Abstract
Cholangiocarcinoma, a malignant neoplasm of the biliary system, poses a significant challenge in clinical practice due to its increasing incidence and diagnostic and therapeutic complexity. This review addresses the epidemiological aspects, risk factors, and classification of this disease. We examine advances in histological diagnosis, highlighting essential criteria for accurate assessment. Additionally, we discuss standard treatment approaches and their efficacy, alongside the latest innovations in therapy, including emerging biomarkers and targeted therapies. By providing a comprehensive overview of these topics, this article aims to enhance understanding and guide the pathological diagnosis and clinical management of this devastating disease.
Similar content being viewed by others
Introduction
General
Cholangiocarcinoma (CCA) arises from the biliary epithelium. Intrahepatic CCA (iCCA) originates within the liver from small ducts or ductules. Perihilar CCA (pCCA) occurs in the hilar region near the bifurcation of the common bile duct. Distal CCA (dCCA) is situated distal to the cystic duct insertion on the biliary tree. Unfortunately, the diagnosis of CCA most frequently occurs at advanced stages of the disease (Ilyas and Gores 2013).
Much like the unexpected revelation in Alexandre Dumas’ novel where the three musketeers are four, studies on CCA reveal that what appears to be one disease is, in fact, at least four distinct conditions, each with unique characteristics and therapeutic targets. Expanding the understanding of morphological, immunohistochemical, and molecular profiles is essential for advancing disease classification and developing targeted therapeutic interventions.
Epidemiology
CCA is considered a rare neoplasm, accounting for 3% of all malignancies arising in the gastrointestinal tract and 10–20% of all primary liver cancers. CCA is known for its aggressive nature, as the disease is often diagnosed in an advanced stage (Siegel et al. 2023).
Most CCAs are sporadic, with approximately 10–15% associated with inherited cancer predisposition syndromes. Currently, genetic testing for CCAs is not an established medical recommendation due to insufficient comprehensive data on the prevalence of germline mutations in this malignancy (Maynard et al. 2020).
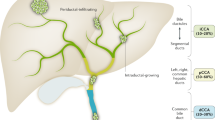
Regarding CCA distribution, iCCA is the least common, accounting for 10–20% of all CCAs. The classic Klatskin tumor, represented by pCCA, corresponds to 50–60% of all CCAs. The remaining 20–30% of CCA cases are represented by dCCA. The term extrahepatic CCA (eCCA) encompasses both pCCA and dCCA (Fig. 1) (Vithayathil et al. 2022).
Misclassification is a significant issue that impacts CCA epidemiology and research, particularly in non-central health institutions, where pCCA (the majority of CCAs) is often incorrectly coded as iCCA. Similarly, iCCA can be misclassified as hepatocellular carcinoma (HCC), and eCCA can be mistakenly coded as gallbladder cancer (Cardinale 2019).
The incidence and mortality of iCCA are increasing globally while eCCA rates remain stable. In Western countries, this rising incidence of iCCA may be associated with obesity, diabetes, and steatotic liver disease. However, these trends may also be influenced by misclassification and should be interpreted with caution (Vithayathil et al. 2022).
The global distribution of CCA is heterogeneous, with higher rates observed in China and Thailand (Vithayathil et al. 2022). In most regions, the peak incidence of CCA occurs in the seventh decade of life, affecting both genders with a slight male preponderance (male-to-female ratio of 2:1 to 3:1). Epidemiological studies focusing exclusively on CCA and its subtypes in Brazil are rare (Santos et al. 2019).
Risk factors and precursor lesions
Multiple studies have demonstrated that iCCA and eCCA share some risk factors, such as PSC, liver fluke infection, lithiasis, and tobacco and alcohol consumption. However, distinct risk factors have also been identified for each subtype. iCCA is additionally associated with hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, cirrhosis, and liver metabolic diseases, especially steatotic liver diseases. In contrast, eCCA is linked to choledochal cyst, and Lynch syndrome (Khan et al. 2019).
Common precursors of CCAs include intraductal papillary neoplasm of the bile duct (IPNB), biliary intraepithelial neoplasia (BilIN), and mucinous cystic neoplasm (MCN), particularly when they exhibit high-grade dysplasia (Nakanuma et al. 2022). These precursor lesions are more commonly associated with large duct intrahepatic iCCA (Nakanuma and Sudo 2017).
Evolution of classifications used in CCA
The classification of primary liver neoplasms is a widely discussed topic in medical literature. Initially, anatomical localization served as the primary method for subclassifying CCAs. However, the classification has evolved to encompass variables such as tumor gross appearance, histologic findings, cell of origin, and more recently, molecular aspects.
In terms of gross appearance, iCCA typically presents as a solid, white, and compact mass-forming tumor (MF type). In contrast, pCCA and eCCA manifest in the hilar region and the extrahepatic biliary tree as either periductal infiltrating (PI type) or intraductal polypoid lesions (ID type). Macroscopic classification facilitates a close radiological correlation to morphological findings (Fig. 2) (Guedj 2022).
Histology demonstrates distinct differences between various types of CCAs (11). More often, dCCA, pCCA, and the large-duct type iCCA (located proximal to the hepatic hilar region) are mucin-producing adenocarcinomas characterized by large ducts and papillary morphologies with columnar cells. In contrast, iCCA located in the periphery of the hepatic parenchyma commonly exhibits tubular or acinar architectures with low columnar to cuboidal cells, organized in a small-duct pattern associated with a desmoplastic stroma. The small duct type of iCCA includes two adicional distinct subgroups: the ductal plate malformation pattern and cholangiolocarcinoma. The ductal plate malformation pattern of iCCA resembles ductal plate malformations (Nakanuma et al. 2012), while the cholangiolocarcinoma subgroup features a more primitive architecture that resembles immature cholangioles (Fig. 3) (Nakanuma and Kakuda 2015; Akita et al. 2017; Sempoux et al. 2011; Liau et al. 2014).
These tumors also have different cells of origin. Generally, peripheral and small-duct type iCCA arises from hepatic progenitor cells or cuboidal (mucin-negative) cholangiocytes, whereas pCCA, dCCA, and central iCCA originate from mature (mucin-producing) cholangiocytes that line the biliary tree (Sigel et al. 2018; Komuta et al. 2008, 2012).
Most common histological subtypes of cholangiocarcinoma and their location in the biliary tree: A) Peri-hilar cholangiocarcinoma, distal cholangiocarcinoma and intrahepatic cholangiocarcinoma, large-duct type (H&E, 200X); B) Intrahepatic cholangiocarcinoma, small-duct type (H&E, 200X); C) Intrahepatic cholangiocarcinoma with ductal plate malformation pattern (H&E, 100X); D) Intrahepatic cholangiocarcinoma with cholangiolocarcinoma pattern (H&E, 100X)
Genomic profiling has identified several oncogenic alterations in CCAs. (Normanno 2022). Based on these molecular discoveries, the US Food and Drug Administration (FDA) has granted accelerated approval for tumor-target therapy independent of the type of the malignant neoplasm (pan-tumor therapy), and that encompasses patients with advanced CCA.
The literature contains numerous efforts to develop comprehensive description of the three entities (iCCA, pCCA, dCCA) (Fig. 4). Many of these aspects are considered in the World Health Organization classification of digestive system tumors (5th edition) that accepts two additional subtypes for iCCA: a small-duct type, which shares many etiologic, pathogenetic, and imaging characteristics with HCC, and a large-duct type resembling eCCA and pancreatic ductal adenocarcinoma (Nagtegaal et al. 2020).
Different aspects of cholangiocarcinoma including anatomic localization, microscopic pattern, main histologic finding, cell of origin and molecular alterations with target therapy. iCCA: intrahepatic cholangiocarcinoma, pCCA: perihilar cholangiocarcinoma, dCCA: distal cholangiocarcinoma (dCCA), eCCA: extrahepatic cholangiocarcinoma, MF: mass-forming tumor, PI: periductal infiltrating tumor, ID: intraductal polypoid tumor
Does the patient have CCA?
Confirming the presence of CCA is a critical diagnostic step before making therapeutic decisions. For the diagnosis of CCA, the exclusion of metastatic adenocarcinomas is essential, as these represent the most prevalent malignant neoplasms in the liver parenchyma. (Fig. 5).
This diagnostic process integrates clinical history, imaging examinations, and histological analysis of both neoplastic and non-neoplastic tissue, including morphological evaluation and immunohistochemical (IHC) studies.
It is important to note that the histological findings and the immunohistochemical profile commonly observed in CCAs are not exclusive to this tumor group. The well-known pancreaticobiliary immunophenotype - characterized by positivity for cytokeratin 7 and 19 combined with negativity for cytokeratin 20 and markers indicative of primary sites outside the biliary system - is shared by adenocarcinomas originating from the intrahepatic and extrahepatic biliary tree, gallbladder, pancreas, and upper gastrointestinal tract (Bellizzi 2020).
Certain immunohistochemical markers, such as S100P, NCAM, N-cadherin, and CRP can aid in distinguishing different subtypes of CCAs. S100P is often expressed in large-duct type adenocarcinomas and can help differentiate these from other subtypes. NCAM (CD56) is typically expressed in cholangiolocarcinomas. Additionally, N-cadherin and CRP are useful markers in conventional small-duct type of iCCA, helping to differentiate this tumor from metastatic adenocarcinomas (Akita et al. 2021; Hayashi et al. 2016).
Recently, in situ hybridization for albumin has emerged as a valuable tool for distinguishing between liver metastases of adenocarcinoma and iCCA (particularly of the small duct morphology, which shows positive staining for this marker). It is crucial to note that albumin expression is also observed in HCC and is described in other rare adenocarcinomas with hepatoid differentiation (Chung et al. 2023).
Predictors of survival in CCA
Predicting survival in individual patients is complex, requiring the evaluation of multiple factors. Early-stage tumors generally carry a more favorable prognosis compared to advanced-stage tumors. Presence of metastasis to distant organs is associated with poor prognosis.
Performance status is a relevant factor because it reflects the patient’s overall health and ability to tolerate treatment. Concurrent liver diseases significantly impact treatment modalities and prognosis in CCA patients. High levels of CA19.9 often indicate poorer outcomes.
The pathology report, particularly from surgical specimens, provides critical information about the tumor, including its localization, size, gross appearance, histological subtype, presence of lymphovascular and perineural invasion, surgical margins and regional lymph node status. For instance, patients with resectable tumors (with negative surgical margins) have better outcomes than those with unresectable tumors. It is also important to note that in the recent eighth American Joint Commission on Cancer (AJCC) edition, iCCA, pCCA, and dCCA have different staging systems (Amin et al. 2017).
Recent studies suggest that the histological subtype of iCCA also have an impact on survival. Patients with small-duct type (mass-forming) iCCA have better survival rates compared to those with large-duct type iCCA. Additionally, there is evidence that cholangiolocarcinoma is associated with even better survival outcomes. Patients with large-duct type iCCA have prognosis more similar to patients with pCCA (Akita et al. 2017).
Impact of pathology on treatment approach
The management of biliary tract cancers is rapidly evolving, drive by the exponential discovery of target therapies recently focused on fibroblast growth factor receptor-2 (FGFR-2) fusions, isocitrate dehydrogenase-1 (IDH-1) mutations, B-Raf proto-oncogene serine/threonine kinase (BRAF V600E) mutations, neurotrophic tyrosine receptor kinase (NTRK) fusions, human epidermal growth factor-2 (ERBB2/HER2) amplifications, and microsatellite instability. An early assessment of the molecular profile allows for adequate therapy planning (Lamarca et al. 2022; Tomczak et al. 2022).
Surgical resection followed by adjuvant chemotherapy with capecitabine is considered the primary treatment for early-stage CCA, particularly for patients with good liver function (Primrose et al. 2019). Hepatectomy is the main surgical approach for iCCA, while surgical options for eCCA include bile duct resection or pancreaticoduodenectomy (Whipple procedure) (Cillo et al. 2019). Chemoradiotherapy may be considered for patients with unresectable disease or lymph node involvement.
In setected centers, liver transplantation (LT) may be considered a treatment option for early stage iCCA or pCCA, especially for patients with underlying liver chronic disease or cirrhosis. LT is typically integrated with other treatment modalities (Mazzaferro et al. 2020).
Systemic chemotherapy is employed for advanced or metastatic disease. Since 2010, cisplatin in combination with gemcitabine has emerged as a standard of care based on findings from the open-label phase III trial (ABC-02) involving 410 patients with locally advanced or metastatic biliary tract tumors (64% CCA). This trial demonstrated significant improvements in overall survival (OS) and progression-free survival (PFS) compared to treatment with gemcitabine alone (Valle et al. 2010).
Two phase III studies analyzed the addition of immunotherapy to the standard chemotherapy. TOPAZ-1 trial investigated the addition of durvalumab to gemcitabine plus cisplatin in 685 patients with treatment-naive advanced or metastatic biliary tract tumors (75% CCA) and no hyperbilirubinemia and has shown significantly improved patient OS. The estimated 24-month OS rate was 24.9% for durvalumab and 10.4% for placebo group, with durable responses (Rimini et al. 2023).
The KEYNOTE-966 trial evaluated the addition of pembrolizumab to gemcitabine plus cisplatin in 1069 patients with previously untreated locally advanced or metastatic biliary tract cancer (78% CCA) and showed improvements in OS, with a median overall survival of 12·7 months in the pembrolizumab group versus 10·9 months in the placebo group (Kelley et al. 2023). Both regimens are considered appropriate as first-line treatment (category 1 by NCCN 2023) (Benson et al. 2023).
Second-line therapy with short-term infusional 5-fluorouracil plus leucovorin and oxaliplatin (FOLFOX) after progression with gemcitabine plus cisplatin is considered the preferred regimen, based on ABC-06 trial. In this phase III trial, FOLFOX was associated with significantly better rates of OS compared with active symptom control (Lamarca et al. 2021).
Patients who are not candidates for surgical and systemic therapies are managed by palliative radiation therapy (RT), RT with concurrent fluoropyrimidine, and palliative care.
Multiple genomic alterations have been identified in CCAs, some of which are now targetable with newly discovered medications. These therapies have the potential to significantly alter the landscape of CCA treatment in the future (Longerich et al. 2024).
New tools in the management of CCAs
Breakthroughs in molecular alterations are linked to emerging tools that influence the management of patients suffering from advanced CCA. For every emerging tool, we elucidate the involved gene (nomenclature, chromosomal localization, type and frequency of molecular alterations; Table 1), its physiological function, detectable molecular alterations in CCA, the aberrant function resulting from genetic and epigenetic events, available molecular testing methodologies, and the corresponding approved target therapy.
Among available molecular testing methodologies, IHC studies are the most cost-effective, employing specific antibodies for initial screening of protein abnormalities. Polymerase chain reaction (PCR)-based testing can be utilized to detect well-known genetic alterations in small panels. Fluorescence in situ hybridization (FISH) can demonstrate fusions and amplifications. Next-generation sequencing (NGS) panels enable simultaneous analysis of multiple genes. These molecular studies allow for comprehensive assessment across multiple targets, aiding oncologists in treatment decision-making and potentially facilitating patient enrollment in ongoing clinical trials.
The amount of viable tumor required for testing varies depending on the technique used. For IHC studies, as low as 10% tumor content is sufficient, as the analysis focuses on protein expression. For PCR-based testing, a minimum of 20% tumor content is often recommended to ensure sufficient DNA yield from the tumor cells. FISH analysis typically requires at least 50–60% tumor content to accurately detect chromosomal abnormalities. For NGS panels, which provide a larger genetic profile, the percentage of tumor cells should ideally be 20–30% or higher, depending on the sequencing platform’s sensitivity. This ensures that the genetic material analyzed predominantly represents the tumor, allowing for precise detection of mutations and other genomic alterations (da Cunha et al. 2021).
These tests can be performed on formalin-fixed, paraffin-embedded tissues. NGS is specifically recommended by NCCN and the European Society for Medical Oncology (Mosele et al. 2020).
It is important to emphasize that CCAs are not always amenable to biopsy because of their localization or involvement with anatomic structures. Additionally, even when a biopsy is feasible, it may not yield sufficient tumor samples for all necessary ancillary tests (Lamarca et al. 2020). In such cases, collaboration between pathologists and oncologists is essential to prioritize tests and optimize the use of available tissue.
IDH1/2 inhibitors
Normal function
IDH1 is found in the cytosol and peroxisomes, while IDH2 is a mitochondrial enzyme. Both IDH1 and IDH2 play crucial roles in the citric acid cycle, also known as the Krebs cycle, which is a central pathway for energy production in cells. IDH1/2 enzymes participate in the conversion of isocitrate to alpha-ketoglutarate, generating NADPH and maintaining the balance of reactive oxygen species in the cell. NADPH in the liver is also important for metabolic processes including synthesis of fatty acids and cholesterol (Molenaar and Wilmink 2022).
Molecular alteration and frequency in CCA
Mutations in IDH1 are hotspot mutations with a broader spectrum in CCA involving various amino acid substitutions. The most common IDH1 mutations in CCA are R132C, which accounts for approximately 50% of IDH1 mutations, followed by R132S (around 20%) and R132G (about 10%). Mutation in IDH2 involves substitution of arginine at position 172 originating the mutant protein IDH2 R172. IDH1 mutations are detected in approximately 10–20% of iCCA and are rare in pCCA and dCCA. IDH2 mutation is rare in all CCA subtypes (Lowery et al. 2018).
Abnormal function
The neomorphic, mutant enzyme, converts alpha-ketoglutarate to the oncometabolite 2-hydroxyglutarate (2HG) both in the cytosol and in the mitochondria. This metabolite causes inhibition of the activity of Ten-Eleven Translocation (TET) enzymes and histone lysine demethylases (KDM). The result is global epigenetic modifications on DNA and histones originating in a hypermethylated phenotype (Golub et al. 2019). The oncometabolite 2HG is particularly relevant for iCCA development because high levels of 2HG block the gene HNF4 alpha and, without a functioning HNF4 transcriptor factor, the hepatic progenitor cell cannot differentiate into hepatocytes. This phenomenon added to mutations in KRAS induce cell expansion and formation of CCA (Saha et al. 2014).
Molecular testing methods
(1) IDH1 immunohistochemistry is not recommended in CCA cases. The IDH1 R132H mutation is the most prevalent hot spot mutation in gliomas and is targeted by the commonly used IDH1 antibody. However, the spectrum of IDH1 mutations in CCA is broader and often involves different amino acid substitutions. The most common IDH1 mutations in CCA are R132C (approximately 50% of IDH1 mutations), R132S (around 20%), and R132G (about 10%). These mutations are not detected by the IDH1 R132H-specific antibody. Therefore, the use of this antibody in CCA is not appropriate for accurate mutation detection and may lead to false-negative results (Rimini et al. 2022; Makawita et al. 2024), (2) PCR-based testing can be utilized to amplify and detect specific IDH1 or IDH2 mutation hotspots, and (3) NGS panels can simultaneously analyze multiple genes, including IDH1/2. Testing should be performed for unresectable or metastatic CCA (subsequent-line therapy if disease progression (Benson et al. 2023).
Target therapy
A multicentre, randomized, double-blind, placebo-controlled, phase III study (ClarIDHy) investigated the efficacy and safety of Ivosidenib (AG-120), a first-in-class, oral, small-molecule inhibitor of mutant IDH1 in patients with advanced CCA. This study included 187 previously treated patients with CCA and IDH1 mutation and resulted in numerically improved overall survival benefits vs. placebo (median OS 10.3 vs. 7.5 months), despite a high rate of crossover. Based on this trial, Ivonidenib has been approved by the FDA and Anvisa (Agência Nacional de Vigilância Sanitária in Brazil) for the treatment of adults with previously treated, locally advanced, or metastatic CCA (Abou-Alfa et al. 2020a).
FGFR inhibitors
Normal protein function
FGFRs belong to a family of cellular transmembrane receptors. All proteins share similar structures consisting of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. FGFRs act in various cellular processes such as cell proliferation, differentiation, migration, and survival. Upon ligand binding, FGFRs undergo dimerization and autophosphorylation, activating downstream signaling pathways such as the MAPK/ERK and PI3K/AKT pathways (Krook et al. 2021).
Molecular alteration and frequency in CCA
Molecular alterations include single-nucleotide variants, copy number amplifications, and gene fusions. FGFR2 fusions and rearrangements are found almost exclusively in iCCA, occurring in 10–16% of patients. In iCCA, the most frequent partner genes involved in FGFR2 fusions are BICC1 (Bicaudal C Homolog 1), AHCYL1 (Adenosylhomocysteinase Like 1), KIAA1217, PPHLN1, and TACC3 (Transforming Acidic Coiled-Coil Containing Protein 3) (Borad et al. 2014).
Abnormal function
The abnormal function allows constitutive activation of FGFR signaling pathways (MAPK/ERK and PI3K/AKT) leading to addictive proliferative advantage to the tumor cell, uncontrolled cell proliferation, survival, and angiogenesis. The signaling for activation can occur through various mechanisms such as ligand-independent activation (gene fusion can directly affect the conformation of the FGFR protein, resulting in its activation without the need for binding to fibroblast growth factors (FGFs), the natural ligands for FGFRs), autocrine/paracrine signaling (tumor cells or neighboring stromal cells may produce and secrete FGF ligands, which can then activate FGFRs in an autocrine/paracrine manner), amplification and overexpression (increase the number of FGFR receptors on the cell surface, thereby enhancing the sensitivity of cells to FGF ligands present in the microenvironment).
Molecular testing methods
(1) Despite its utility as a screening tool, IHC is not suitable for detecting FGFR2 fusions in iCCA because robust antibodies for all targets are not always available, since different fusion partners may affect the result (Saborowski et al. 2020); (2) PCR-based testing can be utilized to detect specific FGFR fusion transcripts. However, the increasing number of fusion partners necessitates the design and use of numerous different primer pairs in a single assay to screen for FGFR2 fusions (Saborowski et al. 2020); (3) Break-apart FISH is suitable for detecting both known and novel gene fusions but does not identify the fusion partner (Saborowski et al. 2020); and (4) NGS panels can simultaneously analyze multiple genes, including FGFR genes. Testing should be performed for unresectable or metastatic CCA (subsequent-line therapy if disease progression) (Benson et al. 2023).
Target therapy
Targeting FGFR2 fusions and rearrangements with specific inhibitors has emerged as a promising therapeutic strategy in CCA treatment. In two single-arm, phase 2 studies (FIGHT-202 and NCT02150967), the use of Pemigatinib and Infigratinibib respectively, oral inhibitors of FGFR 1–3 selective, have shown efficacy in objective response in patients previously treated and have been approved in this scenario (Abou-Alfa et al. 2020b). Phase III studies (FIGHT-302 and NCT03773302) are now ongoing to compare this targeted therapy versus standard chemotherapy. Futibatinib, a next-generation irreversible FGFR1-4 inhibitor, has shown clinical benefit and improved objective response rates and it has also been approved by the FDA for the treatment of locally advanced/metastatic iCCA after one or more systemic therapy (Goyal et al. 2023). The use of Erdafitinib, an oral selective pan-FGFR tyrosine kinase inhibitor, remains investigational in this setting.
NTRK inhibitors
Normal protein function
These proteins have important roles in neuronal development, cell survival, and proliferation
Molecular alteration and frequency in CCA
Mutations can be found, but fusions involving NTRK are the most common mechanism of oncogenic activation. According to available literature, the prevalence of NTRK gene alterations in CCA ranges is rare (< 1%), necessitating physicians’ understanding of managing ‘rare-alteration-in-a-rare-disease’ scenarios (Boilève et al. 2021). Specific data on the prevalence of NTRK gene alterations in iCCA, pCCA, and dCCA individually are not readily available nor consistent across studies.
Abnormal function
The abnormal function permits constitutive activation of signaling pathways (MAPK/ERK and PI3K/AKT), and alteration of cellular processes including differentiation, migration, apoptosis, tumor progression and metastasis since dysregulated NTRK function can promote epithelial-to-mesenchymal transition increasing tumor aggressiveness and dissemination to distant sites.
Molecular testing methods
(1) IHC may detect overexpression of the TRK protein and can be used as initial screening for NTRK fusions, but it can produce false-positive resultsm necessitating confirmation with other techniques ( Zhang et al. 2022); (2) PCR-based testing and FISH can demonstrate rearrangements, and (3) NGS panels can simultaneously analyze multiple genes, including NTRK genes. Testing should be performed for unresectable or metastatic CCA (primary treatment or subsequent-line therapy if disease progression) (Benson et al. 2023).
Target therapy
Larotrectinib, a highly selective tropomyosin receptor kinase (TRK) inhibitor has shown efficacy in a combined analysis of 55 patients with various TRK fusion-positive malignancies enrolled in three trials, including patients with CCA tumors, and is approved by the FDA for solid tumors with an NTRK gene fusion and without a known acquired resistance mutation that is either metastatic or irresectable and has progressed following treatment (Drilon et al. 2018). Similarly, Entrectinib, another TRK inhibitor, has received FDA approval for ten types of cancers with an NTRK gene fusion, including CCA, based on combined analysis from three single-arm clinical trials (Drilon et al. 2018).
Immune checkpoint inhibitors (ICIs)
Normal protein function
PD-L1 is a critical immune checkpoint protein involved in regulating the immune response by interacting with the PD-1 receptor on T cell lymphocytes, causing T cell inhibition and immune evasion by tumor cells. CTLA-4 is expressed in the activated T cells to inhibit T cell activation by competing for binding of the CD28 ligand (Zeng and Chen 2021).
Molecular alteration and frequency in CCA
Studies have reported varying levels of PD-L1 expression in iCCA (tumor or tumor plus immune cell PD-L1 expression), and the reported prevalence ranges from 10 to 40% depending on the cutoff criteria used for defining positivity. Data on PD-L1 expression in pCCA and dCCA are limited (Zeng and Chen 2021).
Abnormal function
PD-L1 is often upregulated in tumor cells and the cells of the microenvironment as a mechanism of immune evasion. When PD-L1 on tumor cells binds to its receptor PD-1 on T cells, it inhibits the cytotoxic activity of T cells and induces T cell exhaustion, reducing antitumor immune response. This strategy allows tumor cells to evade immune surveillance and promote tumor growth and metastasis.
Molecular testing methods
(1) IHC is a widely used method for assessing PD-L1 protein expression in different tumor tissue samples. Although PD-L1 expression is a potential biomarker for immunotherapy response, there is no specific companion diagnostic test approved for CCA. Various studies have employed different antibodies and scoring systems (CPS or TPS). The pathologist should consult the oncologist to select the appropriate antibody. The anatomical report should include a comment stating that the applied score is used in the absence of a specific protocol for PD-L1 testing in CCA (Fontugne et al. 2017; Sangkhamanon et al. 2017; Ahn et al. 2020; Matsumoto et al. 2022) (2) PCR-based testing can be utilized to detect specific PD-L1 alterations, such as mutations or gene rearrangements, (3) FISH can be employed to detect gene amplification or copy number alterations involving the PD-L1 gene, and (4) NGS panels can be used to analyze the entire genomic sequence of the PD-L1 gene and identify various types of alterations, including mutations, amplifications, deletions, and structural variants. Studies are insufficient to warrant a recommendation for testing (Benson et al. 2023).
Target therapy
Phase III studies have demonstrated the efficacy of checkpoint inhibitors like Durvalumab and Pembrolizumab in metastatic CCA, with benefits observed regardless of PD-L1 status. This suggests that additional biomarkers may be important for predicting response rates in CCA treatment (Rimini et al. 2023; Kelley et al. 2023).
Microsatellite instability-high (MSI-H), deficient mismatch repair (dMMR), and tumor burden high (TMB-H) profiles
Normal protein function
The respective proteins are essential in the DNA mismatch repair pathway
Molecular alteration and frequency in CCA
Molecular alterations in CCA that cause MSI-H/dMMR profile include: (1) silencing of MLH1 gene expression due to hypermethylation of its promoter region, (2) mutations in any of the MMR genes (MLH1, MSH2, MSH6, or PMS2), and (3) epigenetic mechanisms, such as histone modifications or altered expression of microRNAs. Reported frequencies of MSI-H/dMMR in CCA subtypes are relatively low compared to other cancer types. The frequencies of dMMR and MSI-H in CCA are 0 to 9.4 and 0‑18%, respectively (Ando et al. 2022).
Abnormal function: dMMR causes
(1) accumulation of errors in repetitive DNA sequences known as microsatellites; (2) elevated mutation rate that contributes to tumor heterogeneity and progression; and (3) formation of neoantigens generated by frameshift mutations that lead to immune recognition and activation.
Molecular testing methods
The tests commonly used to assess dMMR and MSI-H status include: (1) IHC demonstrating loss of expression of one or more MMR proteins; (2) PCR-based testing using the Bethesda panel which includes a set of five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) and two pentanucleotide repeat markers (Penta C and Penta D). MSI-H is defined by the presence of novel or shifted bands in the tumor sample compared to the normal sample; and (3) NGS can detect mutations and alterations in MMR genes and assess MSI status by analyzing microsatellite regions across the genome. Testing should be performed for unresectable or metastatic CCA (primary treatment or subsequent-line therapy if disease progression) (Benson et al. 2023).
Targeted therapy
The efficacy of pembrolizumab in dMMR advanced CCA was shown in the phase II KEYNOTE-158 study. Retrospective evaluation of 149 individuals with various solid tumors and DNA repair enzyme deficiency treated with pembrolizumab resulted in an objective response rate of 40.9% among 22 cases of CCA, confirming that immunotherapy is active in this histology. The Keynote-158 study led to the FDA approval of pembrolizumab for tumors with high mutational burden (≥ 10 mutations/megabase) or MSI-H/dMMR that had progressed following prior treatment, and for which there were no satisfactory alternative treatment options, the first such approval of a tissue-agnostic anticancer treatment (Marabelle et al. 2020). The combined regime with Nivolumab plus Ipilimumab was evaluated in a phase II nonrandomized clinical trial that included patients with advanced rare cancers, including 39 patients with biliary tract cancers. Responses were exclusively observed in patients with intrahepatic CCA and gallbladder carcinoma. Based upon this study, a trial of nivolumab plus ipilimumab is reasonable in the setting of second-line therapy for patients with iCCA who do not have dMMR, high levels of TMB, or PD-L1 overexpression, and who did not receive frontline immunotherapy (Klein et al. 2020).
ERBB2 (HER2) inhibitors
Normal protein function
ERBB2 (HER2) is a member of the epidermal growth factor receptor (EGFR) family. It functions as a receptor tyrosine kinase and activates intracellular signaling pathways upon binding with specific ligands, allowing cell growth, proliferation, and survival.
Molecular alteration and frequency in CCA
ERBB2 (HER2) was amplified in 3.9–8.5% and activating ERBB2 mutations was shown in 2% of CCAs (Czauderna et al. 2021). One study showed that alterations are present in 6.3% of eCCA cases and 0.6% of iCCA cases (Yan et al. 2015).
Abnormal function
Abnormal ERBB2 (HER2) protein signaling contributes to cancer progression by promoting cell proliferation, survival, and metastasis.
Molecular testing methods
(1) IHC may detect protein overexpression; it is important to note that there is no specific guideline to analyze ERBB2 (HER2) protein expression in CCAs, and in most circumstances, pathologists use the guideline developed for gastric and gastroesophageal junction adenocarcinoma as a reference (Fig. 6) (Shen et al. 2013; Galdy et al. 2017); (2) FISH can detect ERBB2 (HER2) gene amplification (Fig. 5); the detection of gene amplification by FISH follows guidelines similar to those used in breast cancer, as outlined by the American Pathologists (ASCO/CAP) guidelines (Wolff et al. 2018); and (3) NGS panels can simultaneously analyze multiple genes, including ERBB2 (HER2) genes. Testing should be performed for unresectable or metastatic CCAs (Benson et al. 2023).
Example of extrahepatic cholangiocarcinoma with amplification for ERBB2 (HER2) gene (ERBB2/CEP17): ≥ 2. A) Score 3 + for ERBB2 (HER2) oncoprotein immunohistochemistry (IHC) pattern in biopsy specimen (following criteria used in the ToGA trial for scoring ERBB2 (HER2) oncoprotein expression by IHC in gastric and gastroesophageal junction adenocarcinoma; B) CEP17; C) ERBB2 (HER2) gene demonstrating amplification
Targeted therapy
For patients with advanced or metastatic ERBB2 (HER2) positive CCA who progress on chemotherapy, regimens based on trastuzumab plus pertuzumab or trastuzumab plus tucatinib have shown good objective response rates with an acceptable toxicity profile in two phase II basket studies (MyPathway and SGNTUC-019). These regimens are preferred options for late-line treatment in this setting (Javle et al. 2021). The efficacy of trastuzumab-deruxtecan (T-DXd), a ERBB2 (HER2)-directed antibody-drug-conjugate in treatment-refractory CCA has been demonstrated in two phase II clinical trials (HERB and DESTINY-PanTumor02). It has FDA approval for patients with unresectable or metastatic ERBB2 (HER2)-positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options (Ohba et al. 2022). However, patients should be cautioned about the risk of interstitial lung disease with this agent.
BRAF inhibitors
Normal protein function
BRAF encodes a protein called B-Raf, which is a serine/threonine kinase involved in the RAS-RAF-MEK-ERK signaling pathway. This pathway regulates cell growth, proliferation, and differentiation.
Molecular alteration and frequency in CCA
BRAF mutations are relatively rare in CCAs, occurring in approximately 1–4% of cases. The most common mutation is the V600E substitution (Chakrabarti et al. 2020; Li et al. 2020).
Abnormal function
The V600E mutation leads to constitutive activation of the BRAF kinase, resulting in dysregulated cell signaling and increased cell proliferation.
Molecular testing methods
(1) One study reveals that VE1 IHC is an approach to screen for BRAF V600E mutation in CCA, facilitating the detection of rare patients who may benefit from BRAF mutation-targeted therapies (Goeppert et al. 2014), and (2) PCR or NSG can simultaneously analyze multiple genes, including BRAF. Testing should be performed for unresectable or metastatic CCAs (Benson et al. 2023).
Target therapy
A phase II basket trial (ROAR) including 43 patients with BRAF V600E-mutated biliary tract showed that the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) appears to be effective on objective response rate in the later line scenario. Based on this trial, the combination of dabrafenib and trametinib was approval from the FDA for the treatment of adult and pediatric patients one year of age and older with unresectable or metastatic solid tumors carrying mutations in BRAF V600E following prior treatment and with no satisfactory alternative treatment options (Subbiah et al. 2023).
RET inhibitors
Normal protein function
RET encodes a receptor tyrosine kinase involved in cell growth, differentiation, and survival, particularly in the development of neural crest-derived tissues.
Molecular alteration and frequency in CCA
RET fusions are found in a subset of CCAs, occurring in approximately 1–2% of cases (Adashek et al. 2021).
Abnormal function
RET fusions result in constitutive activation of the RET kinase, activating aberrant signaling pathways involved in cell proliferation and survival.
Molecular testing methods
Detection of RET fusions typically involves molecular techniques such as FISH, reverse transcription PCR (RT-PCR), or NGS. Testing should be performed for unresectable or metastatic CCAs (Benson et al. 2023).
Target therapy
The phase I/II basket trial LIBRETTO-001 analyses activity in the RET fusion-positive tumour-agnostic population, including CCA, using the RET tyrosine kinase inhibitor selpercatinib (Subbiah et al. 2022). Based on this trial the FDA approved Selpercatinib for adult patients with locally advanced or metastatic solid tumors, with a RET gene fusion and disease progression on or following prior systemic treatment who have no satisfactory alternative treatment options.
KRAS G12C inhibitors
Normal protein function
KRAS protein acts in cell signaling pathways such as signal transduction, cell growth and division, differentiation, apoptosis regulation, and integration between cell and environment.
Molecular alteration and frequency in CCA
In one study KRAS mutations were identified in almost 40% of eCCA (Montal et al. 2020).
Abnormal function
Mutant KRAS protein is associated with oncogenic activity, resistance to apoptosis, increased metastatic potential, altered cellular differentiation, and dysfunctional interaction with the microenvironment.
Molecular testing methods
(1) As molecular testing is performed on formalin-fixed paraffin-embedded samples, immunodetection appears to be an attractive alternative for detecting mutations. However, when tested on colorectal tumor samples with known KRAS status, the KRAS polyclonal antibody showed poor sensitivity and specificity for detecting KRAS mutations (Piton et al. 2015). The literature on this technique for CCA is limited and IHC is not used for this investigationAkita, and (2) PCR-based assay or NGS is performed in this setting. In CCA, testing is not recommended (Benson et al. 2023).
Target therapy
There has been no approved therapy specificity targeting KRAS mutations in CCA until now. Patients with treatment-refractory metastatic CCA that harbors a KRAS G12C mutation should be encouraged to enroll in clinical trials, where available. Adagrasib, an agent target KRASG12C, was evaluated in a phase II trial (KRYSTAL-1) of 64 patients with KRAS G12C mutated, treatment-refractory solid tumors of various histologies, excluding non-small cell lung cancer and colorectal cancer, which includes eight patients with CCA, have shown promising results with an objective response rate of 50% (Pant et al. 2023).
Other molecular alterations
Molecular alterations in genes such as MET, ROS1, and ALK are linked to FDA-approved treatment in other tumor types. They are rare alterations but may be individually and multidisciplinary discussed as options for patients with progressive and refractory metastatic CCAs.
Conclusion
The evolution in CCA studies mirrors the intriguing complexity found in Alexandre Dumas’ novel, where the introduction of a fourth musketeer parallels new CCA subtypes. This analogy underscores that, much like Dumas’ tale, what initially appears to be a single entity often conceals deeper intricacies. Thus, the study of CCA reveals a multifaceted and dynamic landscape. Understanding this disease’s complexity is essential for advancing classification and developing more effective, targeted therapeutic interventions.
Unlike Dumas’ novel, however, the story of CCA is far from complete. New discoveries continuously add layers to our understanding of this disease. To forge a more hopeful future, ongoing debates about classification standards, integration of clinical, radiological, pathological, and molecular data, enhanced patient selection for clinical trials, and improved access to molecular studies at diagnosis are essential (Cardinale et al. 2012; Cardinale et al. 2013).
Data availability
not applicable.
References
Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020a;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. Epub 2020 May 13. Erratum in: Lancet Oncol. 2020;21(10):e462. doi: 10.1016/S1470-2045(20)30547-7. Erratum in: Lancet Oncol. 2024;25(2):e61. https://doi.org/10.1016/S1470-2045(24)00013-5. PMID: 32416072; PMCID: PMC7523268.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020b;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. Epub 2020 Mar 20. Erratum in: Lancet Oncol. 2024;25(1):e3. https://doi.org/10.1016/S1470-2045(23)00642-3. PMID: 32203698; PMCID: PMC8461541.
Adashek JJ, Desai AP, Andreev-Drakhlin AY, Roszik J, Cote GJ, Subbiah V. Hallmarks of RET and co-occuring genomic alterations in RET-aberrant cancers. Mol Cancer Ther. 2021;20(10):1769–76. https://doi.org/10.1158/1535-7163.MCT-21-0329. Epub 2021 Sep 6. PMID: 34493590; PMCID: PMC8492504.
Ahn S, Lee JC, Shin DW, Kim J, Hwang JH, High. PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 2020;10(1):12348. https://doi.org/10.1038/s41598-020-69366-4. Erratum in: Sci Rep. 2020;10(1):21552. doi: 10.1038/s41598-020-78512-x. PMID: 32704067; PMCID: PMC7378166.
Akita M, Fujikura K, Ajiki T, Fukumoto T, Otani K, Azuma T, Itoh T, Ku Y, Zen Y. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol. 2017;30(7):986–97. https://doi.org/10.1038/modpathol.2017.22. Epub 2017 Mar 24. PMID: 28338651.
Akita M, Sawada R, Komatsu M, Suleman N, Itoh T, Ajiki T, Heaton N, Fukumoto T, Zen Y. An immunostaining panel of C-reactive protein, N-cadherin, and S100 calcium binding protein P is useful for intrahepatic cholangiocarcinoma subtyping. Hum Pathol. 2021;109:45–52. Epub 2020 Dec 13. PMID: 33321161.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. Epub 2017 Jan 17. PMID: 28094848.
Ando Y, Kumamoto K, Matsukawa H, Ishikawa R, Suto H, Oshima M, Kamada H, Morishita A, Kobara H, Matsunaga T, Haba R, Masaki T, Suzuki Y, Okano K. Low prevalence of biliary tract cancer with defective mismatch repair genes in a Japanese hospital-based population. Oncol Lett. 2022;23(1):4. Epub 2021 Nov 4. PMID: 34820003; PMCID: PMC8607234.
Bellizzi AM. An algorithmic immunohistochemical Approach to define tumor type and assign site of Origin. Adv Anat Pathol. 2020;27(3):114–63. https://doi.org/10.1097/PAP.0000000000000256. PMID: 32205473; PMCID: PMC7700753.
Benson AB, D’Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21(7):694–704. https://doi.org/10.6004/jnccn.2023.0035. PMID: 37433432.
Boilève A, Verlingue L, Hollebecque A, Boige V, Ducreux M, Malka D. Rare cancer, rare alteration: the case of NTRK fusions in biliary tract cancers. Expert Opin Investig Drugs. 2021;30(4):401–9. Epub 2021 Mar 24. PMID: 33641556.
Borad MJ, Champion MD, Egan JB, Liang WS, Fonseca R, Bryce AH, McCullough AE, Barrett MT, Hunt K, Patel MD, Young SW, Collins JM, Silva AC, Condjella RM, Block M, McWilliams RR, Lazaridis KN, Klee EW, Bible KC, Harris P, Oliver GR, Bhavsar JD, Nair AA, Middha S, Asmann Y, Kocher JP, Schahl K, Kipp BR, Barr Fritcher EG, Baker A, Aldrich J, Kurdoglu A, Izatt T, Christoforides A, Cherni I, Nasser S, Reiman R, Phillips L, McDonald J, Adkins J, Mastrian SD, Placek P, Watanabe AT, Lobello J, Han H, Von Hoff D, Craig DW, Stewart AK, Carpten JD. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10(2):e1004135. https://doi.org/10.1371/journal.pgen.1004135. PMID: 24550739; PMCID: PMC3923676.
Cardinale V. Classifications and misclassification in cholangiocarcinoma. Liver Int. 2019;39(2):260–262. https://doi.org/10.1111/liv.13998. PMID: 30694026.
Cardinale V, Carpino G, Reid LM, Gaudio E, Alvaro D. Cholangiocarcinoma: a cancer in search of the right classification. Hepatology. 2012;56(4):1585-6; author reply 1586. https://doi.org/10.1002/hep.25705. PMID: 22407765.
Cardinale V, Bragazzi MC, Carpino G, Torrice A, Fraveto A, Gentile R, Pasqualino V, Melandro F, Aliberti C, Bastianelli C, Brunelli R, Berloco PB, Gaudio E, Alvaro D. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2(5):272–80. https://doi.org/10.3978/j.issn.2304-3881.2013.10.02. PMID: 24570958; PMCID: PMC3924690.
cBioPortal for Cancer Genomics. Cancer Types Summary [Internet]. cBioPortal for Cancer Genomics. 2024 [accessed May 21, 2024]. https://www.cbioportal.org/results/cancerTypesSummary
Chakrabarti S, Kamgar M, Mahipal A. Targeted therapies in Advanced biliary tract Cancer: an evolving paradigm. Cancers (Basel). 2020;12(8):2039. https://doi.org/10.3390/cancers12082039. PMID: 32722188; PMCID: PMC7465131.
Chung YS, Jeon Y, Yoo JE, Chung T, Ryu HJ, Kim H, Rhee H, Park YN. Albumin, filamin-A and cytokeratin 19 help distinguish intrahepatic cholangiocarcinoma from extrahepatic adenocarcinoma. Hepatol Int. 2023;17(1):77–85. https://doi.org/10.1007/s12072-022-10428-2. Epub 2022 Oct 17. PMID: 36253584.
Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM. Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;Suppl Suppl 1143–55. https://doi.org/10.1111/liv.14089. PMID: 30843343; PMCID: PMC6563077. 39 Suppl 1.
Czauderna C, Kirstein MM, Tews HC, Vogel A, Marquardt JU. Molecular subtypes and Precision Oncology in Intrahepatic Cholangiocarcinoma. J Clin Med. 2021;10(13):2803. https://doi.org/10.3390/jcm10132803. PMID: 34202401; PMCID: PMC8269161.
da Cunha IW, de Almeida Coudry R, de Macedo MP, et al. A call to action: molecular pathology in Brazil. Surg Exp Pathol. 2021;4:15. https://doi.org/10.1186/s42047-021-00096-1.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9. https://doi.org/10.1056/NEJMoa1714448. PMID: 29466156; PMCID: PMC5857389.
Fontugne J, Augustin J, Pujals A, Compagnon P, Rousseau B, Luciani A, Tournigand C, Cherqui D, Azoulay D, Pawlotsky JM, Calderaro J. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(15):24644–51. https://doi.org/10.18632/oncotarget.15602. PMID: 28445951; PMCID: PMC5421876.
Galdy S, Lamarca A, McNamara MG, Hubner RA, Cella CA, Fazio N, Valle JW. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36(1):141–57. PMID: 27981460; PMCID: PMC5385197.
Goeppert B, Frauenschuh L, Renner M, Roessler S, Stenzinger A, Klauschen F, Warth A, Vogel MN, Mehrabi A, Hafezi M, Boehmer K, von Deimling A, Schirmacher P, Weichert W, Capper D. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol. 2014;27(7):1028–34. https://doi.org/10.1038/modpathol.2013.206. Epub 2013 Dec 6. PMID: 24309328.
Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, Modrek AS, Placantonakis DG. Mutant isocitrate dehydrogenase inhibitors as targeted Cancer therapeutics. Front Oncol. 2019;9:417. https://doi.org/10.3389/fonc.2019.00417. PMID: 31165048; PMCID: PMC6534082.
Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, Abrams TA, Furuse J, Kelley RK, Cassier PA, Klümpen HJ, Chang HM, Chen LT, Tabernero J, Oh DY, Mahipal A, Moehler M, Mitchell EP, Komatsu Y, Masuda K, Ahn D, Epstein RS, Halim AB, Fu Y, Salimi T, Wacheck V, He Y, Liu M, Benhadji KA, Bridgewater JA. FOENIX-CCA2 Study Investigators. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N Engl J Med. 2023;388(3):228–239. https://doi.org/10.1056/NEJMoa2206834. PMID: 36652354.
Guedj N. Pathology of Cholangiocarcinomas. Curr Oncol. 2022;30(1):370–80. https://doi.org/10.3390/curroncol30010030. PMID: 36661679; PMCID: PMC9857472.
Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, Kokudo N, Fukayama M. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am J Surg Pathol. 2016;40(8):1021-30. https://doi.org/10.1097/PAS.0000000000000670. PMID: 27259014.
HUGO Gene Nomenclature Committee. Gene Symbol Report [Internet]. genenames.org. 2024 [accessed May 21, 2024]. https://www.genenames.org/data/gene-symbol-report
Ilyas SI, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. https://doi.org/10.1053/j.gastro.2013.10.013. Epub 2013 Oct 15. PMID: 24140396; PMCID: PMC3862291.
Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, Hainsworth J, Meric-Bernstam F, Swanton C, Sweeney CJ, Friedman CF, Bose R, Spigel DR, Wang Y, Levy J, Schulze K, Cuchelkar V, Patel A, Burris H. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22(9):1290–1300. doi: 10.1016/S1470-2045(21)00336-3. Epub 2021 Jul 30. PMID: 34339623.
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A. KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–1865. doi: 10.1016/S0140-6736(23)00727-4. Epub 2023 Apr 16. Erratum in: Lancet. 2023;402(10406):964. https://doi.org/10.1016/S0140-6736(23)01904-9. Erratum in: Lancet. 2024;403(10432):1140. doi: 10.1016/S0140-6736(24)00545-2. PMID: 37075781.
Khan SA, Tavolari S, Brandi G, Cholangiocarcinoma. Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. doi: 10.1111/liv.14095. Epub 2019 Mar 24. PMID: 30851228.
Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, Jackett L, Lum C, Behren A, Palmer J, Tebbutt NC, Carlino MS, Cebon J. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in patients with Advanced biliary tract cancers: Subgroup Analysis of a phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020;6(9):1405–9. https://doi.org/10.1001/jamaoncol.2020.2814. PMID: 32729929; PMCID: PMC7393585.
Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47(5):1544-56. https://doi.org/10.1002/hep.22238. PMID: 18393293.
Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55(6):1876-88. https://doi.org/10.1002/hep.25595. PMID: 22271564.
Krook MA, Reeser JW, Ernst G, Barker H, Wilberding M, Li G, Chen HZ, Roychowdhury S. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 2021;124(5):880–92. https://doi.org/10.1038/s41416-020-01157-0. Epub 2020 Dec 3. PMID: 33268819; PMCID: PMC7921129.
Lamarca A, Kapacee Z, Breeze M, Bell C, Belcher D, Staiger H, Taylor C, McNamara MG, Hubner RA, Valle JW. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and other biliary tract cancers. J Clin Med. 2020;9(9):2854. https://doi.org/10.3390/jcm9092854. PMID: 32899345; PMCID: PMC7563385.
Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW, Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. Epub 2021 Mar 30. PMID: 33798493; PMCID: PMC8082275.
Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. https://doi.org/10.1016/j.esmoop.2021.100378. Epub 2022 Jan 13. PMID: 35032765; PMCID: PMC8762076.
Li W, Cui Y, Yin F, Peng L, Liu X, Shen Y, Guo Y, Wen S, Shi J, Lei M, Javle MM, Wang K, Jiang D. BRAF mutation in Chinese biliary tract cancer patients. J Clin Oncol. 2020;38:e16678–16678.
Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27(8):1163–73. https://doi.org/10.1038/modpathol.2013.241. Epub 2014 Jan 10. PMID: 24406866.
Longerich T, Stenzinger A, Schirmacher P. Molecular diagnostics of hepatobiliary and pancreatic neoplasias. Virchows Arch. 2024;484(2):263–72. https://doi.org/10.1007/s00428-024-03744-5. Epub 2024 Mar 1. PMID: 38429607; PMCID: PMC10948571.
Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, Kemeny NE, O’Reilly EM, El-Dika I, Jarnagin WR, Harding JJ, D’Angelica MI, Cercek A, Hechtman JF, Solit DB, Schultz N, Hyman DM, Klimstra DS, Saltz LB, Abou-Alfa GK. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–61. https://doi.org/10.1158/1078-0432.CCR-18-0078. Epub 2018 May 30. PMID: 29848569; PMCID: PMC6642361.
Makawita S, Lee S, Kong E, Kwong LN, Abouelfetouh Z, Danner De Armas A, Xiao L, Murugesan K, Danziger N, Pavlick D, Korkut A, Ross JS, Javle M. Comprehensive Immunogenomic profiling of IDH1-/2-Altered Cholangiocarcinoma. JCO Precis Oncol. 2024;8:e2300544. https://doi.org/10.1200/PO.23.00544. PMID: 38547421; PMCID: PMC10994443.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in patients with Noncolorectal high microsatellite Instability/Mismatch repair-deficient Cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. Epub 2019 Nov 4. PMID: 31682550; PMCID: PMC8184060.
Matsumoto K, Ohara T, Fujisawa M, Takaki A, Takahara M, Kato H, Yoshida R, Umeda Y, Yagi T, Matsukawa A, Okada H. Diagnostic utility of the PD-L1 immunostaining in biopsy specimens of patients with biliary tract neoplasms. J Gastrointest Surg. 2022;26(6):1213–23. https://doi.org/10.1007/s11605-021-05197-6. Epub 2022 Feb 8. PMID: 35137343; PMCID: PMC9184404.
Maynard H, Stadler ZK, Berger MF, Solit DB, Ly M, Lowery MA, Mandelker D, Zhang L, Jordan E, El Dika I, Kemel Y, Ladanyi M, Robson ME, O’Reilly EM, Abou-Alfa GK. Germline alterations in patients with biliary tract cancers: a spectrum of significant and previously underappreciated findings. Cancer. 2020;126(9):1995–2002. Epub 2020 Feb 3. PMID: 32012241; PMCID: PMC7584349.
Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):364–377. doi: 10.1016/j.jhep.2019.11.020. PMID: 31954498.
Molenaar RJ, Wilmink JW. IDH1/2 mutations in Cancer Stem cells and their implications for differentiation therapy. J Histochem Cytochem. 2022;70(1):83–97. https://doi.org/10.1369/00221554211062499. PMID: 34967233; PMCID: PMC8721574.
Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabró R, Pinyol R, Torres-Martin M, Bassaganyas L, Moeini A, Peix J, Cabellos L, Maeda M, Villacorta-Martin C, Tabrizian P, Rodriguez-Carunchio L, Castellano G, Sempoux C, Minguez B, Pawlik TM, Labgaa I, Roberts LR, Sole M, Fiel MI, Thung S, Fuster J, Roayaie S, Villanueva A, Schwartz M, Llovet JM. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73(2):315–27. Epub 2020 Mar 12. PMID: 32173382; PMCID: PMC8418904.
Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, Scarpa A, Robson M, Meric-Bernstam F, Wagle N, Stenzinger A, Bonastre J, Bayle A, Michiels S, Bièche I, Rouleau E, Jezdic S, Douillard JY, Reis-Filho JS, Dienstmann R, André F. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–505. Epub 2020 Aug 24. PMID: 32853681.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8. https://doi.org/10.1111/his.13975. Epub 2019 Nov 13. PMID: 31433515; PMCID: PMC7003895.
Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: new concepts. Best Pract Res Clin Gastroenterol. 2015;29(2):277–93. https://doi.org/10.1016/j.bpg.2015.02.006. Epub 2015 Feb 17. PMID: 25966428.
Nakanuma Y, Sudo Y. Biliary tumors with pancreatic counterparts. Semin Diagn Pathol. 2017;34(2):167–75. https://doi.org/10.1053/j.semdp.2016.12.013. Epub 2016 Dec 20. PMID: 28109714.
Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, Uehara T, Yamamoto M, Ariizumi S, Park YN, Choi JH, Yu E. Intrahepatic cholangiocarcinoma with predominant ductal plate malformation pattern: a new subtype. Am J Surg Pathol. 2012;36(11):1629–35. https://doi.org/10.1097/PAS.0b013e31826e0249. PMID: 23073321.
Nakanuma Y, Kakuda Y, Sugino T, Sato Y, Fukumura Y. Pathologies of Precursor lesions of biliary tract carcinoma. Cancers (Basel). 2022;14(21):5358. https://doi.org/10.3390/cancers14215358. PMID: 36358777; PMCID: PMC9654669.
Normanno N, Martinelli E, Melisi D, Pinto C, Rimassa L, Santini D, Scarpa A. Role of molecular genetics in the clinical management of cholangiocarcinoma. ESMO Open. 2022;7(3):100505. https://doi.org/10.1016/j.esmoop.2022.100505. Epub 2022 Jun 10. PMID: 35696744; PMCID: PMC9198375.
Ohba A, Morizane C, Ueno M, Kobayashi S, Kawamoto Y, Komatsu Y, Ikeda M, Sasaki M, Okano N, Furuse J, Hiraoka N, Yoshida H, Kuchiba A, Sadachi R, Nakamura K, Matsui N, Nakamura Y, Okamoto W, Yoshino T, Okusaka T. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future Oncol. 2022;18(19):2351–60. https://doi.org/10.2217/fon-2022-0214. Epub 2022 May 5. PMID: 35510484.
Pant S, Yaeger R, Spira AI, Pelster M, Sabari JK, Hafez N, Barve MA, Velastegui K, Yan X, Der-Torossian H, Bekaii-Saab TS. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with advanced solid tumors harboring a KRASG12C mutation. J Clin Oncol. 2023;41:425082–425082.
Piton N, Borrini F, Bolognese A, Lamy A, Sabourin JC. [KRAS and BRAF mutation detection: is immunohistochemistry a possible alternative to molecular biology in colorectal cancer?]. Gastroenterol Res Pract. 2015;2015:753903. https://doi.org/10.1155/2015/753903. Epub 2015 Apr 23. PMID: 25983749; PMCID: PMC4422999.
Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J, BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X. Epub 2019 Mar 25. Erratum in: Lancet Oncol. 2019;20(5):e242. https://doi.org/10.1016/S1470-2045(19)30216-5. PMID: 30922733.
Rimini M, Fabregat-Franco C, Burgio V, Lonardi S, Niger M, Scartozzi M, Rapposelli IG, Aprile G, Ratti F, Pedica F, Verdaguer H, Rizzato M, Nichetti F, Lai E, Cappetta A, Macarulla T, Fassan M, De Braud F, Pretta A, Simionato F, De Cobelli F, Aldrighetti L, Fornaro L, Cascinu S, Casadei-Gardini A. Molecular profile and its clinical impact of IDH1 mutated versus IDH1 wild type intrahepatic cholangiocarcinoma. Sci Rep. 2022;12(1):18775. https://doi.org/10.1038/s41598-022-22543-z. PMID: 36335135; PMCID: PMC9637171.
Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T, Lucchetti J, Giordano G, Pretta A, Tamburini E, Pirrone C, Rapposelli IG, Diana A, Martinelli E, Garajová I, Simionato F, Schirripa M, Formica V, Vivaldi C, Caliman E, Rizzato MD, Zanuso V, Nichetti F, Angotti L, Landriscina M, Scartozzi M, Ramundo M, Pastorino A, Daniele B, Cornara N, Persano M, Gusmaroli E, Cerantola R, Salani F, Ratti F, Aldrighetti L, Cascinu S, Rimassa L, Antonuzzo L, Casadei-Gardini A. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: An early exploratory analysis of real-world data. Liver Int. 2023;43(8):1803–1812. https://doi.org/10.1111/liv.15641. PMID: 37452505.
Saborowski A, Lehmann U, Vogel A. FGFR inhibitors in cholangiocarcinoma: what’s now and what’s next? Ther Adv Med Oncol. 2020;12:1758835920953293. https://doi.org/10.1177/1758835920953293. PMID: 32983265; PMCID: PMC7498964.
Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513(7516):110-4. https://doi.org/10.1038/nature13441. Epub 2014 Jul 2. Erratum in: Nature. 2015;528(7580):152. doi: 10.1038/nature16136. PMID: 25043045; PMCID: PMC4499230.
Sangkhamanon S, Jongpairat P, Sookprasert A, Wirasorn K, Titapun A, Pugkhem A, Ungareevittaya P, Chindaprasirt J. Programmed death-ligand 1 (PD-L1) expression Associated with a high Neutrophil/Lymphocyte ratio in Cholangiocarcinoma. Asian Pac J Cancer Prev. 2017;18(6):1671–4. PMID: 28670887; PMCID: PMC6373788.
Santos FAC, Fernandes FCGM, Santos EGO, Medeiros NBM, Souza DLB, Barbosa IR. Mortality due to malignant neoplasms of the liver and bile ducts in Brazil: Trends and projections until 2030. Revista Brasileira De Cancerologia. 2019;65(4):e–01435.
Sempoux C, Fan C, Singh P, Obeidat K, Roayaie S, Schwartz M, Fiel MI, Thung SN. Cholangiolocellular carcinoma: an innocent-looking malignant liver tumor mimicking ductular reaction. Semin Liver Dis. 2011;31(1):104–10. https://doi.org/10.1055/s-0031-1272838. Epub 2011 Feb 22. PMID: 21344355.
Shen L, Xu JM, Feng FY, Jiao SC, Wang LW, Li J, Guan ZZ, Qin SK, Wang JJ, Yu SY, Wang YJ, Jin YN, Tao M, Zheng LZ, Pan LX. [Trastuzumab in combination with chemotherapy versus chemotherapy alone for first-line treatment of HER2-positive advanced gastric or gastroesophageal junction cancer: a Phase III, multi-center, randomized controlled trial, Chinese subreport]. Zhonghua Zhong Liu Za Zhi. 2013;35(4):295–300. Chinese. https://doi.org/10.3760/cma.j.issn.0253-3766.2013.04.012. PMID: 23985260.
Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics. 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. PMID: 36633525.
Sigel CS, Drill E, Zhou Y, Basturk O, Askan G, Pak LM, Vakiani E, Wang T, Boerner T, Do RKG, Simpson AL, Jarnagin W, Klimstra DS. Intrahepatic Cholangiocarcinomas have histologically and immunophenotypically distinct small and large Duct patterns. Am J Surg Pathol. 2018;42(10):1334–45. https://doi.org/10.1097/PAS.0000000000001118. PMID: 30001234; PMCID: PMC6657522.
Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, Takeda M, Ohe Y, Khan S, Ohashi K, Soldatenkova V, Szymczak S, Sullivan L, Wright J, Drilon A. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23(10):1261–1273. doi: 10.1016/S1470-2045(22)00541-1. Epub 2022 Sep 12. PMID: 36108661.
Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, Wen PY, Dietrich S, de Jonge MJA, Blay JY, Italiano A, Yonemori K, Cho DC, de Vos FYFL, Moreau P, Fernandez EE, Schellens JHM, Zielinski CC, Redhu S, Boran A, Passos VQ, Ilankumaran P, Bang YJ. Dabrafenib plus Trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. 2023;29(5):1103–12. https://doi.org/10.1038/s41591-023-02321-8. Epub 2023 Apr 14. PMID: 37059834; PMCID: PMC10202803.
Tomczak A, Springfeld C, Dill MT, Chang DH, Kazdal D, Wagner U, Mehrabi A, Brockschmidt A, Luedde T, Naumann P, Stenzinger A, Schirmacher P, Longerich T. Precision oncology for intrahepatic cholangiocarcinoma in clinical practice. Br J Cancer. 2022;127(9):1701–8. https://doi.org/10.1038/s41416-022-01932-1. Epub 2022 Aug 19. PMID: 35986087; PMCID: PMC9390961.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-81. https://doi.org/10.1056/NEJMoa0908721. PMID: 20375404.
Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in western countries. J Hepatol. 2022;77(6):1690–8. https://doi.org/10.1016/j.jhep.2022.07.022. Epub 2022 Aug 14. PMID: 35977611.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human epidermal growth factor receptor 2 testing in breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142(11):1364–82. https://doi.org/10.5858/arpa.2018-0902-SA. Epub 2018 May 30. PMID: 29846104.
Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34(1):157–64. https://doi.org/10.1007/s10555-015-9552-6. PMID: 25712293; PMCID: PMC4368842.
Zeng FL, Chen JF. Application of Immune checkpoint inhibitors in the treatment of Cholangiocarcinoma. Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211039952. https://doi.org/10.1177/15330338211039952. PMID: 34528830; PMCID: PMC8450549.
Zhang D, Liao X, Pan -TRK, Immunohistochemistry. NTRK Gene fusions in primary carcinomas of the liver. Appl Immunohistochem Mol Morphol. 2022;30(6):435–40. https://doi.org/10.1097/PAI.0000000000001032. Epub 2022 May 10. PMID: 35587529.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Lidiane Vieira Marins wrote de manuscript and designed the diagrams. Cecília Souza Freire, Camila Motta Venchiarutti Moniz, and Antonio Hugo José Fróes Marques Campos wrote the manuscript. The authorsreviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
We, Lidiane Vieira Marins, Cecília Souza Freire, Camila Motta Venchiarutti Moniz and Antonio Hugo José Fróes Marques Campos authors of the manuscript entitled “The Biliary Tree Musketeers: Cholangiocarcinoma – One Name for All, but at Least Three Different Diseases with Distinct Targets”, declare that we have no conflicts of interest of financial, commercial, political, academic, or personal nature.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marins, L.V., Moniz, C.M.V., Freire, C.S. et al. The biliary tree musketeers: cholangiocarcinoma – one name for all, but at least three different diseases with distinct targets. Surg Exp Pathol 7, 16 (2024). https://doi.org/10.1186/s42047-024-00160-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-024-00160-6