Abstract
Background
Cardiogenic shock (CS) remains a major cause of in-hospital mortality in the setting of acute myocardial infarction (AMI). However, little evidence is available regarding the optimal order of intra-aortic balloon counter-pulsation (IABP) insertion and primary percutaneous coronary intervention (PPCI). The aim of this study was to assess the hospital and short-term survival benefits of two different IABP insertion approaches, before versus after PPCI in patients with acute myocardial infarction and cardiogenic shock.
Results
Total mortality was 80 patients representing 48.4% of the total 165 studied patients; 60 patients died during the hospital admission period, while the remaining 20 patients died post-discharge. In-hospital mortality was significantly higher in Post-PPCI–IABP group 40 (49.4%) versus Pre-PPCI–IABP group 20 (23.8%) (P = 0.001). Moreover, the mortality difference between the two groups was sustained over six-month follow-up period, where 15 patients (18.5%) died in the Post-PPCI–IABP group, while only 5 patients 6.0% died in the Pre-PPCI–IABP (P = 0.001).
Conclusions
Early IABP insertion before PPCI is associated with improved in-hospital and long-term survival when used for patients presenting with AMI complicated by hemodynamic instability.
Similar content being viewed by others
Background
Intra-aortic balloon counter-pulsation (IABP) has been increasingly used as a mechanical circulatory support in cardiogenic shock (CS) patients with post-acute myocardial infarction (AMI) by providing circulatory assistance to the failing left ventricle (LV) [1]. Diastolic inflation and systolic deflation of the counter-pulsation balloon generate kinetic energy in the aortic root that results in LV after-load and cardiac work reduction and thus leads to decrease in myocardial oxygen demand. Moreover, the augmented effect of IABP on diastolic pressure is the increase in coronary perfusion and reduction of LV filling pressure [2]. The reduction of ischemic burden and infarct size post-acute AMI is the net effect of IABP [3]. In the USA, the use of IABP in AMI accounts for one-third of the cardiovascular procedures [4]. The hemodynamic support after cardiac catheterization is the common indication of IABP placement in 20%, followed by CS in 19%, cardiopulmonary bypass weaning in 16%, preoperative use in 13%, and refractory unstable angina in 12% as shown by The Benchmark Registry [5]. Until now, there are no obvious recommendations about the IABP optimal duration post-ST-elevation myocardial infarctions (STEMI) although the use of IABP for more than two days results in a significant increase in vascular complications, gastrointestinal bleeding, and infection [6].
Various clinical randomized-controlled trials demonstrated a better prognosis and short-term survival with simultaneous use of primary percutaneous coronary intervention (PPCI) with IABP in AMI [7,8,9]. However, a few randomized trials and recent meta-analyses showed that IABP insertion after PPCI did not improve in-hospital and short-term survival [10, 11].
In this study, we analyzed the in-hospital and short-term survival benefits of two different IABP insertion approaches, before versus after PPCI in patients with STEMI complicated by CS.
Methods
This study is a single-center non-randomized retrospective trial that aims to assess the hospital and short-term survival benefits of two different IABP insertion approaches, before versus after PPCI in patients with acute myocardial infarction and cardiogenic shock. We retrospectively reviewed the data of 165 patients with hemodynamically unstable acute coronary syndrome treated with IABP insertion and PPCI in King Saud Medical City, a tertiary care hospital between January 2017 to February 2022. Patients' baseline characteristics including demographics, clinical presentation, and procedural and post-procedural complications were collected. The study protocol was approved by the regional ethical committee. The decision and timing of IABP insertion were based mainly on the clinical situation, international guidelines, and operator preferences. All IABPs were inserted under fluoroscopy guidance in the catheterization laboratory using 8-French catheters (Arrow Corp, Reading, PA, USA). Patients referred for emergency or urgent coronary artery bypass graft surgery were excluded from the study. The patients were divided into two groups according to IABP insertion time: The first group, (n = 84 patients) had counter-pulsation support started before PPCI (Pre-PPCI–IABP group) and the second group (n = 81patients) who had the counter-pulsation support started after PPCI (Post-PPCI–IABP group).
Diagnosis of STEMI and non-STEMI was based on symptoms and the European Society of Cardiology electrocardiogram criteria [12, 13]. CS was defined as persistent systolic blood pressure of < 90 mmHg, uses of vasopressors to maintain the systolic blood pressure of > 90 mmHg and evidence of end-organ hypo-perfusion such as altered mental status, oliguria or cold extremities that does not respond to fluid resuscitation [8]. Door-to-balloon time was defined as the interval between the hospital's arrival and the first balloon dilatation of the proposed culprit artery. If required, vasopressors (dopamine, norepinephrine and/or epinephrine) were used and tittered with continuous hemodynamic monitoring. Dual anti-platelets, anticoagulation, and glycoprotein IIb/IIIa were used according to the European Society of Cardiology guidelines [12, 13]. The main outcome was in-hospital mortality and short-term (6 months) mortality.
The data were analyzed using SPSS IBM., Chicago, IL. Continuous normally distributed variables were represented as mean ± SD. with 95% confidence interval, while non-normal variables were summarized as median with 25 and 75 percentiles, and using the frequencies and percentage for categorical variables; a p value < 0.05 will be considered statistically significant. To compare the means of normally distributed variables between groups, the Student’s t test was performed, the Mann–Whitney U test was used in non-normal variables and χ2 test or Fisher’s exact test was used to determine the distribution of categorical variables between groups. Spearman's rank correlation coefficient (r) was used to show the correlation between different parameters in this study. Logistic regression analysis was done to assess the risk factors for the death rate of the studied patients. Significant predictors in the univariate analysis were included in a stepwise forward multivariate analysis (P < 0.05 for entering the model and P < 0.1 for removal from the model) to determine the final predictor factors for death.
Results
In total, 165 patients were recruited in this study. There were no significant differences between the two groups regarding age, body mass index, rate of smoking, diabetes, hypertension, hypercholesterolemia, blood pressure, and cardiac biomarkers. Patients with Post-PPCI–IABP have a significantly higher prevalence of prior MI history compared to Pre-PPCI–IABP (25/81 (30.9%) vs. 5/84 (6.0%), P = 0.001), respectively (Table 1).
Almost all patients had symptomatic heart failure, 65/165(48.1%) with Killip class-III (acute pulmonary edema) and 70/165 (51.9%) with class-IV (cardiogenic shock). Killip class-IV and severely reduced left ventricle ejection fraction (LVEF) of < 35% was more common in the Pre-PPCI–IABP than in the Post-PPCI–IABP group: 64/84 (76%) versus 31/81 (38%), P = 0.001; and 79/81 (94%) vs. 46/84 (56.8%), P = 0.001, respectively. The other clinical characteristics of the two groups are illustrated in Table 1.
STEMI was significantly higher in Post-PPCI–IABP [59/ 84 (70.2%) vs 71/ 81 (87.7%), P = 0.01], while left main coronary artery and/or multi-vessel disease and door-to-balloon time was significantly greater in the Pre-PPCI–IABP when compared to the post-PCI–IABP group: 20/84 (23.8%) versus 44/84 (52.4%), P = 0.04; and (74.3 ± 8.2 vs. 62.0 ± 7.8, p = 0.001), respectively. Complete revascularization was achieved successfully in the Post-PPCI–IABP when compared to the Pre-PPCI–IABP group 25/84 (30%) versus 15/81 (17%), p = 0.04 (Table 2).
During this retrospective study, we did not elucidate any known complications of IABP insertion or early (before intervention) cardiac arrests in our database.
In-hospital and short-term outcome
In total, mortality was 80/165 (48.4%), of whom 60/165 (36.3%) died in-hospital and 20/165 (12.1%) died six months after discharge. The total and out-of-hospital death was significantly higher in the Post-PPCI–IABP group: 25/84 (29.8%) versus 55/81 (67.9%), P = 0.001, and 15/81 (18.5%) versus 5/84 (6%), P = 0.001, respectively, while in-hospital mortality was significantly higher in the Post-PPCI–IABP group versus the Pre-PPCI–IABP group: 40/81 (49%) versus 20/84 (23%), P = 0.001 (Table 2 and Fig. 1).
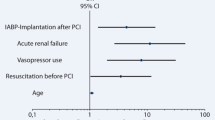
CKMB and creatinine levels were significant predictors of death in the Pre-PPCI–IABP group with an adjusted odd ratio (AOR): 1.002, confidence interval (CI): 1.001–1.03, P = 0.002; and 1.028 (1.014–1.091), P = 0.001, respectively, while in Post-PPCI–IABP, LVEF of < 35% and creatinine level were the most significant predictors of poor outcome: AOR of 5.04, CI 1.66–15.29, P = 0.004 and 1.017 CI 1.004–1.029, P = 0.008, respectively (Table 3).
When analyzing the overall mortality in the whole study population, we found past history of hypertension, lower LVEF and higher creatinine levels were associated with a greater risk of death; in addition, Post-PPCI–IABP group had significantly worse outcomes when compared with Pre-PPCI–IABP (AOR 4.992, P value 0.001) (Table 4).
Discussion
In the present study, we analyze the effect of IABP insertion before and after PPCI in acute myocardial infarction. We found that despite the longer door-to-balloon time with Pre-PPCI–IABP insertion, the in-hospital and 6-month survival was significantly better than that of the Post-PPCI–IABP group.
Hemodynamic instability and CS affect 6–9% of patients presenting with AMI increasing the hospital mortality rate by approximately 50% [14]. In spite of the use of mechanical circulatory support and early revascularization strategies, CS remains the main cause of death in patients with AMI [15].
Currently, IABP is the most used device for mechanical circulatory support in patients with CS related to AMI [16]. Experimental and clinical studies of IABP demonstrated a hemodynamic benefit due to after-load reduction, diastolic augmentation, improvement of coronary and prolonged IABP placement may increase the risk of thrombosis, malignant arrhythmia, heart failure, infection, and pulmonary embolism. Early IABP insertion will result in a faster improvement in coronary perfusion, but it may delay revascularization of the culprit lesion by extending the door-to-balloon time which may increase the perioperative mortality rate. On the other hand, late IABP insertion may delay the positive effect of IABP on coronary perfusion and increase the mortality rate [17].
Reports analyzing the benefits of IABP post-AMI have conflicting results [18]. The SHOCK registry confirmed IABP benefit in reducing in-hospital mortality [19], whereas the IABP-SHOCK II (Intra-aortic Balloon Pump in Cardiogenic Shock II) showed that IABP did not reduce 30-day mortality with no evidence of long-term benefit; however, these results might be affected by the high frequency of crossover (10–30%) between the groups. Additionally, only 13.4% of the IABP were inserted before revascularization which was less than this study (30.9%) [8].
The latest European Society of Cardiology guidelines for myocardial revascularization were largely influenced by the SHOCK II trial results, and have downgraded the recommendation of IABP in treating CS post-AMI to Class IIb, but it did not mention when IABP treatment should start [20].
A recent meta-analysis of 12 randomized trials concluded that IABPs did not reduce the short- or long-term mortality in AMI either with or without CS although the timing of insertion of IABP was not mentioned in many of these trials [21].
In this study patients with Pre-PPCI–IABP suffered more Killip IV, lower systolic blood pressure readings, more prevalence of renal insufficiency, and lower LVEF than the Post-PPCI–IABP. While the Pre-PPCI population has more involvement in left main and three-vessel disease as compared to Post-PPCI–IABP.
Abdel-Wahab et al. analyzed the effect of IABP in 48 patients with AMI and CS and found a better survival rate with Pre-PPCI–IABP than IABP after PPCI [22]. In contrast, a meta-analysis study illustrated that patients who received IABP therapy before PPCI had similar short-term and long-term mortality compared to those who received IABP therapy after PPCI [23]. It is worth mentioning that most of these studies did not report time delays or door-to-balloon time.
In the present study, although patients with Pre-PPCI–IABP had significantly longer door-to-balloon time in comparison with Post-PPCI–IABP, the latter had better overall survival benefit which could be related to early restoration of coronary perfusion with the IABP.
The differences in outcome observed in our study may be explained in part by the early hemodynamic stabilization in Pre-PPCI–IABP, and less prevalence of left main disease and three-vessel diseases. That possibly minimized the need for high doses of inotropes and enabled a higher number of complete revascularizations when compared to Post-PPCI–IABP.
Complete revascularization was achieved in Post-PPCI–IABP more commonly than in the Pre-PPCI–IABP group, which was a protective factor for in-hospital and short/intermediate-term mortality. In agreement with our results, the COMPLETE randomized trial showed 7.8% absolute reduction in cardiovascular death, new MI and revascularization in the complete revascularization group after a 3-year follow-up [24]. On the contrary, the Culprit-Shock trial showed the rate of death from any cause was significantly lower in the culprit-lesion-only PCI group than in the multi-vessel PCI group [25].
In this study, the higher prevalence of low LVEF in Pre-PPCI–IABP LVEF < 30% was a major predictor for mortality of the whole population and was clearly pronounced in Post-PPCI–IABP group, which came in agreement with Brezinov et al. who demonstrated that LVEF is a powerful predictor of 1-year mortality in ACS26.
One of the limitations of this study is a single-center non-randomized retrospective trial that is underpowered to reliably detect a mortality difference between the study groups. Moreover, there is no other investigation (secondary endpoints) like recurrent myocardial infarction, stroke and bleeding. Longer follow-up should have been pursued for the detection of long-term mortality and other secondary endpoints.
Conclusions
This study showed that inserting IABP before PPCI in AMI with hemodynamic instability had a significant survival benefit during the hospital stay that extends to 6 months after hospital discharge. Powered randomized trials are warranted to investigate the relative benefit of the two strategies, that is, IABP inserted before or after PPCI in future.
Availability of data and materials
The data that support the findings of this study are available from the electronic system of the cardiology department in King Saud medical city, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AMI:
-
Acute myocardial infarction
- CS:
-
Cardiogenic shock
- IABP:
-
Intra-aortic balloon counter-pulsation
- MACE:
-
Major adverse cardiac events
- NSTEMI:
-
Non-ST elevation myocardial infarction
- PPCI:
-
Primary percutaneous coronary intervention
- STEMI:
-
ST elevation myocardial infarction
References
de la Espriella-Juan R, Valls-Serral A, Trejo-Velasco B, Berenguer-Jofresa A, Fabregat-Andrés Ó, Perdomo-Londoño D, Albiach-Montañana C, Vilar-Herrero JV, Sanmiguel-Cervera D, Rumiz-Gonzalez E, Morell-Cabedo S (2017) Impact of intra-aortic balloon pump on short-term clinical outcomes in ST-elevation myocardial infarction complicated by cardiogenic shock: a “real life” single center experience. Med Intensiva 41(2):86–93
Gu J, Hu W, Xiao H, Feng X, Song Z, Chen Y, Zhang D (2011) Prophylactic intra-aortic balloon pump reduces C-reactive protein levels and early mortality in high-risk patients undergoing percutaneous coronary intervention. Acta Cardiol 66(4):499–504
Nachlas MM, Siedband MP (1967) The influence of diastolic augmentation on infarct size following coronary artery ligation. J Thorac Cardiovasc Surg 53(5):698–706
Cohen M, Urban P, Christenson JT, Joseph DL, Freedman RJ Jr, Miller MF, Ohman EM, Reddy RC, Stone GW, Ferguson JJ III (2003) Intra-aortic balloon counterpulsation in US and non-US centres: results of the Benchmark® Registry. Eur Heart J 24(19):1763–1770
Ferguson JJ, Cohen M, Freedman RJ, Stone GW, Miller MF, Joseph DL, Ohman EM (2001) The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol 38(5):1456–1462
Boudoulas KD, Bowen T, Pederzolli A, Pfahl K, Pompili VJ, Mazzaferri EL Jr (2014) Duration of intra-aortic balloon pump use and related complications. Acute Card Care 16(2):74–77
Su D, Yan B, Guo L, Peng L, Wang X, Zeng L, Ong H, Wang G (2015) Intra-aortic balloon pump may grant no benefit to improve the mortality of patients with acute myocardial infarction in short and long term: an updated meta-analysis. Medicine 94(19):e876
Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, Webb JG, Steingart R, Picard MH, Menegus MA, Boland J (2001) One-year survival following early revascularization for cardiogenic shock. JAMA 285(2):190–192
Romeo F, Acconcia MC, Sergi D, Romeo A, Muscoli S, Valente S, Gensini GF, Chiarotti F, Caretta Q (2013) The outcome of intra-aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta-analysis. Am Heart J 165(5):679–692
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367(14):1287–1296
Members WC, Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM (2011) 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124(23):e574–e651
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio AL, Crea F, Goudevenos JA, Halvorsen S, Hindricks G (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 42(14):1289–1367
Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS, Investigators NRMI (2005) Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 294(4):448–454
Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, Col J, White HD, for the SHOCK Investigators (2006) Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 295(21):2511–2515
Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, Thiele H, Werdan K, Zeymer U, Prondzinsky R (2015) Intra‐aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev (3)
Shah AH, Puri R, Kalra A (2019) Management of cardiogenic shock complicating acute myocardial infarction: a review. Clin Cardiol 42(4):484–493
Sanborn TA, Sleeper LA, Bates ER, Jacobs AK, Boland J, French JK, Dens J, Dzavik V, Palmeri ST, Webb JG, Goldberger M (2000) Impact of thrombolysis, intra-aortic balloon pump counterpulsation, and their combination in cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol 36(3S1):1123–1129
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P (2018) 2018 ESC/EACTS Guidelines on myocardial revascularization. Kardiologia Polska (Polish Heart J) 76(12):1585–1664
Wan YD, Sun TW, Kan QC, Guan FX, Liu ZQ, Zhang SG (2016) The effects of intra-aortic balloon pumps on mortality in patients undergoing high-risk coronary revascularization: a meta-analysis of randomized controlled trials of coronary artery bypass grafting and stenting era. PLoS ONE 11(1):e0147291
Abdel-Wahab M, Saad M, Kynast J, Geist V, Sherif MA, Richardt G, Toelg R (2010) Comparison of hospital mortality with intra-aortic balloon counterpulsation insertion before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am J Cardiol 105(7):967–971
Cui K, Lyu S, Liu H, Song X, Yuan F, Xu F, Zhang M, Zhang M, Wang W, Zhang D, Tian J (2019) Timing of initiation of intra-aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: a meta-analysis. Clin Cardiol 42(11):1126–1134
Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, López-Sendón J, Faxon DP, Mauri L (2019) Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med 381(15):1411–1421
Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A (2017) PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med 377(25):2419–2432
Brezinov OP, Klempfner R, Zekry SB, Goldenberg I, Kuperstein R (2017) Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: a real world study. Medicine 96(9):e6226
Acknowledgements
This study was approved by the director of the cardiology department of King Saud medical city and cooperation with Dr. Rami abazid.
Funding
All funding for this study came from personal funding.
Author information
Authors and Affiliations
Contributions
AA and WF advised the idea of the paper, data was collected by AA, statistics was done by WE, and WI reviewed the results and collected data. All the authors contributed to the discussion and writing the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research proposal has been reviewed and was approved. The Institutional Review Board (IRB) NO: H1RI-27-Jul21-01committee found that the research met the applicability criteria and was eligible for exempt review. The need for consent is waived by the cardiology department in King Saud Medical City because this research uses secondary electronic data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azazy, A., Farid, W.A., Ibrahim, W.A. et al. Survival benefit of IABP in pre- versus post-primary percutaneous coronary intervention in patients with cardiogenic shock. Egypt Heart J 76, 99 (2024). https://doi.org/10.1186/s43044-024-00527-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00527-w