrodinger molecular modeling software. The calculated parameters were compared with the recommended values of the QikProp properties mentioned in the QikProp User Manual.
Results
Synthesis and spectral data
3-((7-Chloroquinolin-4-yl)amino)-2-(4-fluorophenyl)thiazolidin-4-one (4a)
Yield: 85%, m.p. 282 °C; IR (KBr, cm−1): 3215 (NH), 2924 (CH stretch of CH2), 1668 (C=O), 1612 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.71 (s, 1H, CH-Thiaz), 7.30–7.39 (m, 3H, Ar-H), 7.51 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.84 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.88–7.94 (m, 3H, Ar-H), 8.26–8.40 (m, 3H, Ar-H), 11.42 (s, 1H, NH)
2-(2-Chlorophenyl)-3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one (4b)
Yield: 61%, m.p. 280 °C; IR (KBr, cm−1): 3230 (NH), 2924 (CH stretch of CH2), 1608 (C=O), 1585 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.75 (s, 1H, CH-Thiaz), 7.28–7.30 (m, 3H, Ar-H), 7.46 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.74 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.81–7.93 (m, 3H, Ar-H), 8.30–8.55 (m, 3H, Ar-H), 11.40 (s, 1H, NH)
4-(4-Chlorophenyl)-3-((7-chloroquinolin-4-yl)amino)thiazolidin-2-one (4c)
Yield: 67%, m.p. 150 °C; IR (KBr, cm−1): 3232 (NH), 2924 (CH stretch of CH2), 1608 (C=O), 1577 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.71 (s, 1H, CH-Thiaz), 7.25–7.33 (m, 3H, Ar-H), 7.49 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.79 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.83–7.89 (m, 3H, Ar-H), 8.22–8.48 (m, 3H, Ar-H), 11.40 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-2-(2,3-dichlorophenyl)thiazolidin-4-one (4d)
Yield: 73%, m.p. 300 °C; IR (KBr, cm−1): 3236 (NH), 2904 (CH stretch of CH2), 1664 (C=O), 1618 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.69 (s, 1H, CH-Thiaz), 7.25–7.33 (m, 3H, Ar-H), 7.45 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.72 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.80–7.87 (m, 3H, Ar-H), 8.30–8.51 (m, 2H, Ar-H), 11.48 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-4-(2,4-dichlorophenyl)thiazolidin-2-one (4e)
Yield: 77%, m.p. > 300 °C; IR (KBr, cm−1): 3240 (NH), 2904 (CH stretch of CH2), 1662 (C=O), 1616 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.71 (s, 1H, CH-Thiaz), 7.49–7.53 (m, 2H, Ar-H), 7.58 (d, 1H, J = 11.2 Hz, CH2-Thiaz), 7.94 (s, 2H, Ar-H), 8.16 (d, 1H, J = 8.4 Hz, CH2-Thiaz), 8.46–8.82 (m, 4H, Ar-H), 12.56 (s, 1H, NH)
2-(4-Bromophenyl)-3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one (4f)
Yield: 81%, m.p. 140 °C; IR (KBr, cm−1): 3244 (NH), 2908 (CH stretch of CH2), 1662 (C=O), 1618 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.74 (s, 1H, CH-Thiaz), 7.21-7.30 (m, 3H, Ar-H), 7.43 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.72 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.80–7.91 (m, 3H, Ar-H), 8.26–8.51 (m, 2H, Ar-H), 11.52 (s, 1H, NH)
2-(3-Bromo-4-fluorophenyl)-3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one (4g)
Yield: 66%, m.p. 294 °C; IR (KBr, cm−1): 3238 (NH), 2924 (CH stretch of CH2), 1668 (C=O), 1612 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.71 (s, 1H, CH-Thiaz), 7.28–7.38 (m, 3H, Ar-H), 7.44 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.76 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.88–7.96 (m, 3H, Ar-H), 8.28–8.51 (m, 2H, Ar-H), 11.48 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-4-(4-methoxyphenyl)thiazolidin-2-one (4h)
Yield: 62%, m.p. 260 °C; IR (KBr, cm−1): 3228 (NH), 2895 (CH stretch of CH2), 1670 (C=O), 1612 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.78 (s, 1H, CH-Thiaz), 3.81 (s, 3H, OCH3), 7.01–7.06 (m, 3H, Ar-H), 7.34 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.57 (d, 1H, J = 8.4 Hz, CH2-Thiaz), 7.74–7.84 (m, 3H, Ar-H), 8.37–8.48 (m, 3H, Ar-H), 12.56 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-2-(3,4-dimethoxyphenyl)thiazolidin-4-one (4i)
Yield: 60%, m.p. 130 °C; IR (KBr, cm−1): 3246 (NH), 2893 (CH stretch of CH2), 1610 (C=O), 1550 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.68 (s, 1H, CH-Thiaz), 3.18 (s, 3H, OCH3), 3.34 (s, 3H, OCH3), 7.35–7.44 (m, 2H, Ar-H), 7.56 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.82 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.89–8.05 (m, 3H, Ar-H), 8.33–8.52 (m, 3H, Ar-H), 12.42 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-4-(3,4,5-trimethoxyphenyl)thiazolidin-2-one (4j)
Yield: 50%, m.p. 280 °C; IR (KBr, cm−1): 3226 (NH), 2929 (CH stretch of CH2), 1651 (C=O), 1608 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.72 (s, 1H, CH-Thiaz), 3.71 (s, 3H, OCH3), 3.86 (s, 6H, OCH3), 7.34 (d, 1H, J = 8.0 Hz, CH2-Thiaz), 7.38 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.51–7.98 (m, 4H, Ar-H), 8.33–8.44 (m, 3H, Ar-H), 11.56 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-2-(4-nitrophenyl)thiazolidin-4-one (4k)
Yield: 61%, m.p. > 300 °C; IR (KBr, cm−1): 3334 (NH), 2937 (CH stretch of CH2), 1614 (C=O), 1577 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.75 (s, 1H, CH-Thiaz), 7.37–7.49 (m, 3H, Ar-H), 7.54 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.81 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.90–8.10 (m, 3H, Ar-H), 8.38–8.62 (m, 3H, Ar-H), 11.56 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-4-(4-hydroxyphenyl)thiazolidin-2-one (4l)
Yield: 62%, m.p. > 300 °C; IR (KBr, cm−1): 3192 (NH), 2958 (CH stretch of CH2), 1697 (C=O), 1608 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.72 (s, 1H, CH-Thiaz), 6.82–7.21 (m, 4H, Ar-H), 7.42 (d, 1H, J = 7.6 Hz, CH2-Thiaz), 7.60 (d, 1H, J = 8.0 Hz, CH2-Thiaz), 8.19–8.52 (m, 5H, Ar-H), 9.82 (s, 1H, OH), 11.01 (s, 1H, NH)
4-(3-((7-Chloroquinolin-4-yl)amino)-2-oxothiazolidin-4-yl)benzonitrile (4m)
Yield: 58%, m.p. > 300 °C; IR (KBr, cm−1): 3228 (NH), 2858 (CH stretch of CH2), 1664 (C=O), 1610 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.71 (s, 1H, CH-Thiaz), 7.43 (d, 1H, J = 7.8 Hz, CH2-Thiaz), 7.47–7.95 (m, 4H, Ar-H), 8.10 (d, 1H, J = 8.4 Hz, CH2-Thiaz), 8.53–8.95 (m, 5H, Ar-H), 12.20 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-2-(4-(dimethylamino)phenyl)thiazolidin-4-one(4n)
Yield: 60%, m.p. 195 °C; IR (KBr, cm−1): 3219 (NH), 2899 (CH of CH2), 1664 (C=O), 1608 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.72 (s, 1H, CH-Thiaz), 3.24 (s, 6H, CH3), 6.75–7.33 (d, 4H, J = 8.0 Hz, Ar-H), 7.48 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.61 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.87–8.47 (m, 5H, Ar-H), 11.91 (s, 1H, NH)
3-((7-Chloroquinolin-4-yl)amino)-2-(thiophen-2-yl)thiazolidin-4-one (4o)
Yield: 67%, m.p. > 300 °C; IR (KBr, cm−1): 3192 (NH), 2897 (CH stretch of CH2), 1618 (C=O), 1570 (C=N); 1H-NMR (400 MHz; DMSO-d6): δ = 2.78 (s, 1H, CH-Thiaz), 6.83–7.02 (m, 3H, Ar-H), 7.38 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.65 (d, 1H, J = 8.8 Hz, CH2-Thiaz), 7.70–7.82 (m, 3H, Ar-H), 8.38–8.56 (m, 3H, Ar-H), 11.82 (s, 1H, NH)
Discussion
Chemistry
Various new quinoline-thiazolidinone analogs (4a–o) were synthesized via a two-step process wherein, firstly, 7-chloro-4-hydrazinylquinoline (1) underwent condensation at room temperature with different substituted aromatic aldehydes (2a–o) in the presence of absolute ethanol to yield substituted hydrazone intermediates (E)-4-(2-benzylidenehydrazinyl)-7-chloroquinolines (3a–o) [20]. The subsequent reaction step yielded the target compounds (4a–o) via cyclization between the hydrazone intermediates (3a–o) and thioglycolic acid in N,N-dimethyl formamide (DMF) and zinc chloride (Scheme 1). All the synthesized analogs underwent characterization by FTIR and 1H-NMR spectroscopy. It was observed that the IR spectral data displayed absorption bands around 3201–3236 cm−1 which were attributable to the hydrazinyl NH group. Characteristic peaks for the thiazolidinyl CH of CH2 were observed in the range of 2858–2929 cm−1. The IR spectra also indicated peaks around 1664 cm−1, allocated to C=O group and between 1608 cm−1 and 1618 cm−1 which were attributed to the C=N group. Furthermore, all the synthesized derivatives underwent proton NMR spectral studies. It was evident from the NMR spectra that a singlet signal appeared around 2.72 ppm, which was attributed to the CH proton at the C-2 position in the thiazolidinyl ring. The characteristic peaks of thiazolidinone CH2 protons at the C-5 position were observed as doublet signals in the range of 7.34–7.58 and 7.72–8.16 ppm. Moreover, the NMR spectral data illustrated the presence of a singlet signal which resonated at around 11.01–12.56 ppm, attributable to the hydrazinyl NH proton.
Biological activities
In vitro antitubercular activity
The synthesized derivatives (4a–o) underwent in vitro antitubercular screening against Mycobacterium tuberculosis H37Rv (ATCC 27294) by the MABA method [16, 21]. The antimycobacterial evaluation results are summarized in Table 1 and expressed in terms of minimum inhibitory concentration (MIC). The standard drugs used were ethambutol, pyrazinamide, and ciprofloxacin. It was observed that all the synthesized derivatives exhibited significant antimycobacterial potency within the MIC range of 1.56–12.5 μM. On further structure-activity relationship study, it was seen that the difference in the MIC values of the derivatives was a result of the different substitutions on the phenyl ring attached to the C-2 position of the thiazolidinone ring structure. Among the fifteen synthesized analogs, compounds 4e, 4g, 4n, and 4o were the most potent with a MIC of 1.56 μM. The above four analogs displayed better activity as compared to the standard drugs pyrazinamide and ciprofloxacin (MIC = 3.12 μM). It may be attributed to the presence of two electronegative chloro (-Cl) groups at ortho and para positions of the benzyl ring in compound 4e and the presence of bromo (-Br) group at the meta position and fluoro (-F) group at the para position of the benzyl ring in compound 4g. Compound 4n contains a benzyl ring with dimethyl amino substitution at the benzyl ring para position whereas compound 4o possesses a 2-thiophene substituent instead of the phenyl ring attached to the C-2 position of the thiazolidinone ring structure. Further, compound 4f was shown to inhibit MTB at a MIC of 3.12 μM. This comparatively lowered activity may be due to the presence of a bromo substituent at the para position of the benzyl ring. Compounds 4h and 4i also displayed MTB inhibition at MIC = 3.12 μM. The benzyl ring in compound 4h bears a methoxy group at para position, while the benzyl ring in analog 4i bears two methoxy groups at meta and para positions. It was thus observed that the activity displayed by 4f, 4h, and 4i was similar to the MIC displayed by the standard pyrazinamide and ciprofloxacin (MIC = 3.12 μM). Furthermore, the results revealed that compounds 4a–c and 4k exhibited moderate MTB inhibition at MIC = 6.25 μM. This may be due to the fact that these compounds contain at least one electronegative group attached at the ortho or para position of the benzyl ring. Derivative 4a contains a phenyl ring with 4-fluoro substituent, while derivatives 4b, 4c, and 4k contain 2-chloro, 4-chloro, and 4-nitro substituents, respectively, on the phenyl ring structure. All the other derivatives, 4d, 4j, 4l, and 4m, have displayed comparatively moderate MIC values at 12.5 μM.
MTB DNA supercoiling assay
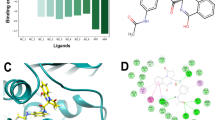
DNA gyrase is one of the two topoisomerases that the MTB genome encodes in order to preserve the DNA topology. Selected synthesized derivatives were assayed to analyze the inhibition of DNA Gyr B by DNA gyrase supercoiling assay [22, 23]. Novobiocin was used as a standard drug. Assay results revealed that four analogs 4e, 4h, 4n, and 4o displayed inhibition of DNA gyrase at 16%, 36%, 68%, and 82%, respectively, at 1 μM (Fig. 1). Thus, significant inhibition of the DNA gyrase was attained at a concentration of 1 μM by the potent compounds 4e, 4h, 4n, and 4o.
Molecular docking studies
Further, all the synthesized derivatives (4a–o) underwent molecular docking studies in order to analyze the binding affinity and binding interactions of the prepared compounds with the active site of Mycobacterium smegmatis GyrB ATPase retrieved from Protein Data Bank (PDB ID – 4B6C). The study of the different docking poses has revealed that most of the synthesized analogs have displayed binding interactions and a varied docking score with various amino acid residues of the receptor molecule (Fig. 2).
The standard drugs ciprofloxacin, pyrazinamide, and ethambutol are well bound to the receptor molecule by different interactions and docking scores of − 6.245, − 3.61, and − 5.913, respectively. Docking scores of all the synthesized derivatives lie between − 3.14 and − 4.533 (Table 1). Most of the synthesized derivatives display a better docking score as compared to the standard drugs. Compounds 4a, 4b, 4g, 4h, 4k, and 4l have displayed a pi-cation interactions with amino nitrogen of ARG 82 (Pi-cation interaction --- NH:ARG 82) and docking scores of − 4.395, − 3.891, − 3.771, − 4.437, − 3.573, and − 4.533, respectively. Analogs 4c and 4e have shown a halogen bond between chlorine and amino hydrogen of ARG 141 residue (4c:Cl --- HN:ARG 141, 4e:Cl --- HN:ARG 141). A hydrogen bond was formed between amino hydrogen of compound 4d and oxygen of ASN 52 residue (4d:NH --- O:ASN 52). Derivative 4f displayed a hydrogen bond between its amino hydrogen and oxygen atom of GLN 102 residue (4f:NH --- O:GLN 102), while derivative 4h has formed a hydrogen bond between its oxygen and amino hydrogen of ARG 141 amino acid residue (4h:O --- HN:ARG 141). It was also observed that an oxygen atom from the nitro group in analog 4k was bound to the amino groups in ARG 141 and ARG 82 residues (4k:O --- HN:ARG 141, 4k:O --- NH:ARG 82). Further, the hydroxyl group from compound 4l and cyano group from compound 4m displayed bond formation with amino group found in ARG 141 residue (4l:HO --- HN:ARG 141, 4m:CN --- HN:ARG 141). Also, derivative 4n exhibited a hydrogen bond between its amino nitrogen and oxygen atom of ASN 52 (4n:HN --- O:ASN 52), and another hydrogen bond between its quinolinyl nitrogen and amino hydrogen of GLY 83 (4n:N --- HN:GLY 83).
In silico ADME predictive study
In addition, various ADME parameters of the synthesized derivatives were calculated to better understand the effect of physicochemical and pharmacokinetic properties on their bioavailability in human bodies [24, 25]. The results (Table 2) displayed that the synthesized derivatives had good oral bioavailability as none of the compounds (4a–o) violated Lipinski’s rule of five (RoF) and most of the derivatives showed 100% of human oral absorption (HOA). Each synthesized analog displayed better oral absorption values as compared to the standard drugs ciprofloxacin, pyrazinamide, and ethambutol. Further, the lipophilicity (QPlog Po/w) and aqueous solubility (QPlogS) values of all the derivatives were within the permissible range. Partitioning through the blood-brain barrier (BBB) which was found to be within the recommended values of − 3.0 to 1.2 and a good score (> 500) of the predicted MDCK cell permeability (QPP MDCK) values for most of the synthesized compounds implied that the synthesized analogs have a potency to penetrate the BBB. In addition, a good amount of intestinal absorption was displayed by a majority of the compounds with better Caco-2 cell permeability (QPPCaco) values. Thus, the results demonstrated that the synthesized derivatives possessed acceptable values of pharmacokinetic and physicochemical parameters and could be considered as potent lead molecules with good membrane permeability and oral bioavailability.
Conclusion
We have demonstrated the synthesis 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one analogs (4a–o) and reported their in vitro MTB H37Rv inhibition potential. Results evidenced that compounds 4e, 4g, 4n, and 4o exhibited potent antimycobacterial activity (MIC of 1.56 μM). DNA gyrase inhibition assay revealed that compound 4o exhibited 82% of DNA gyrase inhibition at 1 μM. Moreover, computational screening study results also substantiated our findings that some of the newly synthesized 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one derivatives could prove to be promising lead molecules for the development of antitubercular agents.
Availability of data and materials
Data and material are available upon request.
Abbreviations
- TB:
-
Tuberculosis
- MTB:
-
Mycobacterium tuberculosis
- GyrA:
-
Gyrase A
- GyrB:
-
Gyrase B
- MIC:
-
Minimum inhibitory concentration
- SEAR:
-
South-East Asia Region
- TLC:
-
Thin layer chromatography
- MABA:
-
Microplate Alamar Blue assay
- DMF:
-
N,N-dimethyl formamide
- IR:
-
Infrared
- NMR:
-
Nuclear magnetic resonance
- ADME:
-
Absorption, distribution, metabolism, and excretion
- PDB:
-
Protein Data Bank
- ARG:
-
Arginine
- ASN:
-
Asparagine
- GLN:
-
Glutamine
- GLY:
-
Glycine
References
Global Tuberculosis Report (2019) – WHO. https://www.who.int/tb/publications/global_report/en/ Accessed 20 Mar 2020
Bending the curve - ending TB: Annual report 2017. World Health Organization; 2017.
World Health Organization (WHO) (2018). Global tuberculosis report, Geneva, 2018.
Tuberculosis Control in the South-East Asia Region: Annual report 2016 World Health Organization. (2016)
De Almeida MV, Saraiva MF, de Souza MVN, da Costa CF, Vicente FRC, Lourenco MCS (2007) Synthesis and antitubercular activity of lipophilic moxifloxacin and gatifloxacin derivatives. Bioorg Med Chem Lett 17:5661–5664. https://doi.org/10.1016/j.bmcl.2007.07.073
WHO Publication (2010) Guidelines for treatment of tuberculosis, 4th edn https://apps.who.int/iris/bitstream/handle/10665/44165/9789241547833_eng.pdf?sequence=1
Medapi B, Janupally R, Saxena S, Jonnalagadda PS, Medidhetti R, Kulkarni P, Yogeeswari P, Sriram D (2015) Design and synthesis of novel quinoline-aminopiperidine hybrid analogues as Mycobacterium tuberculosis DNA gyraseB inhibitors. Bioorg Med Chem 23:2062–2078. https://doi.org/10.1016/j.bmc.2015.03.004
Medapi B, Suryadevara P, Renuka J, Sridevi JP, Yogeeswari P, Sriram D (2015) 4-Aminoquinoline derivatives as novel Mycobacterium tuberculosis GyrB inhibitors: structural optimization, synthesis and biological evaluation. Eur J Med Chem 103:1–16. https://doi.org/10.1016/j.ejmech.2015.06.032
Blanco D, Perez-Herran E, Cacho M, Ballell L, Castro J, González del Río R, Lavandera JL, Remuinan MJ, Richards C, Rullas J, Vazquez-Munz MJ, Woldu E, Zapatero-Gonzalez MC, Angulo-Barturen I, Mendoza A, Barros D (2015) Mycobacterium tuberculosis gyrase inhibitors as a new class of antitubercular drugs. Antimicrob Agents Chemother 59:1868–1875. https://doi.org/10.1128/AAC.03913-14
Nagaraja V, Godbole AA, Henderson SA, Maxwell A (2017) DNA topoisomerase I and DNA gyrase as targets for TB therapy. Drug Discov Today 22(3):510–518. https://doi.org/10.1016/j.drudis.2016.11.006
Chandran M, Renuka J, Sridevi JP, Pedgaonkar GS, Asmitha V, Yogeeswari P, Sriram D (2015) Benzothiazinone-piperazine derivatives as efficient Mycobacterium tuberculosis DNA gyrase inhibitors. Int J Mycobacteriol 4:104–115. https://doi.org/10.1016/j.ijmyco.2015.02.002
Chaudhari K, Surana S, Jain P, Patel HM (2016) Mycobacterium tuberculosis (MTB) GyrB inhibitors: an attractive approach for developing novel drugs against TB. Eur J Med Chem 124:160–185. https://doi.org/10.1016/j.ejmech.2016.08.034
Jain AK, Vaidya A, Ravichandran V, Kashaw SK, Agrawal RK (2012) Recent developments and biological activities of thiazolidinone derivatives: a review. Bioorg Med Chem 20(11):3378–3395. https://doi.org/10.1016/j.bmc.2012.03.069
Tripathi AC, Gupta SJ, Fatima GN, Sonar PK, Verma A, Saraf SK (2014) 4-Thiazolidinones: the advances continue. Eur J Med Chem 72:52–77. https://doi.org/10.1016/j.ejmech.2013.11.01
Manjal SK, Kaur R, Bhatia R, Kumar K, Singh V, Shankar R, Kaur R, Rawal RK (2017) Synthetic and medicinal perspective of thiazolidinones: a review. Bioorg Chem 75:406–423. https://doi.org/10.1016/j.bioorg.2017.10.014
Salve PS, Alegaon SG (2018) Synthesis of new 7’-chloro-4-phenoxyquinoline analogues as potential antitubercular agents. Med Chem Res 27:1–14. https://doi.org/10.1007/s00044-017-1970-2
Salve PS, Alegaon SG, Sriram D (2017) Three-component, one-pot synthesis of anthranilamide Schiff bases bearing 4-aminoquinoline moiety as Mycobacterium tuberculosis gyrase inhibitors. Bioorg Med Chem Lett 27(8):1859–1866. https://doi.org/10.1016/j.bmcl.2017.02.031
Alegaon SG, Alagawadi KR, Sonkusare PV, Chaudhary SM, Dadwe DH, Shah AS (2012) Novel imidazo[2,1-b][1,3,4]thiadiazole carrying rhodanine-3-acetic acid as potential antitubercular agents. Bioorg Med Chem Lett 22:1917–1921. https://doi.org/10.1016/j.bmcl.2012.01.052
Alegaon SG, Alagawadi KR, Dadwe D (2014) Synthesis and antitubercular activity of novel 3,5-diaryl-4,5-dihydro-1H-pyrazole derivatives. Drug Res (Stuttg) 64:553–558. https://doi.org/10.1055/s-0033-1363976
Alegaon SG, Parchure P, Araujo L, Salve PS, Alagawadi KR, Jalalpure SS, Kumbhar VM (2017) Quinoline-azetidinone hybrids: synthesis and in vitro antiproliferation activity against Hep G2 and Hep3B human cell lines. Bioorg Med Chem Lett 27(7):1566–1571. https://doi.org/10.1016/j.bmcl.2017.02.043
Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RHJ (1998) Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol 36:362–366. https://doi.org/10.1128/JCM.36.2.362-366.1998
Jeankumar VU, Renuka J, Santosh P, Soni V, Sridevi JP, Suryadevara P, Yogeeswari P, Sriram D (2013) Thiazole-aminopiperidine hybrid analogues: design and synthesis of novel Mycobacterium tuberculosis GyrB inhibitors. Eur J Med Chem 70:143–153. https://doi.org/10.1016/j.ejmech.2013.09.025
Jeankumar VU, Reshma RS, Vats R, Janupally R, Saxena S, Yogeeswari P, Sriram D (2016) Engineering another class of anti-tubercular lead: hit to lead optimization of an intriguing class of gyrase ATPase inhibitors. Eur J Med Chem 122:216–231. https://doi.org/10.1016/j.ejmech.2016.06.042
Puskullu MO, Celik I, Erol M, Fatullayev H, Uzunhisarcikli E, Kuyucuklu G (2020) Antimicrobial and antiproliferative activity studies of some new quinoline-3-carbaldehyde hydrazone derivatives. Bioorg Chem 101:104014. https://doi.org/10.1016/j.bioorg.2020.104014
Maurya SS, Bahuguna A, Khan SI, Kumar D, Kholiya R, Rawat DS (2019) N-substituted aminoquinoline-pyrimidine hybrids: synthesis, in vitro antimalarial activity evaluation and docking studies. Eur J Med Chem 162:277–289. https://doi.org/10.1016/j.ejmech.2018.11.021
Acknowledgements
The authors are grateful to the principal, KLE College of Pharmacy, Belagavi, for providing the necessary facilities for the research. The authors are also grateful to the NMR Research Center, IISC, Bangalore, India, for providing the spectral data.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. PS: synthesis, characterization, and activity. PP, LA, and RK: synthetic application. SJ: analytical work. DS, VK, and ME: activity work. SA: design, synthesis, characterization and outline of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salve, P.S., Parchure, P., Araujo, L. et al. Design and synthesis of new 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one analogs as Mycobacterium tuberculosis DNA gyrase inhibitors. Futur J Pharm Sci 7, 10 (2021). https://doi.org/10.1186/s43094-020-00162-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-020-00162-7