Abstract
Background
Hearing loss and cognitive impairment are postoperative complications which need more awareness by anaesthesiologists. We set out to investigate whether sevoflurane or propofol would have a negative impact on auditory function, attention, or auditory memory. This is a prospective randomized controlled study which was conducted on patients who were candidates for elective laparoscopic cholecystectomy under general anesthesia with either the sevoflurane or the propofol. All included participants were subjected to cognitive and auditory evaluation preoperative and 1 week after the operation. Cognitive assessment included: Paired Associate Learning test (PALT) and Paced Auditory Serial Addition Test (PASAT). Audiological assessment was done by measuring the auditory brainstem response (ABR).
Results
There was no statistically significant difference between both groups in either age (p value = 0.537) or sex (p value = 0.175). In the propofol group, the postoperative values of LT ABR-I and III were significantly higher than the preoperative ones (p value < 0.001, 0.003), all the postoperative RT ABR waves were significantly higher than the preoperative ones (P < 0.05). In the sevoflurane group, the postoperative values for LTABR-I, III, III–V were significantly higher than the preoperative ones with p value (0.012, 0.008 and 0.009) and the postoperative values for RTABR-III, V, I–III, and III–V were significantly higher than preoperative values (P = 0.041, 0.029, 0.005 and < 0.001). There were no statistically significant differences between the propofol and sevoflurane groups in all waves of ABR on both sides (P > 0.05). There was a significant worsening between pre- and postoperative PASAT scores in the propofol and sevoflurane groups, respectively, with p value (< 0.001) with no statistically significant difference between both groups (p value = 0.906). In addition, there was a significant worsening between pre- and postoperative PALT scores in the propofol group only (p value = 0.01) with a statistically significant difference between both groups (p value = 0.038).
Conclusions
There was a statistically significant postoperative impairment in auditory function and attention following both the propofol and sevoflurane anesthesia with no significant difference between the two drugs. Whereas, the auditory memory was significantly impaired following the propofol only.
Similar content being viewed by others
Background
Hearing loss is one of the postoperative complications, which happens more commonly than most anaesthesiologists expect. Perioperative hearing loss may be unilateral or bilateral, conductive or sensorineural, and temporary or permanent [1]. Several reports suggested that some anesthetics such as propofol can cause instability of hemodynamics due to hypotension which may occur during the operation with transient vertebrobasilar hypoperfusion which reduces blood supply to the cochlea producing cochlear injury and hearing loss [2]. Traumatic, mechanical causes, and cerebrospinal fluid (CSF) pressure fluctuations were also reported as contributing factors for perioperative hearing loss [1].
Perioperative hearing loss may be subclinical and may pass unobserved unless audiometry is performed [1]. The (ABR) can evaluate the behavioral audiogram shape and so, it is a very useful method for the assessment of hearing sensitivity. It involves a series of voltage peaks which occur within the first few milliseconds (ms) after the auditory stimulus and represent the progressive propagation of neural activity through the ascending auditory pathway [3]. The use of the propofol was reported to cause a significant increase in the latencies of ABR waves III and V and the interpeak intervals I–V and III–V [4].
Postoperative cognitive dysfunction (POCD) is a well-known complication of anesthetic drugs [5]. Subtle deficiencies in a variety of neurocognitive functions, including attention, auditory memory, executive function, visuospatial abstraction, and psychomotor speed are typical symptoms. POCD can be moderate and only be identified by psychometric testing, but it can occasionally have substantial negative effects on functional status and quality of life [6].
In an international multicenter study [International Study of POCD (ISPOCD1)], older persons with major non-cardiac surgery had an incidence of POCD of 25.8% at 1 week, 9.9% at 3 months and 1% at 1–2 years [7, 8]. While post-operative cognitive dysfunction was reported to be transient and self-limiting, strong evidence indicated that elderly patients that are three times more likely to suffer from permanent cognitive impairment or dementia [9].
The role of neuroinflammation and neurodegeneration in the pathogenesis of POCD has been implicated by a substantial and expanding body of evidence, but the findings were not entirely conclusive due to the heterogeneity of the animal models and human populations studied, as well as the variability in the clinical assessment tools [10, 11].
General anesthetics such as propofol and sevoflurane are frequently utilized in clinical settings.The incidence of POCD was reported to be significantly higher in patients undergoing major surgery under inhalational anesthesia with sevoflurane in comparison with those maintained on intravenous propofol [12, 13]. The detrimental effects of both sevoflurane and the propofol on neuronal cell integrity were addressed in multiple animal studies. Exposure of newborn rat’s brain to sevoflurane has been shown to induce apoptotic neurodegeneration [14]. In addition, direct experimental evidence indicated that the anesthetic dose of the propofol-induced neuronal cell death in the cortex and thalamus of the developing rat brain [15].
We aimed to evaluate the probable harmful effect of the propofol versus sevoflurane on attention, auditory memory and function in patients undergoing laparoscopic cholecystectomy before and 1 week postoperative.
Methods
Patients who were candidates for elective laparoscopic cholecystectomy under general anesthesia participated in this prospective randomized controlled experiment. Participants were randomly assigned to either the sevoflurane or propofol groups. We used a closed opaque envelope technique for randomization. Between June 2021 and December 2021, the patients who were included in the study were recruited from Beni-Suef university hospital, Beni-Suef University. All of the patients that were involved provided written informed consent. The Helsinki Declaration of Biomedical Ethics, as updated, was followed in the study's design. The research ethics committee of the faculty of medicine, Beni-Suef University approved this study with (approval number: FMBSUREC/12062018). On May 5, 2021, the study's protocol was entered on ClinicalTrials.gov. The identification number is NCT04874545.
According to the American Society of anesthesiologists physical status classification system (ASA), we included in our study ASA I–II patients who were candidates for elective laparoscopic cholecystectomy from both genders, aged from 20 to 70 years (ASA-I is defined as normal healthy patients and ASA-II is defined as patients with mild systemic disease). These patients were excluded from our research: patients with conductive or sensorineural hearing loss, patients using drugs are known to be ototoxic or neurotoxic, patients who have experienced trauma or infection to ears, patients exposed to intraoperative major hemodynamic fluctuations, patients with severe bleeding or postoperative shock, patients who are allergic to any drug used in the study, patients with neurodegenerative disorders and patients with medical or metabolic disorders which may affect cognition. Illiterate patients and pregnant females were also excluded from the study. A flow diagram is demonstrated in Fig. 1
Patients were submitted to clinical assessment comprising the patient’s hemodynamics; heart rate, mean arterial blood pressure, SPO2, and end-tidal CO2. Routine preoperative biochemical and hematological testing, along with electrocardiograms were performed for all the included patients. All included participants were subjected to cognitive and auditory evaluation preoperative and 1 week after the operation.
Cognitive assessment: included: Paired Associate Learning test (PALT) and Paced Auditory Serial Addition Test (PASAT).
(PALT) was used to evaluate auditory–verbal memory using the idea of semantic cueing. Ten-related pairs were uttered aloud by the inspector in front of the applicant. Six of these pairs are semantically related and four are not, with these pairs containing 6 well-matched semantically related pairs. The candidate was given the first word in each pair after 1 min, and was then asked to recall the second word. Three times were given the test. Correctly paired incompatible pairs received a score of 1, while correctly paired compatible pairs received a score of 0.5. The overall score was between 0 and 21 [16].
(PASAT) was used to evaluate attention and auditory working memory. On an audiotape, a series of 61 single-digit, digits were stated one every 3 s. Each number had to be added to the one before it, without providing a running total, and the candidate was to report the total verbally. The total score, which varies from 0 to 60, is the accumulation of all right responses [17].
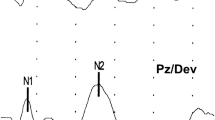
Audiological assessment: Interacoustic Eclipse's "EP25" was used to measure brainstem auditory evoked potentials (BAEPs). The ground electrode was placed on the lower mid-frontal region, the active electrode was placed on the scalp at the vertex (Fz position of the 10–20 International System of electroencephalogram (EEG) electrode placement), and the reference electrodes were placed on the right (A2) and left (Al) mastoids (Fpz position). On skin that had been rubbed raw with a skin-prepping gel, Ag/AgCl electrodes loaded with conductive paste were affixed. There were inter-electrode impedances of less than 2 k and electrode impedances of less than 5 k. The candidate was in a calm mood. Using TDH39 headphones, a click was obtained. At an intensity of 80 dB HL (hearing loss in decibels), rarefaction polarity click stimuli were provided at a rate of 21.1 stimuli per second and potentials on average to 1200 clicks. To guarantee that the waveforms could be replicated, two recordings were collected. With the use of BAEPs, the latencies of waves I, III, and V as well as interpeak latencies I–V, I–III, and III–V (IPLs) were estimated.
Anesthetic technique: A 18 G intravenous cannula was placed when the patient entered the operation room, and intravenous (IV) crystalloid fluids were started. For the purpose of taking preoperative readings, the monitor was linked to the patients. Fentanyl 2 mg/kg, propofol 1.5–2.5 mg/kg, and atracurium 0.5 mg/kg for muscular relaxation was injected to induce anesthesia. For performing laryngoscopy and endotracheal intubation, an oral cuffed tube lubricated with 2% lidocaine jelly was used. Depending on the situation, sevoflurane or propofol was used to maintain anesthesia. The propofol total intravenous anesthesia (TIVA) 6–12 mg/kg/h and a combination of oxygen and air were used to maintain anesthesia in the propofol group (70:30). Sevoflurane 1.5–2% and a combination of oxygen and air were used to maintain anesthesia in the sevoflurane group (70:30). Propofol and sevoflurane concentrations were altered according to patient’s vital signs.
Both groups' ventilation was artificially regulated to maintain an end-tidal partial pressure of carbon dioxide between 35 and 45 mmHg. Neostigmine 0.04 mg/kg and atropine 0.02 mg/kg were administered intravenously to reverse neuromuscular blockade following surgery. When the patient reacts to commands, the trachea has been extubated. The patients were then monitored and supplied 3–4 L/min of oxygen through a face mask.
The primary endpoint is the occurrence of a significant decline in auditory function, attention, and auditory memory in both the propofol and sevoflurane groups 1 week following surgery.
The secondary endpoint is the occurrence of significant differences between the propofol and sevoflurane groups in the postoperative decline in auditory function, attention, and auditory memory.
Sampling: Based on the outcomes of a pilot study we conducted before beginning our study, we computed the sample size. G*Power version 3.1.9.2 software was used to calculate the sample size. Type I error (α) was 5%, the effect size was 0.65, non-centrality parameter λ was 2.842, critical t was 1.992, and df was 74.394. To achieve a statistical power (1-) 80%, each group needed a total of forty participants.
Statistical analysis: The data were examined using IBM SPSS (Statistical Package of Social Science) version 25 ‘IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA). Numbers and percentages were used to express categorical variables. The median and interquartile ranges were used to express quantitative factors (IQR). Quantitative data were compared between groups using the Mann–Whitney test, while categorical data were compared using the Chi-squared test. Quantitative data from both the pre- and post-operative periods in each group were compared using the Wilcoxon test. Quantitative data from the two groups' pre- and post-operative periods were compared using a mixed ANOVA test. P values lower than 0.05 were regarded as statistically significant. There were two tails on each exam.
Results
This prospective randomized controlled trial was conducted on 80 patients who were candidates for elective laparoscopic cholecystectomy under general anesthesia; 40 patients received the propofol (propofol group), and 40 patients received the sevoflurane (sevoflurane group).
Regarding the demographics of the patients, There was no statistically significant difference between both groups in either age (p value = 0.537) or sex (p value = 0.175) (Table 1). As regards the intraoperative clinical data of the patients, There was no statistically significant difference between both groups regarding the median value of B/P (p value = 0.558), HR (p value = 0.456), SPO2 (p value = 0.305), or ETCO2 (p value = 0.559) (Table 2).
There was a significant difference between pre- and postoperative PASAT scores in the propofol and sevoflurane groups, respectively, with p value (< 0.001) with no statistically significant difference between both groups (p value = 0.906) (Table 3).
There was a significant difference between pre- and postoperative PALT scores in the propofol group (p value = 0.01), but there was no significant difference between pre- and postoperative PALT scores in the sevoflurane group (p value = 0.488); moreover, there was a statistically significant difference between both groups (p value = 0.038) (Table 3).
In the propofol group, there was a statistically significant difference between pre- and postoperative RT ABR I, III, V, I–III, I–V, III–V with p value (0.011, 0.001, 0.004, 0.046, 0.007and 0.019), respectively. In the sevoflurane group, there was a statistically significant difference between pre- and postoperative RT ABR III, V, I–III, III–V with p value (0.041, 0.029, 0.005 and < 0.001), respectively (Table 4).
In the propofol group, there was a statistically significant difference between pre- and postoperative LT ABR I, III with p value (< 0.001, 0.003), respectively. In the sevoflurane group, there was a statistically significant difference between pre- and postoperative LT ABR I, III, III–V with p value (0.012, 0.008 and 0.009), respectively. Finally, there were no statistically significant differences between the propofol and sevoflurane groups in all waves of ABR in both sides p value > 0.05).
Discussion
This study was conducted to detect the possible deleterious effect of propofol versus sevoflurane on auditory function, attention and auditory memory. A total of 80 patients were enrolled in this study (n = 40 in each group). Our results revealed that there was a significant delay in the latency of most of the waves of postoperative ABR than preoperative assessment for both the propofol and sevoflurane groups separately with no significant difference between the two drugs regarding ABR parameters.
Similar findings were obtained by Mun and Cho who reported that propofol can cause transient vertebrobasilar hypoperfusion-producing cochlear injury resulting in hearing loss [2]. Propofol increases cerebral vascular resistance by 50% and decreases systolic blood pressure by 20–30% [18]. It was also reported to change the evoked otoacoustic emissions (OAE) thresholds [19]. In contrast, Gungor and colleagues reported that transient evoked otoacoustic emissions (TEOAE) were not significantly affected after propofol [20].
A drop in ABR wave I amplitude, a marker for ribbon-synapse functionality, was found in mice whose hearing had been reportedly impaired by sevoflurane exposure. Sevoflurane exposure did not appear to harm hair cells, but it did produce a drop in cochlear ribbon synapses in children at postnatal day 15 and a partial recovery at postnatal day 30. This was explained by the cochlear explants' higher mitochondrial reactive oxygen species stress and decreased autophagy [21]. However, sevoflurane, described by other authors to be safe in terms of ototoxicity, (TEOAE) were not significantly changed after sevoflurane, but propofol, while having a similar effect on blood pressure to sevoflurane, has less of a protective effect on inner ear microcirculation. They also noticed that sevoflurane has a hypotensive effect without changing cochlear blood flow [22]. The decline of hearing function could also be explained by that anesthetic agents can cause the dysfunction of the eustachian tube cilia and resultant hearing dysfuction. Previous studies reported that ear volume and ear pressure to increased on tympanography after the induction of general anaesthesia [1].
Changes in middle ear pressure (MEP) could affect auditory function measurements besides agents’ direct pharmacological effects on cochlear micromechanics and hemodynamics. Both the tympanic membrane and the conducting tissues can be damaged by abrupt changes in MEP. The round window may burst as a result of excessive MEP, seriously impairing hearing. Furthermore, middle ear injuries were caused by rapid transmission of high pressure through the Eustachian tube during intense mask ventilation [23].
Sevoflurane was reported increased MEP. Therefore, the propofol may be used in middle ear operations more safely than sevoflurane [24].
In addition, according to a number of reports, the cause of sensorineural hearing loss (SNHL) following general anesthesia during non bypass surgery is the microembolic phenomenon. Hearing impairment by microembolisms, which can cause ischemia of the stria vascularis and hair cells, has often been linked to unilateral hearing loss. In addition, cholecystectomy may liberate microemboli that obstruct the cochlear division of the internal auditory artery and cause hearing loss [25] and this may support our results that there was no significant difference in postoperative hearing impairment in either the propofol or sevoflurane.
Furthermore, medication used during anesthesia may either worsen the ototoxic effects of drugs administered prior to anesthesia or induce ototoxicity themselves. [26]
And so our findings in postoperative hearing impairment after anesthesia with either the propofol or sevoflurane can be explained by vascular pathology, changes in MEP, embolism, or ototoxic drugs.
The anesthesiologist can actively participate in preventing or limiting drug-induced hearing deficits by being aware of patients who are at high risk for developing ototoxicity, such as those with impaired renal function, those with preexisting ototoxic drug serum levels, those with preexisting SNHL, and those who could receive a synergistic combination of ototoxic drugs [25].
Early detection of ototoxicity can be important to the anesthesiologist. Audiometry is the best tool for early detection of drug-induced ototoxic effects on the cochlea. The prognosis for recovery depends on a number of variables, including patient age, the presence of vertigo at onset, degree of hearing loss, audiometric configuration, and the interval between the loss's occurrence and treatment [27]. Within 7 days of the onset of symptoms, prompt therapy is linked to a positive prognosis and hearing improvement in 49–79% of patients. The greatest improvement in hearing usually occurs during the first 2 weeks with little benefit after 4–6 weeks [25].
The choice of general anesthetic medications and their relationship to the occurrence of POCD is still debatable issues. In the present study, there was a statistically significant postoperative impairment in attention (assessed by PASAT) following both the propofol and sevoflurane anesthesia with no significant difference between the two drugs. Whereas, the auditory memory (assessed by PALT) was significantly impaired following the propofol but not sevoflurane anesthesia.
Sun and colleagues observed that sevoflurane has a shorter half-life and is rapidly excreted, whereas propofol has a more noticeable negative effect on postoperative cognitive function. Laboratory research also demonstrated that pretreatment with sevoflurane at high concentrations could successfully protect against focal cerebral ischemia in rats, lowering neurological impairment scores, cerebral infarction volume, and cerebral edema regions, and that sevoflurane may have a more subtle impact on elderly patients' cognitive function due to the up-regulation of the expression levels of NR1 and NR2 subunits of hippocampal N-methyl-d-aspartate receptors [28]. Schoen and colleagues also discovered that, despite having similar pre- and intraoperative risk profiles, patients who underwent sevoflurane-based anesthetic performed better in four independent cognitive assessments than those who underwent propofol-based anesthesia. This finding implies that sevoflurane has neuroprotective effects [29]. In addition, a prior study found that sevoflurane had no significant impact on cerebral blood flow as measured by transcranial Doppler sonography when compared to propofol, indicates the safety of sevoflurane [30]. These findings reflect our results that, in contrast to the propofol group, the sevoflurane group did not experience postoperative impairment in auditory–verbal memory as measured by PALT. Nevertheless, sevoflurane was found to have a stronger negative effect on cognitive function than the propofol, according to Tang and colleagues who explained that in the absence of a particular ongoing inflammatory process (which acts via lipopolysaccharide [LPS] activation), propofol and sevoflurane have both been shown to only slightly alter the production of cytokines in resting microglia. However, sevoflurane allowed the LPS response to continue unabatedly, while propofol demonstrated dramatically diminished anti-inflammatory effects once these microglia were activated by LPS stimulation. Given that sevoflurane anesthesia can worsen the degree of impairment in patients with mild cognitive impairment (MCI), this finding suggests that propofol may be a preferable general anesthetic in terms of minimizing the neuroinflammatory response [31].
In animal experiments, inhaled anesthetics were found to increase amyloid b oligomerization and cytotoxicity in phenochromocytoma cells. Propofol, on the other hand, significantly increased amyloid b oligomerization at very high doses and may mitigate sevoflurane-induced cytotoxicity. As a result, it has been claimed that sevoflurane may increase the neuropathogenesis of Alzheimer's disease. Furthermore, following 2 years of follow-up, sevoflurane may increase the advancement of amnestic MCI in older patients [32]. High concentrations of sevoflurane were found to induce serious cognitive dysfunction through changing the integrity of the blood–brain barrier (BBB) and increasing an intracerebral oxidative stress response [33]. In addition, a previous study discovered that the incidence of POCD was considerably reduced in the propofol group over the sevoflurane group at D1 and D3. Plasma concentrations of S-100β and Aβ1-40 were considerably higher in the propofol group at T1(after extubation) and T2(1 h), but not at T3 (24 h) [34]. Isoflurane and sevoflurane have been shown to increase Aβ oligomerization, raise Aβ levels, and amplify Aβ-induced cytotoxicity. Propofol, on the other hand, has a lower effect on Aβ and can even reduce isoflurane-induced caspase-3 activation [35].
Guo and colleagues discovered in another trial that there was no appreciable difference in the incidence of POCD in elderly patients undergoing sevoflurane- or propofol-based general anesthesia which can be explained by the fact that prolonged sevoflurane and propofol exposure causes neurodegeneration and apoptosis in the developing rats' brains [36, 37]. In addition, Erdem and colleagues noted that both sevoflurane and propofol-based anesthesia had high levels of the neurodegenerative markers S100 and neuron-specific enolase (NSE), which indicate severe neuronal damage [38].
Sevoflurane and propofol were also known to stimulate GABAA receptors, raise intracellular calcium, and cause turbulences in mitochondrial membrane potential, which result in neuronal dysfunction and death. These effects also increase the production of reactive oxygen species (ROS), which worsens ischemic brain damage. Therefore, avoidance of excessive hyperoxic ventilation or administration of antioxidants, while under anesthesia may protect against anesthetic neurotoxicity [39]
In a different study, the incidence of POCD was evaluated in patients who received either sevoflurane or propofol for maintaining anaesthesia during total hip replacement surgery after receiving a spinal anaesthia. A neuropsychological test battery was used to evaluate POCD postoperatively at day 7, 3 months, and 12 months. They discovered no statistically significant difference between sevoflurane and propofol in terms of the occurrence of POCD at any timepoint [40].
It must be noted that Terri and colleagues showed that POCD can develop in adult patients of any age following non-cardiac surgery. One thousand sixty-four patients, aged 18 or older and classified as young (18–39), middle-aged (40–59), or elderly (60 years or older), participated in their study. The findings showed that POCD was present at hospital discharge in 117 (36.6%) young, 112 (30.4%) middle-aged, and 138 (41.4%) elderly patients, but being older was a risk factor for POCD 3 months following surgery independently of other factors [41].
One of the most significant factors that affect POCD is advanced age, since it is frequently accompanied by alterations in (BBB) permeability brought on by chronic inflammatory processes, which are a part of the pathology of POCD. Increased (BBB) permeability adds to the load of chemicals, infections, and inflammation entering the brain, which speeds up neurodegenerative processes and reduces brain reserve, making the brain more vulnerable to POCD [42]. In addition, as people age, their ability to eliminate medicines from the body declines, which increases the risk of neurodegenerative diseases when such chemicals enter the brain [43].
To avoid the advanced age contributor to POCD, we kept the median age of our trial participants at 36 for the sevoflurane group and 37 for the propofol group at 37 years.
Conclusions
This study was conducted to detect the possible harmful effect of the propofol versus sevoflurane on auditory and cognitive functions (attention and auditory memory) which were assessed for the included patients before and 1 week after the operation.
There was a statistically significant postoperative impairment in auditory function and attention following both the propofol and sevoflurane anesthesia with no significant difference between the two drugs. Whereas, auditory memory was significantly impaired following the propofol only. This study is regarded as the first to explore the effect of propofol versus sevoflurane anesthesia on attention, auditory memory, and auditory function. This study, however, had certain drawbacks. First, there is the brief follow-up time. Second, we only assessed attention and auditory memory and did not test the other cognitive domains. Third, we did not investigate the effect of anesthesia intensity or duration on ABR alterations and cognitive functions. The anesthesiologist must have a better awareness of the incidence, etiology, and prognosis of perioperative hearing loss and POCD.
Availability of data and materials
The data sets generated and/or analyzed during the current study are not publicly available due to the current Cairo University regulations and Egyptian legislation but are available from the corresponding author on reasonable request and after institutional approval.
Abbreviations
- ABR:
-
Auditory brain stem response
- TIVA:
-
Total intravenous anesthesia
- POCD:
-
Postoperative cognitive dysfunction
- PASAT:
-
Paced Auditory Serial Addition Test
- PALT:
-
Paired Associate Learning Test
- ms:
-
Milliseconds
- BAEPs:
-
Brainstem auditory evoked potentials
- ASA:
-
American society of anesthesiologists physical status classification system
- EEG:
-
Electroencephalogram
- dB HL:
-
Hearing loss in decibels
- SPSS:
-
Statistical Package of Social Science
- IQR:
-
Inter quartile range
- SNHL:
-
Sensorineural hearing loss
- CSF:
-
Cerebrospinal fluid
- TEOAE:
-
Transient evoked otoacoustic emissions
- MEP:
-
Middle ear pressure
- ROS:
-
Reactive oxygen species
- GABAA:
-
Gamma-aminobutyric acid
- IV:
-
Intravenous
- MCI:
-
Mild cognitive impairment
- BBB:
-
Blood–brain barrier
References
Thompson CS, Gohil R, Montague ML. Sudden onset hearing loss following intra-abdominal surgery: an unusual association. BMJ Case Rep. 2020;13(9): e234793. https://doi.org/10.1136/bcr-2020-234793.
Mun S, Cho S. Sudden sensorineural hearing loss after laparoscopic cholecystectomy under general anesthesia. J Med Cases. 2013;4(11):742–5.
Cui J, Zhu B, Fang G, Smith E, Brauth SE, Tang Y. Effect of the level of anesthesia on the auditory brainstem response in the Emei Music Frog (Babina daunchina). PLoS ONE. 2017;12(1): e0169449. https://doi.org/10.1371/journal.pone.0169449.
Abulebda K, Patel VJ, Ahmed SS, Tori AJ, Lutfi R, Abu-Sultaneh S. Comparison between chloral hydrate and propofol-ketamine as sedation regimens for pediatric auditory brainstem response testing. Braz J Otorhinolaryngol. 2019;85(1):32–6. https://doi.org/10.1016/j.bjorl.2017.10.003.
Brodier EA, Cibelli M. Postoperative cognitive dysfunction in clinical practice. BJA Educ. 2021;21(2):75–82. https://doi.org/10.1016/j.bjae.2020.10.004.
Kahl U, Callsen S, Beck S, Pinnschmidt H, von Breunig F, Haese A, et al. Health-related quality of life and self-reported cognitive function in patients with delayed neurocognitive recovery after radical prostatectomy: a prospective follow-up study. Health Qual Life Outcomes. 2021;19(1):64. https://doi.org/10.1186/s12955-021-01705-z.
Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction (Erratum in: Lancet 1998;351(9117):1742). Lancet. 1998;351(9106):857–61. https://doi.org/10.1016/s0140-6736(97)07382-0.
Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, et al. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. ISPOCD investigators. International study of post-operative cognitive dysfunction. Acta Anaesthesiol Scand. 2000;44(10):1246–51. https://doi.org/10.1034/j.1399-6576.2000.441010.x.
Sprung J, Roberts RO, Weingarten TN, Nunes CA, Knopman DS, Petersen RC, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316–23.
Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–56. https://doi.org/10.1016/j.ebiom.2018.10.021.
Hussein M, Fathy W, Nabil T, Abd ER. Postoperative cognitive dysfunction and the possible underlying neurodegenerative effect of anaesthesia. Int J Neurosci. 2019;129(8):729–37. https://doi.org/10.1080/00207454.2018.1561451.
Zhang Y, Shan GJ, Zhang YX, Cao SJ, Zhu SN, Li HJ, et al. First Study of Perioperative Organ Protection (SPOP1) investigators. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br J Anaesth. 2018;121(3):595–604. https://doi.org/10.1016/j.bja.2018.05.059.
Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;23(15):154. https://doi.org/10.1186/s12871-015-0130-9.
Yang B, Liang G, Khojasteh S, Wu Z, Yang W, Joseph D, et al. Comparison of neurodegeneration and cognitive impairment in neonatal mice exposed to propofol or isoflurane. PLoS ONE. 2014;9(6): e99171. https://doi.org/10.1371/journal.pone.0099171.
Milanović D, Pešić V, Popić J, Tanić N, Kanazir S, Jevtović TV, et al. Propofol anesthesia induces proapoptotic tumor necrosis factor-α and pro-nerve growth factor signaling and prosurvival Akt and XIAP expression in neonatal rat brain. J Neurosci Res. 2014;92(10):1362–73. https://doi.org/10.1002/jnr.23409.
Scorpio KA, Islam R, Kim SM, Bind R, Borod JC, Bender HA. Paired-Associate Learning. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology, Springer, Cham; 2018. https://doi.org/10.1007/978-3-319-57111-9_1137.
Correia S. Paced auditory serial attention test. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. Springer, New York, NY; 2011. https://doi.org/10.1007/978-0-387-79948-3_1319.
Costa ND, Pereira DA, Azevedo P, Duarte D. Sudden unilateral hearing loss after non-otologic surgery. WebmedCentral Otorhinolaryngol. 2016;7(12): WMCC005247.
Wang WL, Bai YR, Zheng Q, Zheng S, Liu XY, Ni GJ. Otoacoustic emission and its application in anesthesia. Eur Rev Med Pharmacol Sci. 2022;26(15):5426–35. https://doi.org/10.26355/eurrev_202208_29411.
Gungor G, Sutas BP, Yener HM, Yilmaz YZ, Sarı E, Atas A, et al. Comparison of anesthetic agents on otoacoustic emissions in children: propofol vs ketamine. Paediatr Anaesth. 2016;26(7):752–8. https://doi.org/10.1111/pan.12936.
Yuan X, Liu H, Li Y, Li W, Yu H, Shen X. Ribbon synapses and hearing impairment in mice after in utero sevoflurane exposure. Drug Des Devel Ther. 2020;14:2685–93. https://doi.org/10.2147/DDDT.S253031.
Aladag I, Kaya Z, Gurbuzler L, Eyibilen A, Songu M, Ates D, et al. The effects of hypotensive anaesthesia on otoacoustic emissions: a prospective, randomized, double-blind study with objective outcome measures. Eur Arch Otorhinolaryngol. 2016;273(1):73–9. https://doi.org/10.1007/s00405-014-3488-2.
Sprung J, Bourke DL, Contreras MG, Warner ME, Findlay J. Perioperative hearing impairment. Anesthesiology. 2003;98(1):241–57.
Dogan M, Duger C, Uysal IO, Kol IO, Yuce S. Middle ear pressure changes with sevoflurane and propofol-remifentanil. B-ENT. 2015;11(3):219–22.
Mun S, Cho S. Sudden sensorineural hearing loss after laparoscopic cholecystectomy under general anesthesia. J Med Cases. 2013;4(11):742–5.
Szczepek AJ, Stankovic KM. Editorial: emerging ototoxic medications and their role in cochlear and vestibular disorders. Front Neurol. 2021;12: 773714. https://doi.org/10.3389/fneur.2021.773714.
Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3 Suppl):S1-35.
Sun H, Zhang G, Ai B, Zhang H, Kong X, Lee WT, et al. A systematic review: comparative analysis of the effects of propofol and sevoflurane on postoperative cognitive function in elderly patients with lung cancer. BMC Cancer. 2019;19(1):1248. https://doi.org/10.1186/s12885-019-6426-2.
Schoen J, Husemann L, Tiemeyer C, Lueloh A, Sedemund AB, Berger KU, et al. Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth. 2011;106(6):840–50. https://doi.org/10.1093/bja/aer091.
Banevičius G, Rugytė D, Macas A, Tamašauskas A, Stankevičius E. The effects of sevoflurane and propofol on cerebral hemodynamics during intracranial tumors surgery under monitoring the depth of anesthesia. Medicina. 2010;46(11):743.
Tang N, Ou C, Liu Y, Zuo Y, Bai Y. Effect of inhalational anaesthetic on postoperative cognitive dysfunction following radical rectal resection in elderly patients with mild cognitive impairment. J Int Med Res. 2014;42(6):1252–61. https://doi.org/10.1177/0300060514549781.
Tang JX, Eckenhoff MF, Eckenhoff RG. Anesthetic modulation of neuroinflammation in Alzheimer’s disease. Curr Opin Anaesthesiol. 2011;24:389–94.
Hu N, Guo D, Wang H, Xie K, Wang C, Li Y, et al. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 2014;10(1551):13–24. https://doi.org/10.1016/j.brainres.2014.01.015.
Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: a randomized controlled trial. J Clin Anesth. 2017;38:165–71.
Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, et al. Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Aβ oligomerization. PLoS ONE. 2011;6(11): e27019.
Guo L, Lin F, Dai H, Du X, Yu M, Zhang J, et al. Impact of sevoflurane versus propofol anesthesia on post-operative cognitive dysfunction in elderly cancer patients: a double-blinded randomized controlled trial. Med Sci Monit. 2020;26: e919293. https://doi.org/10.12659/MSM.919293.
Tong D, Ma Z, Su P, Wang S, Xu Y, Zhang LM, et al. Sevoflurane-induced neuroapoptosis in rat dentate gyrus is activated by autophagy through NF-κB signaling on the late-stage progenitor granule cells. Front Cell Neurosci. 2020;14: 590577. https://doi.org/10.3389/fncel.2020.590577.
Erdem AF, Sahin YN, Dogan N, Umudum Z, Bayar F, Bulut C, et al. Effects of sevoflurane and propofol on S100β and neuron-specific enolase protein levels during cardiopulmonary bypass. Niger J Clin Pract. 2016;19(2):278–83. https://doi.org/10.4103/1119-3077.164346.
Jevtovic TV, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesthesia. 2013;111(2):143–51.
Konishi Y, Evered LA, Scott DA, Silbert BS. Postoperative cognitive dysfunction after sevoflurane or propofol general anaesthesia in combination with spinal anaesthesia for hip arthroplasty. Anaesth Intensive Care. 2018;46(6):596–600.
Terri GM, Craig W, Cyndi WG, Duane ED, Maria T, Kenneth MH, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30.
Bredesen DE, Amos EC, Canick J, Ackerley M, Raji C, Fiala M, et al. Reversal of cognitive decline in Alzheimer’s disease. Aging (Albany NY). 2016;8(6):1250.
Kinirons MT, Omahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57(5):540–4.
Acknowledgements
The authors acknowledge subjects for their participation and cooperation in this study
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MH: research idea, manuscript writing, WF: research idea, manuscript writing, RAK: data acquisition, data analysis and interpretation,. HE: writing manuscript and editing, DAR: research idea, manuscript writing, all authors have read and approved the final manuscript, HAA: data acquisition, data interpretation and manuscript writing and reviewing, HE: Manuscript reviewing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An informed written consent was taken from each patient. All data obtained from every patient were confidential and were not used outside the study. The patients have rights to withdraw from the study at any time without giving any reason. All the cost of the investigations was afforded by the researcher. Our study was approved by ethical committee of the Department of Anaesthesiology, faculty of medicine, Beni-Suef University (approval number FMBSUREC/12062018). The study design followed the requirements of revised Helsiniki declaration of biomedical ethics, Also the protocol of this study was registered in clinical trial registration: www.ClinicalTrials.gov, identifier: NCT04874545 in 5/5/2021.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, M., Fathy, W., Koura, R.A. et al. Effect of propofol versus sevoflurane on auditory and cognitive functions: a randomized controlled trial. Egypt J Neurol Psychiatry Neurosurg 59, 77 (2023). https://doi.org/10.1186/s41983-023-00680-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00680-0