Abstract
Background
Mineralizing microangiopathy represents one of the delayed complications of radiotherapy and chemotherapy. We reviewed clinical and radiological data of pediatric cancer patients who presented with mineralizing microangiopathy. This is a retrospective analysis of the medical records of 37 cancer children treated with chemoradiotherapy presented with imaging criteria suspected of mineralizing microangiopathy admitted to our hospital during the period 2015–2020. The CT was reviewed for distribution of calcification and MRI for signal criteria.
This study aims to raise awareness among radiologists about radiological features of mineralizing microangiopathy during the sequential routine follow-up brain scans of pediatric cancer patients who received chemo, radio, or combined chemoradiotherapy and to identify changes as a long-term delayed complication of therapy and avoid misdiagnosis.
Results
Thirty-seven pediatric cancer patients (17 female and 20 males, aged 1.5–18 years) who had mineralizing microangiopathy were thoroughly investigated. Most of them (32 patients) had brain tumors and 5 patients had leukemia. Cranial radiotherapy and systemic chemotherapy were given to 33 patients, while nine patients received intrathecal chemotherapy. The interval needed to develop mineralizing microangiopathy ranged from 1 to 10 years after the end of treatment. CT detected calcification in the basal ganglia, being the most common location (32 cases), followed by cerebral gray–white matter interface in 26 patients, cerebellum (18), brain stem (13), thalamus (5), and caudate nucleus (4), while dural calcifications were found in only one patient. MRI was considered “positive” when T1 hyperintensity was noted in the anatomical location of CT detected calcification; it was positive in 29 cases.
Conclusion
Mineralizing microangiopathy is one of the delayed complications of chemoradiotherapy among pediatric cancer patients. The awareness of its radiological criteria is essential to avoid misdiagnosis. Early detection can alert pediatric oncologists to monitor neurotoxicity and help prevent long-term neurological sequels.
Similar content being viewed by others
Background
Malignant tumors are the second most common cause of death among children in the USA, preceded by accidents [1]. The most common cancer in children is acute lymphoblastic leukemia (ALL) (26%), central nervous system tumors (21%), neuroblastoma (7%), and non-Hodgkin lymphoma (NHL) (6%) [2].
Cancer therapy, including chemotherapy with or without cranial radiotherapy, is potentially toxic to the nervous tissue [3]. If chemo and radiotherapy are used concurrently, it is difficult to evaluate each one's contribution to the toxic effect on brain tissue and they may have a synergistic effect. It is recognized that chemo agents can be radiosensitizers lowering the toxic threshold and radiotherapy affects the integrity of the blood–brain barrier potentiating the effect of the chemo agent given [4]. Recently with increased cure rate and survivors, the recognition of the delayed therapeutic complication is increased. Therapy-related sequelae are a major cause of mortality in the survivors of childhood cancer [5, 6].
Mineralizing microangiopathy is recognized as one of the uncommon delayed complications of cancer therapy [7]. It occurs most commonly with combined radiotherapy and chemotherapy, with children more susceptible than adults. Therefore, the radiologist should be aware that these toxicities can be asymptomatic and his prompt reporting may be of great value to the referring physician and his patients.
Histologically, mineralizing microangiopathy is characterized by calcium deposition in small vessels due to fibrinoid necrosis [8]. It is present in the walls of precapillary arterioles, capillaries, venules, and smaller arteries such as the lenticulostriate artery. It also develops in the perivascular neural tissue secondary to mineralization of plasma fluids, which leak out of the damaged vessels, and regional ischemia resulting from the circulatory impairment. An autopsy study of radiotherapy-treated leukemic children showed mineralizing microangiopathy in 28 (17%) of 163 patients [9].
We aimed to highlight the imaging findings consistent with mineralizing microangiopathy in pediatric patients surviving cancer treatment.
Methods
Inclusion criteria
Retrospective analysis of the electronic medical records of 37 childhood cancer patients treated with chemotherapy and/or cranial radiotherapy presented with imaging criteria suspected of mineralizing microangiopathy admitted to our hospital during the period 2015–2020.
Exclusion criteria
This study excluded any child with large intracranial residual or recurrent lesion (which may mask the calcification and hinder their assessment) and children who had a history of intracranial infection (the most common cause of pediatrics intracranial calcifications).
Machines and techniques
All CT scans were performed at our institution using (SOMATOM Definition AS + Siemens Healthcare GmbH, Germany). The images were recorded at 0.6 cm slice thickness, 80 kV voltage, 75 mA currents, 20 s/scan, 360o rotation, and 5 mSv radiation dose. General anesthesia was administered to the patients during CT scan if required. All images were converted to patients’ DICOM file formats (field of view [DFOV] 51.2 × 61.5 cm).
All subjects underwent MRI examinations at our institution using a superconducting 3 Tesla unit (Philips, Achieva, Best, The Netherlands). A standard head circularly polarized coil was used for MRI while the patients lay in a supine position using the following sequences: axial T1 FSE (TR = 450 ms, TE = 12 ms), axial T2 SE (TR = 4540 ms, TE = 96 ms), and sagittal T1SE (TR = 430 ms, TE = 10 ms). Other MRI parameters included slice thickness of 5 mm with a 1–2 mm gap and a matrix size of 256 × 256 mm.
Evaluation of performed examinations
Two radiologists (S.H and A.Y) with 8 and 15 years of experience in neuroradiology evaluated the studies independently from each other. We evaluated all scans undergone by the patient before and after chemoradiotherapy treatment to detect the first traces of calcifications and analyzed all the following scans within the period of the study to define the progress of development.
Clinical data analysis included demographic data, primary malignancy, clinical symptoms, and signs at the time of diagnosis of mineralizing microangiopathy, systemic chemotherapy regimen, radiotherapy field and dose, local intrathecal chemotherapy that is given, and the time interval between the end of initial treatment and the date of mineralizing microangiopathy diagnosis (Table 1).
Statistical analysis
Data were coded and entered into the SPSS software for statistical analysis. Quantitative variables were described as mean and median, while qualitative variables as frequency and percent and compared using the chi-square test and p value of significance if less than 0.01.
Results
Clinical characteristics
The clinical characteristics of the 37 children with mineralizing microangiopathy are shown in Table 1. The median age was 8 years (range 1.5–18y). Thirty-two (86%) patients had brain tumors: medulloblastoma was the most common (n = 20 patients) followed by atypical teratoid rhabdoid tumor (ATRT) (n = 4 patients), while five patients were diagnosed with acute leukemia.
Seven patients (19%) were asymptomatic, while headache was the most common clinical presentation (n = 13, 35%), followed by convulsion (n = 11, 30%), ataxia (n = 2, 5%), slurred speech, hearing impairment, squint and behavioral changes in one patient each. Most patients (92%) received radiotherapy to the brain as part of their treatment except in three leukemia patients, while 9 (24%) patients received intrathecal chemotherapy. Thirty-three (89%) patients received systemic chemotherapy.
The median time interval between the end of treatment and the diagnosis of mineralizing microangiopathy was 4 years (range 1–10 years).
Radiological findings
CT detected calcification in all patients, with the basal ganglia being the most common location (n = 32), followed by cerebral gray–white matter interface (n = 26), cerebellum (n = 18), and thalamus in five patients, caudate nucleus in four patients and one patient had dural calcifications (Figs. 1, 2). Table 2 summarizes the distribution of CT-detected calcifications. The absence of any edema or mass effect excludes the possibility of neoplastic or inflammatory processes.
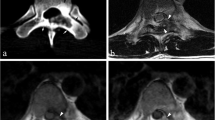
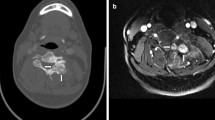
A 9-year-old male patient with acute lymphocytic leukemia, CT a, b shows faint calcifications located at the basal ganglia bilaterally and right temporal lobe. Follow-up CT 3 years later c, d and e shows progression of the basal ganglia calcifications, newly developed asymmetrical frontal and parietal as well as temporal subcortical calcifications. Axial T1WI f only detected bright signal at the basal ganglia bilaterally and the subcortical calcifications are hardly seen
A 16-year-old female with treated medulloblastoma, CT done 6 year after end of therapy a–d shows calcifications located at the left cerebellum a, pons b basal ganglia and thalami bilaterally as well as gray–white matter interface c and d. Axial T1WI e and f shows foci of bright signal at the basal ganglia and thalami bilaterally (e) as well as gray–white matter interface (f)
On the other hand, MRI was considered “positive” when T1 hyperintensity was noted at the anatomical location of the CT detected calcification; it was positive in 29 cases with the basal ganglia showing the most evident abnormalities (Figs. 1f and 2f).
Discussion
The most common long-term sequel after cancer therapy is brain tissue atrophy and volume loss. However, mineralizing microangiopathy is considered one of the delayed complications of chemoradiotherapy. It represents dystrophic calcification within the brain substance and usually develops after combined chemotherapy and central nervous system (CNS) radiation treatment in childhood [5].
The direct effects of delayed radiation-induced damage on the brain and spinal cord include focal CNS necrosis, diffuse white matter injury, CNS atrophy, mineralizing microangiopathy, hemorrhagic telangiectasia, optic neuritis, and large vessel vasculopathy. These entities result mainly from injury to the small vessels. Mamlouk MD et al. [10] described the mineralizing microangiopathy as a non-inflammatory deposition of calcium and mucopolysaccharides in non-tumorous areas of the brain. These deposits occur in the vasculature. In the arteries, they are confined to the intima, while the entire wall is affected in the veins.
The risk of this complication is more remarkable in children less than 10 years of age who have undergone radiotherapy as stated by Vázquez E et al. [5]. In our study, about 70 percent of the cases (n = 26/37) are younger than 10 years old.
Mineralizing microangiopathy has been reported after radiation treatment for patients with craniopharyngiomas as described in a case report by Srinivasan KG et al. [7], in addition to medulloblastoma and CNS leukemia, as illustrated in Torrisi JM et al. [8] who listed the tumors may be associated with mineralizing microangiopathy, also described radiation-induced mineralizing microangiopathy in a case of recurrent craniopharyngioma. In our study, brain tumors followed by leukemia are the primary tumors of the patients who developed mineralizing microangiopathy after therapy. In the present study, medulloblastoma is the most common among brain tumors.
Some of the present study patients were asymptomatic and were incidentally discovered during the routine follow-up of their original disease; however, the headache was the most common among the symptomatic patients (35%) followed by convulsion, ataxia, and slurred speech. In concordance with many studies [7], 10, 11] reported that microangiopathy was asymptomatic in most cases and is an incidental finding on follow-up CT examinations [12]. On the other hand, it may be associated with the development of focal epilepsy, neurological deficits, abnormal electroencephalogram (EEG) tracings, impaired intellectual function, and abnormal behavior [7], 10, 11].
As expected, CT is more sensitive in the detection of calcification compared to MR as described by Espagnet MC et al. [6] and Suzuki S et al. [13]. In the present study, basal ganglia were the most common location of calcification followed by the gray–white matter interface of different cerebral lobes, cerebellum, brain stem, and thalami which were also affected. Lewis E and Lee YY [14] described seven patients with mineralizing microangiopathy with dystrophic calcification present in the corticomedullary junction, lentiform nucleus of the basal ganglia, corticomedullary junction, and dentate nucleus of the cerebellum, respectively [14]. Also, many review articles described the same pattern [11&14].
As MR was recently used as the standard technique for imaging of the brain in post-therapeutic follow-up, the effect of calcium deposition on signal intensity in MR images has generally been thought to be of low signal on T1-weighted and T2-weighted images, due to a paucity of mobile protons. However, in our series as well as in the series of Suzuki S et al. [13], calcification demonstrated an increased signal on T1-weighted sequences.
Such paradoxically increased signal on T1-weighted images has been attributed to a surface relaxation mechanism associated with particulate calcium, resulting in shortening of both T1 and T2 relaxation times [15].
The limitations of this study were its retrospective nature; MRI was done without T2* or susceptibility-weighted images which are more sensitive than T1WI in the detection of calcification. Prospective studies including all patients who received chemotherapy and/or cranial radiation to detect the prevalence of mineralizing microangiopathy in children after cancer therapy are warranted. Ongoing surveillance and long-term follow-up for such children with mineralizing microangiopathy are needed to identify the squeal of such not uncommon complication.
Conclusions
An elevated level of suspicion is needed to recognize the imaging features of CNS complications after cancer therapy. Radiologists need familiarity with the features of mineralizing microangiopathy in order to accelerate the imaging diagnosis and minimize the associated morbidity and avoid the misdiagnosis of other lesions. A full understanding of the pathology and mineralizing microangiopathy predisposing factors may eventually decrease its incidence and lead to improvement in the quality of life of long-term cancer survivors particularly children.
Availability of data and materials
Available upon request.
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- ATRT:
-
Atypical teratoid rhabdoid tumor
- CNS:
-
Central nervous system
- CT:
-
Computed tomography
- EEG:
-
Electroencephalogram
- MRI:
-
Magnetic resonance imaging
- NHL:
-
Non-Hodgkin lymphoma
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. CA Cancer J Clin 65(1):5–29
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A (2014) Childhood and adolescent cancer statistics. CA Cancer J Clin 64(2):83–103
Rossi A, Morana G, Gandolfo C, Severino M (2010) Neuroradiology of chemotherapeutic. Neurotox Child Neuroradiol J 23(2):183–190
Rane N, Quaghebeur G (2012) CNS effects following the treatment of malignancy. Clin Radiol 67(1):61–68
Vázquez E, Delgado I, Sánchez-Montañez A, Barber I, Sánchez-Toledo J, Enríquez G (2011) Side effects of oncologic therapies in the pediatric central nervous system: update on neuroimaging findings. Radiographics 31(4):1123–1139
Espagnet MC, Pasquini L, Napolitano A et al (2017) Magnetic resonance imaging patterns of treatment-related toxicity in the pediatric brain: an update and review of the literature. Pediatr Radiol 47(6):633–648
Srinivasan KG, Ramprabananth S, Ushanandhini KP, Srividya S, Praveen Kumar M (2010) Radiation-induced mineralizing microangiopathy in a case of recurrent craniopharyngioma. Case Rep Neuroradiol J 23(4):412–415
Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H (2011) CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 258(1):41–56
Katsura M, Sato J, Akahane M, Furuta T, Mori H, Abe O (2021) Recognizing radiation-induced changes in the central nervous system: where to look and what to look for. Radiographics 41(1):224–248
Mamlouk MD, Handwerker J, Ospina J, Hasso AN (2013) Neuroimaging findings of the post-treatment effects of radiation and chemotherapy of malignant primary glial neoplasms. Neuroradiol J 26(4):396–412
Murphy ES, Xie H, Merchant TE, Yu JS, Chao ST, Suh JH (2015) Review of cranial radiotherapy-induced vasculopathy. J Neurooncol 122(3):421–429
Barkovich AJ (2005) Pediatric neuroimaging. Lippincott Williams & Wilkins, Philadelphia
Suzuki S, Nishio S, Takata K, Morioka T, Fukui M (2000) Radiation-induced brain calcification: paradoxical high signal intensity in T1-weighted MR images. Acta Neurochir 142(7):801–804
Lewis E, Lee YY (1986) Computed tomography findings of severe mineralizing microangiopathy in the brain. J Comput Tomogr 10(4):357–364
Kanda T, Wakabayashi Y, Zeng F et al (2018) Imaging findings in radiation therapy complications of the central nervous system. Jpn J Radiol 36(9):519–527
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AY contributed to conceptualization and design of the work and primarily prepared the manuscript; YM performed analysis and interpretation of the patients’ data regarding the clinical aspects; MZ reviewed the manuscript; SA performed analysis and interpretation of the patients’ data regarding the radiological aspects. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval from SMAC community at Children Cancer Hospital, Egypt 57357.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Youssef, A., Madney, Y., Zaghloul, M. et al. Mineralizing microangiopathy: radiological features of a "not uncommon" complication of chemoradiotherapy in pediatric cancer patients. Egypt J Radiol Nucl Med 53, 129 (2022). https://doi.org/10.1186/s43055-022-00806-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00806-z