Abstract
Background
Presence of Germline mutations in the BRCA1 and BRCA2 genes is the most significant epidemiological factor for breast cancer (BC), where germline BRCA1 (gBRCA 1) mutation increases the risk for BC by 59–87% and gBRCA 2 mutation increases the risk by 38–80%. In this retrospective study, we have analyzed NGS-based genetic data for samples received at our laboratory for genetic testing over a three-year period to understand the prevalence and pattern if any of BRCA1 and BRCA2 mutations in Indian breast cancer patients.
Results
BRCA gene sequencing using NGS was performed in 395 consecutive cases of BC referred for testing to our lab between 2021 and 2023. Genetic analysis of mutations BRCA 1 and BRCA 2 genes resulted in 115 (29%) positive patients. Out of 115 patients, 79 reported BRCA1 mutations, whereas 36 had BRCA2 mutations. Exon 10 (57.3%) of BRCA1 and exon 11 (52%) of BRCA2 were the most mutated exons observed in this study. The c.1961delA (26.4%) variant, followed by the c.68_69delAG (22.7%) variant in BRCA1, and the c.6373delA (20.5%) variant in BRCA2, were the most common mutations found in our study. Our data shows positive correlation of younger age group (20–45 years) with BRCA positive status (Chi-square p value = 0.001).
Conclusion
BRCA mutation prevalence was 29.1% in our data which is higher than Western countries. Based on our findings BRCA screening looks imperative for women with BC especially younger women (< 50 years), as family history based BRCA testing would miss out many BRCA positive candidates which could benefit from PARP therapy options.
Similar content being viewed by others
Introduction
Incidences of Breast Cancer (BC) are on rapid rise (by 39% over past two decades) making it the most common malignancy among Indian women, accounting for 28.2% of all female cancers [1,2,3]. Also, as compared to western countries, the burden of avoidable deaths from BC disproportionately affects low-income and middle income countries, where more than 70% of breast cancer deaths occur in people younger than 70 years of age. This is because 70% of BC cases are reported in the advanced stages [4, 5]. The epidemiology of BC, incidence rate, clinical outcomes and mortality rate differ significantly in Indian women when compared with the Western population [2] but limited data availability hinders objective comparison [2, 6, and 7]. Furthermore, genetic epidemiology data for BC in Indian patients is even scarce.
Presence of Germline mutations in the BRCA1 and BRCA2 genes is the most significant epidemiological factor for BC, where germline BRCA1 (gBRCA 1) mutation increases the risk for BC by 59–87% and gBRCA 2 mutation increases the risk by 38–80%. [8, 9]. BRCA 1/2 gene test not only helps in risk prediction for BC, but also helps in treatment regime planning with Poly-Adp Ribose Polymerase inhibitors (PARPi) [10, 11]. Various studies both clinical and preclinical, showed that BRCA is an important factor affecting chemotherapy response and treatment toxicity in breast cancer patients [12, 13]. Therefore, screening for BRCA mutations almost becomes a prerequisite for early intervention in women with a family history of breast cancer or among young BC patients. However, for the management of those with BRCA-mutated BC, it is essential that healthcare providers understand the burden of BRCA-mutated disease and the prevalence in the given population, as it is noted that the frequency of mutations in these genes is higher in certain populations [9].
To date, the knowledge about the prevalence of BRCA1/2 mutations in BC patients belonging to some regions of India is poor. Compilation and understanding of genetic data along with correlation of treatment follow-up could help in reducing time and cost ensuring better reach to mass Indian population.
Therefore, in this retrospective study, we have analyzed NGS-based (Next Generation Sequencing) genetic data for samples received at our laboratory for genetic testing over a three-year period to understand the prevalence and pattern if any of BRCA1 and BRCA2 mutations in Indian breast cancer patients.
Materials and methods
Study population: Informed consent was obtained for a total of 395 patients diagnosed with breast cancer, and subsequently referred for germline BRCA 1/ 2 gene testing to our laboratory between January 2021 and December 2023. Patients were not selected for clinical characteristics/ cancer subtype or family history. All patients were females in the age ranging from 22 to 87 years.
DNA extraction and high-throughput sequencing
3 ml peripheral blood was obtained from each patient and genomic DNA was extracted using a DNA Blood Mini kit (Qiagen). The target regions in the BRCA1 and BRCA2 genes were amplified using the Ion AmpliSeq Library kit plus and Oncomine BRCA Research Assay panel (ThermoFisher Scientific) according to manufacturer’s protocol (Detailed methods has been provided in supplementary data). Next generation sequencing was performed on Ion Torrent S5 NGS platform (ThermoFisher Scientific).
Bioinformatics analysis and variant interpretation
The sequence reads were aligned to hg19/GRCh37 using Torrent suite software v.50. Variant calling and annotation was performed using Ion Reporter Software 5.12 (BRCA Oncomine 5.12, Thermo Fisher Scientific). Only exonic and splice site variants (Indels/SNVs) found in the Oncomine BRCA assay were used for clinical interpretation. Variants were classified according to the American College of Medical Genetics and Genomics (ACMG) recommendations [14, 15]. Statistical analyses were performed in R programming language and Vassar stats [16].
Results
A total of 395 female breast cancer patients were considered for the study. The women belonged to different age groups, with youngest patient being tested at 18 years and the oldest patient at 76 years. Among the studied 395 patients, majority belonged to premenopausal age group; n = 218 (55.1%) and 177 (44.8%) women were in post-menopausal stage. Overall mean age at diagnosis was observed to be 45 years. The mean age at diagnosis for the premenopausal women was 40 years and 60 years in the post-menopausal group.
The NGS sequencing of these 395 patients had an average of 0.25million reads per patient, with the mean read length being 106 bp. The average read depth was ~ 500X, with the mean percentage of reads on target being > 98.6%. Genetic analysis of mutations BRCA 1 and BRCA 2 genes resulted in 115 (29%) positive patients. BRCA positive patients were defined as patients who were identified with Pathogenic or Likely Pathogenic variant in one of the BRCA genes. Current analyses have not considered VUS (variants of uncertain significance) due to their unknown effects at this point of time. Among the 115 positive patients, a total of 34 SNVs and 82 Indels (short insertions/deletions) in the exonic region BRCA1 and BRCA2 genes were identified.
Out of 115 patients, 79 reported BRCA1 mutations, whereas 36 had BRCA2 mutations. Among the 79 positive BRCA1 patients, 76 (96.2%) variants were classified as pathogenic and 3 (3.79%) were likely pathogenic. Similarly. Out of 36 positive BRCA2 mutations, 34 (94.7%) Pathogenic and 2 (5.71%) Likely pathogenic variants were detected. Interestingly, the co-presence of two different BRCA2 variants; c.475 + 1G > A and c.476-2A > G was observed in one patient. Some patients showed presence of an additional VUS along with a pathogenic BRCA mutation.
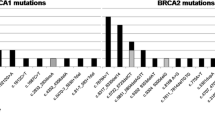
Exon 10 (57.3%) of BRCA1 and exon 11 (52%) of BRCA2 were the most mutated exons observed in this study. The c.1961delA (26.4%) variant, followed by the c.68_69delAG (22.7%) variant in BRCA1, and the c.6373delA (20.5%) variant in BRCA2, were the most common mutations found in our study. The most common types of mutation are attributed to small insertion/deletion frameshift (66.7%), nonsense mutations (27.8%) and missense mutations (5.2%). Mutational landscape details of pathogenic mutations in BRCA1/2 genes is shown in Fig. 1 (Mutational landscape of pathogenic variants in BRCA1/2 genes). Figure 2 (Heatmap representing commonly found mutations) shows a heatmap of mutations in each positive patient.
Chi-square test was performed to determine correlation between age categories and BRCA mutation status in the BRCA positive patients (Table 1). We stratified patients into three groups based on age at diagnosis. Our data shows positive correlation of younger age group (20–45 years) with BRCA positive status (Chi-square p-value ≥ 0.0001) Fig. 3. Of the 395 patients, 218 (55.1%) women were premenopausal and 177 (44.8%) women were in post-menopausal stage. The mean age at diagnosis for the premenopausal women was 45 years and 60 years for the post-menopausal group.
Discussion
Several studies have reported how the prevalence of germline mutations and gene specific risk evaluations depend, not only on factors such as family history of cancer or tumor molecular subtype, but also on factors like age, race/ ethnicity and geographical location [17, 18].
In this retrospective study, we report our observations on the prevalence of BRCA gene mutations in BC patients from different geographical regions of India tested in our laboratory. However, the patients for this study were referred by clinicians based on their BC diagnosis, irrespective of age and geographical location. However, we noticed, most patients were from Northern part of the country (North India ~ 57.9%, South India ~ 25.5%, East 10.3% and West 6.07%). Since, the patients from other Indian locations (Southern/Eastern/Western or Central) were not enough for appropriate representation we did not proceed with any location specific analyses. Only patients with clinically diagnosed Breast cancer (n = 395) were included in the study. Our data for the course of 2 years (2021–2023) shows, 29.1% patients were detected as BRCA positive. Chedda et al. [19] reported prevalence (31.9%) of pathogenic variants in BRCA1/ 2 genes in 160 HBOC Indian patients. Previous study conducted by Mittal et al. [20] reported a prevalence (18.64%) of P/LP mutations in 236 BC patients from the northern part of India [20]. Kulkarni et al. [21] with 94 BC patients showed prevalence of 25.5%, whereas Ajay Gogia et al. [22] reported a 16.6% prevalence of BRCA1 and BRCA2 mutations in 210 BC patients.
Even with larger sample size as compared to previous Indian studies our data too aligns with presence of higher prevalence rate (29.1%) of BRCA mutations. This also holds up an overall indication that the prevalence rate of BRCA gene mutations in Indian BC patients is much higher than the Western population which is reported in range of (5–10%) [21]. Study by Singh et al. [23] with 1010 Breast and ovarian cancer patients reported prevalence of 30.1% but this study was not exclusive for BRCA gene mutations.
Indian scenario is characterized by a younger median age of onset (< 50 years) and a higher prevalence of triple-negative breast cancer (TNBC) [20]. In our study, we observed the mean age at diagnosis of the premenopausal women was 45 years and among these 60.8% were BRCA positive. Similarly, for the post-menopausal women the mean age at diagnosis was 60 years and 39.1% were BRCA positive. The correlation analyses also throws light on presence of BRCA gene mutation and age at diagnosis. Our data shows positive correlation of younger age group (20–45 years) with BRCA positive status (Chi-square p-value = 0.001). Our observations align with other Indian studies to show peculiarity of Indian BC data in terms of peak age. This observation is also in line with the fact that germline mutations drive early onset and most often an aggressive cancer.
The most frequent BRCA1 gene mutations were c.1961delA and c.68_69delAG found in our study. The c.1961delA mutation, also known as 185delAG was seen in 22.7% cases (n = 18) in this study. This is also a common mutation reported in the Central and Southern European populations [8], but it has also been reported among several other Peruvian, Russian, Egyptian, Korean, Iranian [24, 25], and some of the Indian populations [26]. Another frequent recurrent mutation in BRCA1 was c.68_69delAG, seen in 21.5% cases (n = 17), which was also reported in two different studies conducted in India [27, 28]. Also, the c.68_69del BRCA1 mutation was first reported in Ashkenazi Jews [28, 29], consequently in Argentina, Brazil (0.3%), Chilean (0.6%), Peru (2.6%) and Russian populations. In addition, this c.68_69del mutation is described as a founder mutation in Egyptian and Hungarian BC patients [30]. Generally, the BRCA1 c.68_69delAG mutation is also found more commonly in Asian, Arabic, African, European, and American populations than the c.1961delA mutation [31]. Mannan et al. [32] reported that c.68_69delAG mutation is associated mainly with Northern and Southern parts of India [28, 33,34,35]. A previous study suggested that the origin of the c.68_69delAG mutation in Indian population is independent of that of Ashkenazi Jews based on haplotype analysis [36]. Further studies will be helpful in determining the frequency of the c.68_69delAG mutation and its origin in Indian population.
Six known BRCA1 pathogenic mutations were identified in twelve patients. Among them: (1) c.5074 + 1G > A variant of exon 16 was n = 4 (3.47%) (2) Both c.3607C > T and c.1352C > G variants of exon 10 in n = 3 (2.6%) each, (3) c.5509 T > C, c.2214_2215insT and c.441 + 1_441 + 2insA in exon 23, 10 and 6, respectively in n = 2 (1.7%) each. The c.5074 + 1G > A which was earlier reported as an Icelandic founder mutation was found in n = 4 (3.47%) [37]. Saxena et al. [35] and Mannan et al. [32] from India reported the same mutation in three patients suggesting that this could be due to common ancestry or they migrated from the same place. The c.3607C > T variant which produces the amino acid change p.Arg1203Ter has been associated with an increased risk of breast and ovarian cancer in earlier studies. It was reported previously in Greece, Italy, Turkey and Israel and also mentioned as dominant variant of North Western Romania [32, 38, and 39]. Among all mutations which were found in our BC cases, exon 10 seems to be more influenced by single base pair change, multiple insertions and deletions and by frameshift mutations in BRCA1 gene.
In BRCA2 gene, we identified the highest recurrence variant as c.6373delA, which represented 11.1% cases (n = 4) among other mutations. This mutation has been reported rarely in Indian studies but is a common or founder mutation in Danish population [28, 40]. In addition, Karami et al. [8] reported that, it is not prevalent in the USA or other European countries. The c.1855C > T mutation in exon 10 and c.92G > A in exon 3 were seen in two patients (5.5%) each.
Several studies have reported that the contribution of BRCA2 mutations in familial BC seems to be rather low in India, which supports our data [35, 37]. We have shown some BRCA1 and BRCA2 novel pathogenic variants (Tables 2 and 3, respectively). To our knowledge, our study is the first to report such mutations in Indian women with familial breast cancer.
Altogether, these findings may suggest genetic and ethnic associations among distinct populations or that these mutations occur in mutational hotspots. Nevertheless, various novel and specific mutations in BRCA1/2 genes occurring at a high frequency in different populations have been reported to date. So, knowledge of most recurrent mutations in BRCA1/2 according to ethnicity and population, treatment and diagnosis options for BC patients become better through efficient genetic testing methods.
Our study suggests a high prevalence rate (29.1%) of BRCA gene mutations in an Indian BC cohort. We believe with higher prevalence rate like this, BRCA sequence variant screening is imperative for Indian women with BC especially in younger women (< 50 years), as BRCA testing recommendations based on family history alone would miss out potential BRCA positive patients. Hence, reducing the mortality rate of BC in Indian women requires a multidisciplinary approach that includes education campaigns, preventive measures, early detection screening programs including BRCA genetic screen. Identification of a BRCA mutation is of paramount importance not only for providing appropriate genetic counseling and discussing risk-reducing interventions, but also for determining treatment options in patients with metastatic disease. A BRCA positive patient is eligible for PARP inhibitor therapy, leading to better survival rates/outcomes. At the same time, detection of BRCA mutation in a patient also, alerts testing of close relatives due to autosomal dominant mode of inheritance of this gene. This is in turn, inevitably encourages early screen and management of potential at risk individuals.
Our analysis includes female BC patients from across India, not selected for age, histologic subtypes or family history, thus presenting a more generalized results of this patient population. This adds concurring information on higher prevalence rate of BRCA gene variants and positive correlation between onsets of age-BRCA mutation status in Indian BC patients. We strongly believe, burden of BRCA gene variants in Indian BC patients may be different from Western BC patients and hence the need for designing the population specific testing criteria and protocols for BRCA1/2 and other cancer predisposing genes.
Study limitations
Limited access to clinical data: The study uses data generated in a referral diagnostic laboratory. Very often data like clinical details, family history, patient ethnicity or follow-up on treatment responses is not available to us as we depend on the physician for collection of this data. Due to lack of such data, the study is unable to correlate current genetic findings with above mentioned factors as a result of design constraint. Future studies will strive to achieve this study design. However, these limitations do not impact the gross findings of the study which focuses on peculiarity of overall Indian BC patients with regards to higher BRCA mutation burden, and correlation of young age with BRCA mutation status.
Conclusion
Our results highlight
-
BRCA mutation prevalence was 29.1% in our data, which is higher than Western countries.
-
Median age of women with cancer was 49 years, whereas median age for BRCA positive patients was 45 years. Young age category (20–45 Years) was found to be significantly associated with BRCA positive status.
-
Most common mutations were c.1961delA and c.68_69delAG in BRCA 1 and c.6373delA in BRCA 2 gene. Most common exon was exon 10 in BRCA1 and exon 11 in BRCA 2 gene.
-
Based on above BRCA screening, it is imperative for women with BC especially younger women (< 50 Years) as family history based BRCA testing would miss out many BRCA positive candidates.
Availability of data and materials
Data will be available on request.
References
International Agency for Research on Cancer, World Health Organization. Global Cancer Observatory. Cancer Today. International Agency for Rsearch on Cancer.2023;22.
Mehrotra R, Yadav K (2022) Breast cancer in India: present scenario and the challenges ahead. World J Clin Oncol 13(3):209–218
Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P (2022) Cancer incidence estimates for 2022 & projection for 2025: result from National Cancer Registry Programme, India. Indian J Med Res 156(4&5):598–607
Soerjomataram I, Cabasag C, Bardot A, Fidler-Benaoudia MM, Miranda-Filho A, Ferlay J, Parkin DM, Ranganathan R, Piñeros M, Znaor A, Mery L, Joko-Fru YW, Dikshit R, Sankaranarayanan R, Swaminathan R, Bray F; SURVCAN-3 collaborators (2023) Cancer survival in Africa, central and south America, and Asia (SURVCAN-3): a population-based benchmarking study in 32 countries. Lancet Oncol 24(1):22
Sathishkumar K, Vinodh N, Badwe RA, Deo SV, Manoharan N, Malik R, Panse NS, Ramesh C, Shrivastava A, Swaminathan R, Vijay CR, Narasimhan S, Chaturvedi M, Mathur P (2021) Trends in breast and cervical cancer in India under National Cancer Registry Programme: an age-period-cohort analysis. Cancer Epidemiol 74:101982
Sung H, Ferlay J, Siegel RL, Laversanne M et al (2020) Global cancer statistics: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66:15–23
Karami F, Mehdipour P (2013) A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res Int 928562
Mehrgou A, Akouchekian M (2016) The importance of BRCA1 and BRCA2 genes mutations in breast cancer development. Med J Islam Repub Iran 30:369
Fu X, Tan W, Song Q, Pei H, Li J (2022) BRCA1 and breast cancer: molecular mechanisms and therapeutic strategies. Front Cell Dev Biol 10:813457
Cortesi L, Rugo HS, Jackisch C (2021) An overview of PARP inhibitors for the treatment of breast cancer. Target Oncol 16(3):255–282
Huszno J, Kołosza Z, Grzybowska E (2019) BRCA1 mutation in breast cancer patients: analysis of prognostic factors and survival. Oncol Lett 17(2):1986–1995
Górski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Pluzańska A, Bebenek M, Fischer-Maliszewska L, Grzybowska E, Narod SA, Lubiński J (2000) Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet 66(6):1963–1968
Richards S, on behalf of the ACMG Laboratory Quality Assurance Committee. Aziz N, Bale S, Bick D, Das S, et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–23
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Aguilera MA, Meyer R et al (2019) VarSome:the human genomic variant search engine. Bioinformatics 35:197880
Ganggayah MD, Taib NA, Har YC, Lio P, Dhillon SK (2019) Predicting factors for survival of breast cancer patients using machine learning techniques. BMC Med Inform Decis Mak 19:48
Murthy NS, Agarwal UK, Chaudhry K, Saxena S (2007) A study on time trends in incidence of breast cancer—Indian scenario. Eur J Cancer Care (Engl) 16(2):185–186
Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, Zaguia A, Koundal S, Belay A (2022) Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int 2022:9605439
Chedda P, Pande S, Dama T, Vinarkar S, Chanekar M, Limaye S et al (2020) Spectrum of germline BRCA mutations in hereditary breast and ovarian cancer syndrome in Indian population: a central reference laboratory experience. Cancer Res Stat Treat 3(1):32–41
Mittal A, Deo SVS, Gogia A, Batra A, Kumar A, Bhoriwal S, Pramanik R (2022) Profile of pathogenic mutations and evaluation of germline genetic testing criteria in consecutive breast cancer patients treated at a north indian tertiary care center. Ann Surg Oncol 29:1423–1432
Kulkarni SS, Nag S, Patra A, Pant HB, Agiwal V, Nirupama AY et al (2023) Prevalence of germline mutations in women with breast and/or Ovarian cancer in a tertiary care center in Pune, India. Int J Mol Immuno Oncol 8:65–71
Gogia A, Nithin SG, Pramanik R, Gupta R, Deo SVS, Mathur S, Sharma D, Batra A, Prasad CP, Sagiraju HKR (2023) Prevalence of BRCA1 and BRCA2 mutations in an unselected cohort of Indian women with breast cancer. American Society of Clinical Oncology. Breast Cancer—Metastatic. May 31, 2023
Singh J, Thota N, Singh S, Padhi S, Mohan P, Deshwal S, Sur S, Ghosh M, Agarwal A, Sarin R et al (2018) Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: prevalence of BRCA1/2 and non-BRCA mutations. Breast Cancer Res Treat 170:1
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A (2021) Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers 13(17):4287
Ferreyra Y, Rosas G, Cock-Rada AM, Araujo J, Bravo L, Doimi F, Casas J, Clavo MD, Pinto JA, Belmar-López C (2023) Landscapeofgermline BRCA1/BRCA2 variants in breast and ovarian cancer in Peru. Front Oncol 13:1227864
Felix GE, Zheng Y, Olopade OI (2018) Mutations in context: implications of BRCA testing in diverse populations. Fam Cancer 4:471–483
Mehta A, Vasudevan S, Sharma SK, Kumar D, Panigrahi M, Suryavanshi M, Gupta G (2018) Germline BRCA1 and BRCA2 deleterious mutations and variants of unknown clinical significance associated with breast/ovarian cancer: a report from North India. Cancer Manag. Res. 10:6505–6516
Mannan AU, Singh J, Lakshmikeshava R, Thota N, Singh S, Sowmya TS, Mishra A, Sinha A, Deshwal S, Soni MR, Chandrasekar A, Ramesh B, Ramamurthy B, Padhi S et al (2016) Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: implications of embracing a multi-gene panel in molecular diagnosis in India. J Hum Genet 61:515–522
Rashid MU, Muhammad N, Naeemi H, Khan FA, Hassan M, Faisal S, Gull S, Amin A, Loya A, Hamann U (2019) Spectrum and prevalence of BRCA1/2 germline mutations in Pakistani breast cancer patients: results from a large comprehensive study. Hered Cancer Clin Pract. 17:27
Surakasula A, Nagarjunapu GC, Raghavaiah KV (2014) A comparative study of pre- and post-menopausal breast cancer: risk factors, presentation, characteristics and management. J Res Pharm Pract 3:12–18
Ferreyra Y, Rosas G, Cock-Rada AM, Araujo J, Bravo L, Doimi F, Casas J, Clavo MD, Pinto JA, Belmar-López C (2023) LópezLandscapeof germline BRCA1/BRCA2 variants in breast and ovarian cancer in Peru. Front Oncol 13:1227864
Mannan AU, Singh J, Lakshmikeshava R et al (2016) Detection of high frequency of mutations in a breast and/or ovarian cancer cohort: implications of embracing a multi-gene panel in molecular diagnosis in India. J Hum Genet 61(6):515–522
Hansa J, Kannan R, Ghosh SK (2012) Screening of 185DelAG, 1014DelGT and 3889DelAG BRCA1 mutations in breast cancer patients from North-East India. Asian Pac J Cancer Prev 13:5871–5874
Hedau S, Jain N, Husain SA, Mandal AK, Ray G, Shahid M et al (2004) Novel germline mutations in breast cancer susceptibility genes BRCA1, BRCA2 and p53 gene in breast cancer patients from India. Breast Cancer Res Treat 88:177–186
Saxena S, Chakraborty A, Kaushal M, Kotwal S, Bhatanager D, Mohil RS et al (2006) Contribution of germline BRCA1 and BRCA2 sequence alterations to breast cancer in Northern India. BMC Med Genet 7:75
Kadalmani K, Deepa S, Bagavathi S, Anishetty S, Thangaraj K, Gajalakshmi P (2007) Independent origin of 185delAG BRCA1 mutation in an Indian family. Neoplasma 54:51–56
Guidugli L, Shimelis H, Masica DL, Pankratz VS, Lipton GB, Singh N, Hu C, Monteiro AN, Lindor NM, Goldgar DE, Karchin R, Iversen ES, Couch FJ (2018) Assessment of the clinical relevance of BRCA2 missense variants by functional and computational approaches. Am J Hum Genet 102(2):233–248
Vidra R, Ciuleanu TE, Nemeș A, Pascu O, Heroiu AM, Antone N, Vidrean AI, Oprean CM, Pop LA, Berindan-Neagoe I, Eniu R (2022) Spectrum of BRCA1/2 mutations in romanian breast and ovarian cancer patients. Int J Environ Res Public Health 19:4314
Goidescu IG, Eniu DT, Caracostea GV, Cruciat G, Stamatian F (2018) Associations of pathogenic mutations responsible for breast cancer risk with histology and immunohistochemistry in Romanian population. Rev Romana Med Lab 26:165–175
Janavičius R (2010) Founder BRCA1/2 mutations in the Europe: implications for hereditary breast-ovarian cancer prevention and control. EPMA J 1:397–412
Kansuttiviwat C, Lertwilaiwittaya P, Roothumnong E et al (2024) Germline mutations of 4567 patients with hereditary breast-ovarian cancer spectrum in Thailand. npj Genom Med 9:9
Imagawa E, Fattal-Valevski A, Eyal O, Miyatake S, Saada A, Nakashima M, Tsurusaki Y, Saitsu H, Miyake N, Matsumoto N (2016) Homozygous p.V116* mutation in C12orf65 results in Leigh syndrome. J Neurol Neurosurg Psychiatry 87(2):212–216
Pramanik R, Chitikela S, Deo SVS, Gogia A, Batra A, Kumar A, Gupta R, Thakral D, Ramprasad VL, Mathur S, Sharma DN, Sharma A, Mishra A, Bansal B (2024) Comprehensive germline profiling of patients with breast cancer: initial experience from a Familial Cancer Clinic. Ecancermedicalscience 18:1670
Sharma-Oates A, Shaaban AM, Tomlinson I, Wynne L, Cazier JB, Sundar S (2018) Corrigendum to: Heterogeneity of germline variants in high risk breast and ovarian cancer susceptibility genes in India. Precis Clin Med 1(3):134
Kim YC, Zhao L, Zhang H, Huang Y, Cui J, Xiao F, Downs B, Wang SM (2016) Prevalence and spectrum of BRCA germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget 7(8):9600–9612
Torres-Mejía G, Royer R, Llacuachaqui M, Akbari MR, Giuliano AR, Martínez-Matsushita L, Angeles-Llerenas A, Ortega-Olvera C, Ziv E, Lazcano-Ponce E, Phelan CM, Narod SA (2015) Recurrent BRCA1 and BRCA2 mutations in mexican women with breast cancer. Epidemiol Biomarkers Prev 24(3):498–505
Verma A, Julka PK, Kaur J (2020) Mutations in BRCA1/2 genes: unexpected higher prevalence in Indian Patients. Cancer Res Stat Treat 3:376–377
Heramb C, Wangensteen T, Grindedal EM, Ariansen SL, Lothe S, Heimdal KR, Mæhle L (2018) BRCA1 and BRCA2 mutation spectrum: an update on mutation distribution in a large cancer genetics clinic in Norway. Hered Cancer Clin Pract 16:3
Son BH, Ahn SH, Kim SW, Kang E, Park SK, Lee MH, Noh WC, Kim LS, Jung Y, Kim KS, Noh DY, Moon BI, Suh YJ, Lee JE, Choi DH, Kim SY, Jung SH, Yom CK, Lee H, Yang JH (2012) Prevalence of BRCA1 and BRCA2 mutations in non-familial breast cancer patients with high risks in Korea: the Korean Hereditary Breast Cancer (KOHBRA) Study. Breast Cancer Res Treat 133:1143–1152
Lotfy MM, Youssef AS, Nassar A, Kamel M, Hassan ZK, Abou-Bakr AA, Farahat A, Gomaa MM, Zekri AR (2021) Identification of Somatic BRCA1/2 mutation profile in tissue-based and liquid biopsy-based next generation sequencing in Egyptian patients with breast cancer. Cancer Res 81:2175
Youssef AS, Zekri AR, Mohanad M, Loutfy SA, Abdel Fattah NF, Elberry MH, El Leithy AA, El-Touny A, Rabie AS, Shalaby M, Hanafy A et al (2023) Deleterious and ethnic related BRCA1/2 mutations in tissue and blood of Egyptian colorectal cancer patients and its correlation with human papilloma virus. Clin Exp Med 23:5063–5088
Donenberg T, George S, Ali J, Bravo G, Hernandez K, Sookar N, Ashing KT, Narod SA, Akbari MR, Hurley J (2019) A clinically structured and partnered approach to genetic testing in Trinidadian women with breast cancer and their families. Breast Cancer Res Treat 174(2):469–477
Acknowledgements
We acknowledge the support provided by Bioserve Biotechnologies India Private Limited and Reprocell group.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Rosy Chikkala, made substantial contributions towards literature research, and drafted the manuscript followed by editing and revision. Deepak Bhayal carried out bioinformatics and statistical analysis. Nikki Rani was involved in wet lab/ experimental work ranging from DNA extraction to NGS sequencing. Dr. Bhawna Dubey conceived the idea and design of the study, drafting the manuscript, revising it critically for important intellectual content and final submission. Rama Modali & Dr. Kishor Bhatia provided their support critical inputs and review for improvement of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from all patients.
Consent for publication
All authors are aware of its submission and the paper has not been submitted elsewhere.
Competing interests
The authors declare no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chikkala, R., Bhayal, D., Rani, N. et al. Mutational landscape of BRCA gene mutations in Indian breast cancer patients: retrospective insights from a diagnostic lab. Egypt J Med Hum Genet 25, 101 (2024). https://doi.org/10.1186/s43042-024-00567-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-024-00567-6