Abstract
Background
Alpha-mangostin (AM) has been shown to have hypoglycemic activity. This study aimed to analyze the effects of AM at a dose of 100 mg/kg and 200 mg/kg to alleviate hyperuricemia and renal dysfunction on high-fat/high-glucose diet and low dose streptozotocin (HF/HG/STZ) injection-induced IR rat model. IR was induced in male Wistar rats by giving a HF/HG diet for 11 weeks and single injection of STZ (35 mg/kg, i.p.), then divided randomly into IR rats, IR rats treated with AM 100 and 200 mg/kgBW given by gavage for 8 weeks. At the end of the 11th week, all rats were killed, and the kidneys were taken to be analyzed for urate transporters 1 (URAT1) and glucose transporters 9 (GLUT9). We also assessed serum uric acid, proteinuria, BUN, creatinine clearance, HOMA-IR, and fasting blood glucose (FBG).
Results
We have found the significant increase in HOMA-IR and FBG levels of the IR rats, in comparison with its control groups, which were decreased significantly after AM administration at both doses. URAT1 and GLUT9 mRNA and protein expressions in kidney in the IR + AM at both doses groups also decreased compared those in the IR without treatment group, though the decrease in GLUT9 did not appear to be statistically significant. Consequently, hyperuricemia and renal dysfunction were attenuated by AM treatment at both doses.
Conclusion
After considering all findings, AM might be a potential candidate to ameliorate IR-induced hyperuricemia and renal dysfunction at least in part by modulating the renal URAT1.
Similar content being viewed by others
1 Background
Previous studies have demonstrated that increasing serum uric acid (SUA) levels often occur in patients with insulin resistance (IR) [6, 9]. Uric acid (UA) has also been reported to be one of the important factors in the occurrence of impaired kidney function [24]. Previous study has reported that in healthy normotensive adults, increase in SUA level was linked to subsequent deteriorating of kidney function [4]. Basically, the levels of SUA are governed by the UA transporters system in the proximal tubules of the kidney [10]. Briefly, UA after filtration process in the glomeruli is reabsorbed in the proximal tubules through URAT1 transporter on the apical side of the proximal tubules and then transcellularly transported through GLUT9 transporter which is on the basolateral membrane side which will then enter the systemic circulation [1, 22]. Previous study has reported that in metabolic syndrome rats there was an increase in the expression of UA transporters, namely URAT1 and GLUT9 which lead to hyperuricemia [19].
At present, herbal medicine get full attention as protector of IR complications, one of which is alpha mangostin (AM) [8]. The main xanthone isolated from the bark and dried sap of the Garcinia mangostana plants was AM. Multiple reports have shown that AM has several biological effects, including anti-oxidants, anti-inflammatory, pro-apoptotic, anti-proliferative, neuroprotective, hypoglycemic, and anti-obesity [15, 20]. We also previously reported that AM treatment has significant beneficial effects on High-Fat/High-Glucose/streptozotocin (HF/HG/STZ)-induced IR rats, which are obtained by decreasing plasma glucose, improving cardiac hypertrophy and fibrosis [23]. Even though recent evidence proposes that AM yielded potent anti-oxidative, anti-inflammatory, and hypoglycemic properties, not widely known regarding its effect on ameliorating hyperuricemia and renal dysfunction in IR. Therefore, the aim of this study is to evaluate the effects of AM to overcome hyperuricemia and renal dysfunction in rat model of IR.
2 Methods
2.1 Materials
All reagents used were of analytical grade and purchased from Sigma (CA, USA). High-fat diet (TestDiet, 58V8 rat chow) containing 46.1% fat, 35.8% carbohydrate, and 18.1% protein with total energy 4.60 kcal/g was purchased from Richmond, USA. Alpha mangostin was obtained from Aktin Chemical, Inc., Chengdu, China. Primary antibody against URAT1 was obtained from Abbiotec, USA (cat. # 250521), and GLUT9 was obtained from Invitrogen (cat. # PA5-22966).
2.2 Animal
Male Wistar rats (150–250 g; obtained from Animal Laboratory of National Institute of Health Research and Development, Indonesian Ministry of Health Indonesia) were housed in colony cages, maintained on a 12-h light/12-dark cycle. Rats in IR group were fed with high-fat (HF) diet along with 20% high-glucose (HG) drinking water for 3 weeks. Low-dose streptozotocin (STZ) (35 mg/kg) was injected by intraperitoneal (i.p.) after 3 weeks of giving a HF and HG diet. Streptozotocin was freshly prepared by dissolved in 0.01 M citrate buffer, pH 4.5. Fasting blood glucose (FBG) levels were measured 72 h after STZ injection, rats that have FBG levels ≥ 250 mg/dL can be included in the study. If the FBG has not reached the target, the injection of STZ can be repeated once with a half-dose of the initial dose. Then, the HF and HG diet were continued until the 11th week. As a result, thirty rats were allocated into control (C, n = 6), control + AM 200 (C + AM 200, n = 6), IR (n = 6), IR + AM100 (n = 6), and IR + AM 200 (n = 6). Alpha mangostin was dissolved in 1 mL of corn oil and administered orally for 8 weeks. The C and C + AM 200 rats were received 0.5 mL of distilled water alone. In addition, rats in the C and C + AM 200 groups received a standard diet and drinking water without glucose throughout the study and at Week 3 were given NaCl injection to mimic the actions performed in the treatment groups. At 11 weeks, all rats were anesthetized with a single i.p. injection of ketamine/xylazine 0.15 mL/100 g body weight, and euthanized by cervical dislocation, and their kidneys were excised and decapsulated. Protein analysis was performed on a portion of the kidney which has been frozen immediately in liquid nitrogen and stored at − 80 °C. Half of the kidneys were fixed in 10% formalin, and some were unfixed for morphological studies.
2.3 Estimation of biochemical parameters
Blood samples of all rats were withdrawn from the heart via heart puncture at the time of sacrifice and placed in a serum separator vacutainer tube. To separate serum sample, the blood was sit for 30 min at room temperature to clot, and then, blood was centrifuged at 1000 g, 4 °C, for 10 min. The serum, then, was used for the estimation of glucose, uric acid, urea, creatinine, and insulin. Twenty-four hours urine was also collected for each rat prior to killing by placing each rat in the metabolic cages for the measurement of creatinine using Jaffe method and protein concentration using Bradford method. Blood glucose was measured using Glucometer 4 Accu-check, CA, and was done at baseline, week-3, 5, 8, and 11, whereas serum uric acid (SUA) and urea were analyzed using the respective spectrophotometric diagnostic kit obtained from Diasys, Diagnostic Systems GmbH, Germany. Calculation of blood urea nitrogen (BUN) was done by multiplying the urea levels (mg/dL) by 0.467 [3]. Insulin was quantified with rat-specific ELISA kit (Mercodia, Uppsala, Sweden; Linco Research, St. Charles, MO, USA). HOMA-IR (homeostasis model assessment insulin resistance) was done using the following formula: HOMA-IR = blood glucose (mM) × insulin (mU/L)/22.5 [18]. The spectrophotometric analysis was performed using a UV–Vis Optizen. The calculation of creatinine clearance was carried out by the following formula: clearance creatinine (mL/min) = urine creatinine (mg/dL) × urine volume (mL)/plasma creatinine (mg/dL) × time (1440 min) [2].
2.4 Histopathological analysis
The kidney tissues were cut transversely with a thickness of 2-mm and then fixed in 10% formalin and stained with hematoxylin and eosin (HE) to demonstrate nucleus pyknotic and thickening of capillary wall in the kidney. Formalin-fixed, paraffin-embedded kidney tissues were used for immunohistochemical staining. The slides, then, were deparaffinized and hydrated and followed by washed in Tris-buffered saline (TBS; 10 mM/L Tris HCl, 0.85% NaCl, pH 7.5) which contain 0.1% bovine serum albumin. Activity of endogenous peroxidase was quelled by incubating the slides in methanol and 0.3% H2O2 in methanol. After being incubated overnight with primary antibodies, that are, URAT1 and GLUT9 (1:50 dilution) at 4 °C, the slides were washed in TBS buffer, added HRP conjugated secondary antibody (Santa Cruz), and incubated at room temperature for 45 min. Hematoxylin was used to counterstained the slides, and diaminobenzidine tetrahydrochloride (DAB) was used to visualize immunostaining. Twenty-five random fields per section were checked for all sections, and 3 animals per groups were used to assess immunostaining results.
2.5 Polymerase chain reaction
Kidney tissues were maintained by immersion in RNAlater (ThermoFisher, USA) immediately after sampling. Total RNA extraction was accomplished after homogenization using Ultra TurraxT8 (IKA Labortechnik, Staufen, Germany) in Quick-RNA Miniprep Plus Kit (Zymo Research) in accordance with the standard protocol. The total level of RNA is measured using a NanoDrop spectrophotometer at a wavelength of 260/280 nm and is considered pure if the purity index > 2.0. cDNA synthesis was carried out from equal quantities of RNA using ReverTra Ace™ qPCR Master Mix with gDNA Remover (Toyobo).
To analyze the URAT1 and GLUT9 mRNA, splicing polymerase chain reaction was performed in a qRT-PCR Light Cycler 480 using Thunderbird SYBR qPCR Mix kit (Toyobo). The total number of DNA templates used in each sample was 100 ng. Table 1 is the primer list used in this study. The conditions of PCR were as follows: pre-denaturation at 95 °C for 1 min, 40 cycles at 95 °C for 3 s, and 60 °C for 20 s. The quantity of mRNA was calculated via the cycle threshold (Ct) values, and the mRNA expression levels were measured according to the Livak method [16].
2.6 Statistical analysis
Data were reported as means ± standard error of the mean (SEM). Differences between groups were analyzed using one-way analysis of variance (ANOVA), followed by multiple comparisons using Tukey’s test. All data were analyzed using SPSS 20.0 software, and the results were said to be statistically significant when p value < 0.05.
3 Results
3.1 Beneficial effect of AM on HOMA-IR and fasting blood glucose in HF/HG/STZ-induced IR rats
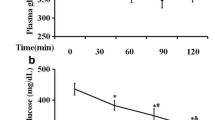
In order to determine the occurrence of IR, we assessed the HOMA-IR and FBG in HF/HG/STZ-induced IR rats. The vehicle-treated IR rats were noticed to have raised HOMA-IR (Fig. 1A) in comparison with control and control + AM 200 rats. The AM dose-dependently and significantly attenuated the HOMA-IR (p = 0.03) in HF/HG/STZ-induced IR rats. These results suggest that AM improves IR in HF/HG/STZ-induced IR rats. At week 3, the baseline value of FBG revealed that there were no significant changes among groups (Fig. 1B). From the week 5, after the injection of STZ, the HF/HG/STZ rats were shown significant changes in FBG in comparison with control and control + AM 200 rats. Interestingly, there were decreased in the FBG of rats that were given AM at both doses at week 8 and 11, even though it did not reach a statistically significant (p = 0.22) as compared to that of vehicle-treated IR rats.
3.2 Effect of AM on renal dysfunction in HF/HG/STZ-induced IR rats
In order to investigate the role of AM on renal dysfunction in HF/HG/STZ-induced IR rats, we examined proteinuria, BUN, and creatinine clearance. At 8 weeks after HF/HG/STZ administration, the vehicle-treated IR rats were shown significantly elevated in the proteinuria (p < 0.01) (Fig. 2A) and BUN (p < 0.01) (Fig. 2C) levels in comparison with control and control + AM 200 rats. The AM-treated IR rats showed significant changes in proteinuria (Fig. 2A) and BUN (Fig. 2C) in comparison with vehicle-treated IR rats. In contrary, our vehicle-treated IR rats did not significantly affect the creatinine clearance level, and AM had no effect as well on the creatinine clearance in IR rats (Fig. 2B).
3.3 Effect of AM on SUA in HF/HG/STZ-induced IR rats
We next focused on renal UA handling. Interestingly, SUA in the vehicle-treated IR rats was significantly increased compared with that of the control and control + AM 200 rats (p < 0.001) (Fig. 2D). The SUA levels of AM-treated IR rats were decreased significantly both at a dose of 100 mg/kg/day (p < 0.03) and of 200 mg/kg/day as compared to that of vehicle-treated IR rats (p < 0.001) (Fig. 2D).
3.4 Effect of AM on the mRNA expression of URAT1 and GLUT9 in HF/HG/STZ-induced IR rats
As shown in Fig. 3A, the levels of renal mRNA expression of URAT1 were significantly increased in the vehicle-treated IR rats as compared to that of control and control + AM 200 rats. In line with the mRNA expression, the protein expression of URAT1 was increased in the vehicle-treated IR rats (Fig. 3B), and AM at both doses evidently decreased the expression of URAT1 in the HF/HG/STZ-induced IR rats (Fig. 3A, B). In contrary, mRNA expression of GLUT9 did not show any difference between groups (Fig. 3C, D).
Effect of AM on A URAT1 mRNA expression, B URAT1 performed by IHC in kidney sections, C GLUT9 mRNA expression, and D GLUT9 performed by IHC. Both expression of URAT1 and GLUT9 mRNA and protein were upregulated in animals fed with HF/HG/STZ injection. **p < 0.01 versus C and C + a-MG 200; ††p < 0.01 versus IR; $$p < 0.01 versus IR. (B and D, magnification × 200)
3.5 Effect of AM on tubulointerstitial injury in HF/HG/STZ-induced IR rats
To assess changes in the kidneys after HF/HG/STZ administration and AM intervention, we performed kidney histological analysis in HF/HG/STZ-induced IR rats. As shown in Fig. 4, vehicle-treated IR rats demonstrated thickening of capillary wall in renal artery and pyknosis of the nuclei tubules (Fig. 4C). AM administration prevented the occurrence of these lesions as indicated by a decrease in the percentage of tubular damage in HF/HG/STZ-induced IR rats (Fig. 4D, E).
Effect of AM on HE staining of histological kidney sections. Control and control + AM 200 kidney have normal architecture (A, B), while IR group (C) showed thickening of capillary wall and nuclear pyknotic (arrows). Alfa-mangostin administration at both doses showed similar architecture to control groups (D, E). Magnification × 200
4 Discussion
In this study, we investigated the effects of AM to alleviate hyperuricemia and renal dysfunction in the HF/HG/STZ-induced IR rat model. It has been demonstrated that hyperuricemia and IR influence each other based on pathophysiological and metabolic point of view. Hyperuricemia can cause IR at least in part by reducing the bioavailability of nitric oxide and generation of mitochondrial oxidative stress through several mechanisms [12, 14]. Conversely, IR can induce hyperuricemia by increasing the reabsorption of UA in the renal tubules as manifested by up-regulation URAT1 expression [25]. The current study firstly demonstrated that IR has occurred as evidenced by an increase in HOMA-IR and FBG in experimental animals receiving a HF/HG diet and low-dose STZ injection for 11 weeks. Our results also indicate that treating IR rat with AM at doses of 100 and 200 mg/kg/day for 8 weeks significantly reduced HOMA-IR and FBG and led not only to improved hyperuricemia but also ameliorated renal dysfunction and pathological changes with attenuated of nuclear pyknosis of tubules and thickening of capillary wall of renal artery compared with those in vehicle-treated IR rats. In the kidney, we found that the URAT1 and GLUT9 transporters were upregulated in the vehicle-treated IR rats. Interestingly, administration of AM at both doses could decrease them as compared to that of vehicle-treated rats.
IR and hyperuricemia are positively correlated with the progression of cardiorenal and metabolic diseases. In fact, hyperuricemia can arise due to reduced renal or extrarenal excretion or overproduction of UA [5]. However, more than 90% of all cases of hyperuricemia are the result of the diminished renal excretion of UA. Facchini et al., demonstrated that the extent of IR is associated with increased urinary UA reabsorption in human [30]. Through a genome-wide association studies, it is known that urate transporters in the kidneys play an important role in UA handling in the kidneys [21, 28]. In fact, UA is filtered in the glomeruli followed by its reabsorption in the proximal tubules through URAT1 at the apical membrane of proximal tubules and transcellularly transported via GLUT9 at the basolateral membrane [17]. Wu et al. have reported that on hyperuricemia mice there was an increased in plasma UA levels, mRNA expression of renal URAT1, and GLUT9 [29]. Also, Ng et al. have demonstrated that the hyperglycemia and hyperuricemia greatly linked with the increase in renal glucose and UA transporters [19]. In line with the previous studies, we have shown that the IR kidney rats had significant increase in the mRNA expression of kidney URAT1 (Fig. 3A), whereas although there was an increase in mRNA expression of kidney GLUT9 (Fig. 3C), the increase was not significant. Our present study is in accordance with previously reported data that HF diet increased the protein level of URAT1 kidney transporter without any changes in the protein level of GLUT9 kidney transporter in mice, whereas in leptin-deficient mice, both of the kidney transporters, URAT1 and GLUT9, were increased. In fact, hyperuricemia was associated with the increased protein expression of URAT1 transporter without increased expression of GLUT9, which was enhanced by fat [26], similar to our study that used HF diet to induce IR. In this study, we also observed that the administration of AM at both doses has significant favorable effects on HF/HG/STZ-induced IR, which are obtained by blocking the upregulation of urate transporter. Previous study has demonstrated that AM administration to obese mice could improve insulin sensitivity and glucose tolerance, as well as ameliorated adipose inflammation and hepatic steatosis through inhibition of C–C chemokine receptor 2 [13]. In line with the previous study, we also showed that AM could improve insulin sensitivity and reduce the levels of FBG. However, to the best of our knowledge, our study was the first to evaluate the effects of AM in alleviating hyperuricemia and kidney dysfunction in the IR rat model.
IR, obesity, hypertension, and diuretics used have been shown to decrease renal excretion of UA which leads to elevated SUA level. A prospective cohort study involving 900 healthy and normotensive adults showed that high SUA levels were related with worsening renal function [4]. In addition, it has been demonstrated that the activation of the renin-angiotensin system as well as cyclooxygenase-2 pathways is strongly correlated with increased SUA level as shown by preglomerular arterial disease, renal inflammation, and hypertension [11]. In the present study, the HF/HG/STZ-induced IR rats displayed significantly increased proteinuria, as well as a decreased creatinine clearance compared with the normal rats, and AM at both doses treatment significantly reduced the proteinuria and elevated creatinine clearance, though it was not significantly different with the HF/HG/STZ-induced IR rats (Fig. 2A, B). Moreover, we observed significant decreases in the levels of BUN and SUA, after AM treatment in these rats (Fig. 2C, D). Chae et al. have shown that in diet-induced obesity mice, AM administration was able to attenuate hepatic steatosis, reduced serum glucose, induced weight loss, and improved lipid profile through sirtuin 1-AMP-activated protein kinase and peroxisome proliferator-activated receptor (PPAR) gamma pathways [7]. Moreover, Watanabe et al. reported that mangosteen extract which contained alpha and gamma mangostins could potentially represent a treatment of obesity and insulin resistance in obese female patients [27].
5 Conclusion
Given the promising results we showed, we suggest possible role of AM in selectively decreasing renal transporter URAT1 which leads to ameliorate hyperuricemia in the rat model of IR, though we failed to demonstrate a statistically significant difference of GLUT9, creatinine clearance as well as dose-dependency of AM doses as compared to that of control group.
Availability of data and materials
Not applicable.
Abbreviations
- AM:
-
Alpha mangostin
- BUN:
-
Blood urea nitrogen
- DAB:
-
Diaminobenzidine tetrahydrochloride
- FBG:
-
Fasting blood glucose
- HE:
-
Hematoxylin and eosin
- HF/HG/STZ:
-
High-fat/high-glucose/streptozotocin
- HG:
-
High-glucose
- HOMA-IR:
-
Homeostasis model assessment insulin resistance
- IR:
-
Insulin resistance
- i.p:
-
Intraperitoneal
- SUA:
-
Serum uric acid
- STZ:
-
Streptozotocin
- TBS:
-
Tris-buffered saline
- UA:
-
Uric acid
References
Auberson M, Stadelmann S, Stoudmann C et al (2018) SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney. Pflugers Arch Eur J Physiol 470:1739–1751
Bagheri F, Gol A, Dabiri S, Javadi A (2011) Preventive effect of garlic juice on renal reperfusion injury. Iran J Kidney Dis 5:194–200
Bazzano T, Restel TI, Porfirio LC, Souza AS, Silva IS (2015) Renal biomarkers of male and female Wistar rats (Rattus norvegicus) undergoing renal ischemia and reperfusion. Acta Cir Bras 30:277–288
Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M (2010) Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis 56:264–272
Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT et al (2015) Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 33:1729–1741
Castro VMF, Melo AC, Belo VS, Chaves VE (2017) Effect of allopurinol and uric acid normalization on serum lipids hyperuricemic subjects: a systematic review with meta-analysis. Clin Biochem 50:1289–1297
Chae HS, Kim YM, Bae JK, Sorchhann S, Yim S, Han L et al (2016) Mangosteen extract attenuates the metabolic disorders of high-fat-fed mice by activating AMPK. J Med Food 19:148–154
Chasan T, Muth A (2021) The diverse bioactivity of α-mangostin and its therapeutic implications. Future Med Chem 13(19):1679–1694. https://doi.org/10.4155/fmc-2021-0146
Chen J, Ge J, Zha M, Miao JJ, Sun ZL, Yu JY (2020) Effects of uric acid-lowering treatment on glycemia: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 11:577
Feng Y, Sun F, Gao Y, Yang J, Wu G, Lin S, Hu J (2017) Taurine decreased uric acid levels in hyperuricemic rats and alleviated kidney injury. Biochem Biophys Res Commun 489:312–318
Galán I, Goicoechea M, Quiroga B, Macías N, Santos A, García de Vinuesa MS et al (2018) Hyperuricemia is associated with progression of chronic kidney disease in patients with reduced functioning kidney mass. Nefrologia (Engl Ed) 38:73–78
Johnson RJ, Stenvinkel P, Martin SL, Jani A, Sánchez-Lozada LG, Hill JO et al (2013) Redefining metabolic syndrome as a fat storage condition based on studies of comparative physiology. Obesity (Silver Spring) 21:659–664
Kim HM, Kim YM, Huh JH, Lee ES, Kwon MH, Lee BR et al (2017) α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C–C chemokine receptor 2. PLoS ONE 12:e0179204
Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T et al (2012) Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 7:e47948
Lazarus G, Alexander S, Kusuma GO, Wijaya K, Soetikno V (2020) Antioxidative activities of alpha-mangostin in high-fat/high-glucose diet and streptozotocin-induced insulin-resistant rodents. J App Pharm Sci 10:035–039
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Mandal AK, Leask MP, Estiverne C, Choi HK, Merriman TR, Mount DB (2021) Genetic and physiological effects of insulin on human urate homeostasis. Front Physiol 2(12):713710
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Ng HY, Lee YT, Kuo WH, Huang PC, Lee WC, Lee CT (2018) Alterations of renal epithelial glucose and uric acid transporters in fructose induced metabolic syndrome. Kidney Blood Press Res 43:1822–1831
Ovalle-Magallanes B, Eugenio-Pérez D, Pedraza-Chaverri J (2017) Medicinal properties of mangosteen (Garcinia mangostana L.): a comprehensive update. Food Chem Toxicol 109(Pt 1):102–122
Ruiz A, Gautschi I, Schild L, Bonny O (2018) Human mutations in SLC2A9 (Glut9) affect transport capacity for urate. Front Physiol 18(9):476
Sangkop F, Singh G, Rodrigues E, Gold E, Bahn A (2016) Uric acid: a modulator of prostate cells and activin sensitivity. Mol Cell Biochem 414:187–199
Soetikno V, Murwantara A, Andini P, Charlie F, Lazarus G, Louisa M et al (2020) Alpha-mangostin improves cardiac hypertrophy and fibrosis and associated biochemical parameters in high-fat/high-glucose diet and low-dose streptozotocin injection-induced type 2 diabetic rats. J Exp Pharmacol 12:27–38
Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS (2018) Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis 71:362–370
Tan RJ, Zhou D, Xiao L, Zhou L, Li Y, Bastacky SI et al (2015) Extracellular superoxide dismutase protects against proteinuric kidney disease. J Am Soc Nephrol 26:2447–2459
Tanaka Y, Nagoshi T, Takahashi H, Oi Y, Yoshii A, Kimura H et al (2022) URAT1-selective inhibition ameliorates insulin resistance by attenuating diet-induced hepatic steatosis and brown adipose tissue whitening in mice. Mol Metab 55:101411
Watanabe M, Gangitano E, Francomano D, Addessi E, Toscano R, Costantini D et al (2018) Mangosteen extract shows a potent insulin sensitizing effect in obese female patients: a prospective randomized controlled pilot study. Nutrients 10:586
Wu Y, He H, Yu K, Zhang M, An Z, Huang H (2019) The association between serum uric acid levels and insulin resistance and secretion in prediabetes mellitus: a cross-sectional study. Ann Clin Lab Sci 49:218–223
Wu XH, Ruan JL, Zhang J, Wang SQ, Zhang YW, Pallidifloside D (2014) A saponin glycoside constituent from Smilax riparia, resist to hyperuricemia based on URAT1 and GLUT9 in hyperuricemic mice. J Ethnopharmacol 157:201–205
Yu P, Huang L, Wang Z, Meng X, Yu X (2021) The association of serum uric acid with beta-cell function and insulin resistance in nondiabetic individuals: a cross-sectional study. Diabetes Metab Syndr Obes 14:2673–2682
Acknowledgements
We thank Chiswyta and Annisa for assistance in conducting the gene analysis.
Funding
This work was supported by Universitas Indonesia Grants (BA-1239/UN2.RST/PPM.00.03.01/2020).
Author information
Authors and Affiliations
Contributions
VS participated in the design of the study, performed statistical analysis, drafted the manuscript, and carried out the qRT-PCR studies. AM carried out the qRT-PCR and histopathology studies and helped the statistical analysis. AAJ participated in histopathological studies and helped drafted the manuscript. ML performed statistical analysis, helped drafted the manuscript, and participated in the design of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and Consent to participate
Animal studies have been approved by the Ethics Committee of Universitas Indonesia (ethical clearance # 0084/UN2.F1/ETIK/2018), and all efforts were made to minimize suffering. Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soetikno, V., Murwantara, A., Jusuf, A.A. et al. Alpha-mangostin counteracts hyperuricemia and renal dysfunction by inhibiting URAT1 renal transporter in insulin resistance rat model. Beni-Suef Univ J Basic Appl Sci 11, 95 (2022). https://doi.org/10.1186/s43088-022-00275-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00275-3