Abstract
Prosopis tamarugo Phil. is a legume tree native to the Atacama Desert, Chile. Tamarugo has physiological characteristics that are highly adapted to extreme life conditions in the Pampa del Tamarugal. Null precipitation makes tamarugo completely dependent on groundwater, developing in areas where the groundwater depth is closest to the surface. Groundwater extraction for domestic consumption, mining, and agriculture affects the desert ecosystem by lowering the water table. Measuring and describing the impacts on vegetation through the monitoring of physiological variables along with groundwater depletion in salt flats where extraction wells are located has contributed to a better understanding of tamarugo response to this stress factor. Integrated variables such as green canopy fraction, normalized difference vegetation index (NDVI), 18O isotope enrichment in foliar tissue, and twig growth proved to be far more reactive toward groundwater depth increase and presented lower error values. These variables respond to mechanisms that tamarugo has to maintain a stable water condition when water offer (water table depth (WTD)) decreases regarding water demand (transpiration). Defoliation along with twig growth diminishment would combine toward a canopy reduction strategy in order to reduce water demand. Green biomass loss, beyond a certain WTD, would lead to complete drying of the tamarugo. Up to 10 m of groundwater table depth, Tamarugo grows, has photosynthetic activity, and has the ability to perform pulvinary movements. Beyond 20 m of water table depth, tamarugo survival is compromised and hydraulic failure is inferred to occur. The current scenario is of groundwater over-exploitation; if economic efforts will be made to conserve and/or restore tamarugo, habitat groundwater extraction is a key element in effective management. Reaching of the thresholds depends on the adequate authority management of groundwater. The objectives of this review are (a) to review information collected from scientific studies regarding tamarugo condition and its response, over time, to WTD increase, (b) to identify WTD thresholds that affect tamarugo’s functioning, and (c) to propose a sequence of physiological events triggered by groundwater (GW) depletion.
Similar content being viewed by others
Introduction
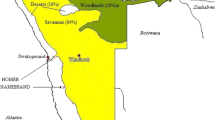
Prosopis tamarugo Phil. (P. tamarugo) is a native and endemic legume tree species that grows in the Pampa del Tamarugal located in the Atacama Desert, Chile (19° 33' S to 21° 50' S) (Habit 1985) (Fig. 1). This area has tropical hyper-desertic bio-climate (Pliscoff and Leubert 2006) with almost complete absence of rainfall, low relative humidity, and wide daily temperature variation (Campillo and Hojas 1975). It is an endorheic basin composed of an underground lake with a water table that fluctuates between 4 and 20 m (Habit 1985). The high evaporation from soil surface generates the presence of salt deposits in an active or partially active state (IREN Instituto de Investigación de Recursos Naturales, Chile 1976). Thus, La Pampa aquifer is associated with salt flats ecosystems (Santibañez et al. 1982).
Location of salt flats where tamarugos are distributed. Sector I: Pintados and Bellavista salt flats; sector II: Llamara salt flat; sector III: Zapiga salt flat (PRAMAR 2007)
P. tamarugo is a strict phreatophyte (Aravena and Acevedo 1985) highly adapted to the conditions of high temperature and radiation of the Pampa del Tamarugal (Lhener et al. 2001), and described as water stress-resistant species (Acevedo et al. 1985). However, at present, this species is at risk due to groundwater (GW) extraction for human and mining consumption (Rojas and Desargues 2007), which has induced a decline in the water table of all the aquifer, so now, it is considered as an officially protected species (“almost under threat” (UICN), “vulnerable” (Ministry of Environment 2012)). The water flux into the aquifer is estimated to be from 880 to 1000 l/s while the water outflow is estimated to be as much as 4000 l/s (Chávez 2014), including 1100 l/s used by evaporation and evapotranspiration, about 2000 l/s of water extraction for human consumption, particularly the city of Iquique, and about 900 l/s for mining and others.
There are impacts of GW extraction on natural vegetation (Elmore et al. 2006), particularly if overexploited. These impacts can be observed in various scales; individual plants may show biomass reduction facing water stress which in a greater scale can be observed through lower normalized difference vegetation index (NDVI) values, and in more severe drought scenarios, when tolerance thresholds have been overcome, specific plant populations may decrease, changing community composition and ecosystem dynamics permanently. It is important to monitor these impacts since desert vegetation provides ecosystem services such as the regulation of the hydrological cycle, conservation and nursery of endemic and rare species, and the provision of an oasis for local settlements, grazing, and small-scale agriculture (Ezcurra 2006).
Tamarugos are subject to multiple stress factors, which may be biotic or abiotic in nature and that affect plant productivity. Abiotic stresses can affect growth through various physiological processes (Thomas and Packham 2007; Pallardy 2008). If a stress factor exceeds the threshold of physiological performance, the survival of the plant depends on the activation of physiological and biochemical mechanisms of avoidance or tolerance, on the flexibility of these mechanisms, on the compensatory abilities, and on the intensity and duration of the stressor (Mandre 2002). Therefore, an understanding of the mechanisms triggered under stressful conditions is needed to preserve this fragile forest ecosystem. The objectives of this review are (i) to review information collected from scientific studies regarding tamarugo condition and its response, over time, to water table depth (WTD) increase, (ii) to identify WTD thresholds that affect P. tamarugo in their condition, and (iii) to propose a sequence of physiological events triggered by GW depletion.
Review
The tamarugo ecophysiology
Tamarugo grows in a desertic climate, with a broad thermal oscillation between night and day, extreme temperatures, frequent winter frosts, low daytime relative humidity, high solar radiation, almost absolute absence of precipitation registering an annual mean of 5 mm per year, saline and alkaline soils (54–500 dS m−1 and pH of 8.4) (Santibañez et al. 1982), and variable WTD (Acevedo et al. 2007). P. tamarugo has adapted its physiology to these extreme conditions (Acevedo et al. 2007). P. tamarugo trees are distributed in areas where the WTD is between 4 and 18 m (Acevedo and Pastenes 1983) in the Bellavista, Zapiga, Pintados, and Llamara salt flats (Fig. 1). P. tamarugo root system is composed by long pivoting roots reaching the groundwater table and a root mat at less than a meter depth from the soil surface (Sudzuki 1969). Soil moisture is almost null at surface level, covered by salt crusts, but it increases at about 30 to 80 cm soil depth where the root mat is located (Ortiz 2010). Mooney et al. (1980) proposed that during summer and spring, the lowest water potential values (Ψ) registered during daytime are at plant level followed by the root mat. Due to this gradient, water passes from the capillary fringe to the roots and then toward the plant. At night, as atmospheric evaporative demand decreases and the Ψ of the plant rises, the lowest Ψ values are registered in the high salinity root mat zone. Water moves both from the capillary fringe and from the crown of the tree to this zone, explaining greater soil moisture in this area. The root mat zone would serve as a water reservoir for the winter season when WTD drops (Mooney et al. 1980). Through the study of isotopic composition of water in the tamarugo soil-plant-atmosphere system, Aravena and Acevedo (1985) proved that the water comes from the aquifer.
P. tamarugo is a halophyte species (Mooney et al. 1980); the salt tolerance mechanisms of P. tamarugo have not been studied directly. Studies on other Prosopis species conducted by Kahn (1987) and Reinoso et al. (2004) in P. strombulifera and Ramoliya et al. (2006) in P. cinerea indicate that Na+ was accumulated by the plants in the roots. On the other hand, roots exposed to higher salinity presented higher renewal rates in P. strombulifera, suggesting root absorption as desalting mechanism (Ramoliya et al. 2006). Like these members of the Prosopis gender, P. tamarugo would present a Na+ exclusion/accumulation mechanism at root level and the capacity to undergo osmotic adjustment to endure soil Ψ reduction produced by the osmotic activity of salts in the soil solution (Acevedo et al. 2007).
Acevedo et al. (1985c) demonstrated that P. tamarugo have the ability to perform osmotic adjustment maintaining turgor at about 0.8 MPa when varying the soil Ψ from −0.06 to −3.0 MPa. This adjustment allows the plant to maintain higher relative water content in leaves at low values of Ψ, favoring a positive pressure potential and an active metabolism. Researches have described the behavior of P. tamarugo stomatal resistance during a daytime period (Ortiz 2010). During early morning, the stomatal resistance decreases. From 10 am toward midday, this resistance has an abrupt change of direction and increases in line with an increase in vapor pressure deficit (vpd) toward a maximum resistance (Ortiz 2010). Mooney et al. (1980) described active stomatal control of the water balance of tamarugo as a key aspect of the P. tamarugo enduring harsh atmospheric conditions.
P. tamarugo can adjust the leaf angle to avoid direct irradiation and photoinhibition (Chavez et al. 2013a, b, Chávez 2014), in a process where cells of one side of the pulvinus swell osmotically while those on the opposite side shrink, effecting a curvature of the pulvinus through turgor-based volume changes (Liu et al. 2007, Pastenes et al. 2004).
Twig growth is directly related to water conditions necessary for cell elongation. Restrictions in water or nutrient transport are related to inhibition of twig growth (Wilson 2000). P. tamarugo has been observed to respond to groundwater depletion by reducing twig growth rate (Squella 2013). Another biomass reduction mechanism performed by P. tamarugo is defoliation. Chlorophyll degradation, loss of foliage, and irregular canopy structure are typical symptoms of forest decline (Deshayes et al. 2006), but to our knowledge, they have not been studied in P. tamarugo. The magnitude of defoliation, observed through canopy-related variables, is apparently quantitative and reflects the severity of water stress (Rood et al. 2003). Ortiz et al. (2012) negatively associated WTD with P. tamarugo plant vigor, suggesting a biomass reduction strategy as a response of tamarugo to water stress.
Water table depth, physiological responses, and tamarugo decline
Since 1988, the authorized GW extractions have exceeded the aquifer’s natural recharge, generating a depletion of the GW table in the aquifer (Rojas et al. 2013). The aquifer’s natural recharge is estimated at 880 l/s (DICTUC 2006); and in 2011, estimated water extraction would have reached 4000 l/s (DGA 2011). The Chilean water service (Dirección General de Aguas (DGA)) officially declared the Pampa del Tamarugal aquifer as a restricted area in 2009 (Res. DGA Number 245), and therefore no new GW extraction rights were granted thereon. Nevertheless, pre-existing extraction rights are still available assets in the “water market” and can be acquired.
P. tamarugo was mainly located in areas with less than 15 m of WTD, and 50 % of the trees were in areas with WTD less than 10 m. WTD maps for the 1960–1993 period built by the JICA-DGA-PCI Project (1995) concluded that no significant GW depletion occurred for that period. Pumping was concentrated in the northeastern part of the study area (Canchones) for the 1993–1998 period, GW depletion occurred, thus, especially in that area leaving few P. tamarugo locations with less than 10 m GWD. By 1998, no P. tamarugo stands in areas with WTD greater than 20 m. In 2005, a new cluster of wells appeared in the northern part of the study area (El Carmelo), the major WTD depletion occurred around this new cluster from 2005 to 2013. By 2013, only a few areas where P. tamarugo were distributed had less than 10 m WTD and the lines of 20 m WTD got close to the P. tamarugo settling in the north and the east of the study area reaching natural forests in the north. According to the information collected by Chávez (2014), groundwater depth would have increased approximately 5 m from 1988 to 2013. The aquifers in question are the only source of drinking water for urban areas, as well as a source for agricultural irrigation and mining enterprises, all of which are activities that have increased extraction rates through the years (DGA 2011).
Chilean environmental authorities have consensus on the fact that the decline of the P. tamarugo trees is due to GW depletion. Studies on the magnitude of the impacts and the extent and the thresholds for P. tamarugo’s survival are required for environmental impact assessment and water as well as basin management (Chávez et al. 2013b).
Physiological responses
The mechanisms of tree mortality as a result of water stress are related to the plant capacity to regulate carbon and water balance under drought (McDowell et al. 2008). Carbon starvation is expected to affect plants that close their stomata at relatively low xylem water tensions (relatively isohydric plants) whereas hydraulic failure is expected to occur in plants that keep their stomata open during drought (relatively anisohydric plants) (McDowell et al. 2008). Adjustments in stomatal resistance reduce transpiration in response to declining hydraulic conductance helping plants to avoid Ψ that may lead to cavitation (Sperry 2000). Isohydric and anisohydric mechanisms in response to drought vary depending on the combination of intensity and duration factors, long-term moderate stress, short-term intense stress, etc. Studies indicate that water stress in the Pampa del Tamarugal would be better described as long-term moderate stress (Ortiz 2010).
For a better understanding of the water relations of P. tamarugo, it is possible to discriminate between death by carbon starvation and hydraulic failure due to harsh water conditions. P. tamarugo performs stomatal closure at midday in response to harsh environmental conditions, but monitoring over a long period of time, they maintained relatively stable minimum stomatal resistance values. Ortiz (2010) and Squella (2013) did not observe significant differences in minimum stomatal resistance in a monitoring of four groups of P. tamarugo trees from different WTD scenarios (two groups enduring GWD increase and two groups with no GWD increase). On the other hand, these authors observed that there was no significant difference in predawn Ψ values between consecutive years, but only variations between seasons, within a year that could be related to the P. tamarugo’s phenological stage (Sudzuki 1985; Mooney et al 1980; Acevedo and Pastenes 1983), specifically, to a decrease of the green cover fraction of the trees during winter (Acevedo et al. 2007). Nevertheless, in some cases, the lowest Ψ values were registered in the group of trees that experienced the most significant WTD descent. This shows that the water status of P. tamarugo at the studied WTDs is a relatively conserved variable. The minimum control of the leaf stomatal resistance between different WTDs at the observed differences in water potential supports the anisohydric behavior of P. tamarugo, eventually generating death by cavitation.
Ortiz (2010) and Squella (2013) monitored 13C discrimination (∆13C) in P. tamarugo plants. Through the use of ∆13C, it is possible to trace photosynthetic activity in plants (Farquhar et al. 1982). Measuring isotopic composition in leaf tissue would allow inferring on photosynthetic activity as higher values of ∆ would evidence greater photosynthetic rate. Neither of the studies observed variation in isotopic 13C discrimination in trees subject to decrease in the water table. There might be a threshold of water availability beyond which isotopic discrimination may suffer alterations (Squella 2013).
Regarding stomatal resistance, 18O isotope concentrations in foliar tissue of P. tamarugo can describe the processes occurring in trees. It has been demonstrated that leaf tissue 18O is a very sensitive indicator of stomatal resistance. As stomata partially close, 18O increases. The advantage of 18O enrichment is that it gives an integrated value of stomata resistance/conductance over time (Barbour 2007). When monitoring P. tamarugo response to GW depletion, 18O is a sensitive variable. A set of trees facing similar vpd conditions throughout the day that record different values of δ18O could lead to understand that these differences are related to differences in WTD. Squella (2013) studied the effect of WTD on P. tamarugo water parameters at Llamara salt flat which has an independent confined aquifer. The trees away from a pumping well along a transect (higher WTD) presented lower values of δ18O in leaf tissue than trees experiencing higher WTD. The study suggests that P. tamarugo trees facing drought conditions may have partial stomatal closure that would increase δ18O (Barbour et al. 2000, Barbour 2007). This change in integrated stomatal resistance indicated by the increase in δ18O is not observed with the point diffusion resistance measurements at the time of minimum stomatal resistance.
In terms of growth, Squella (2013) associated linear and negatively twig growth with WTD in the Llamara salt flat, where the twig growth reached null values at table depths of 11.7 m. Ortiz (2010) described the relationship between foliage loss of P. tamarugo trees and WTD. The objective was to study the spatial and temporal variability of the WTD and its impact on the P. tamarugo forest located at the Pintados salt flat. WTD was modeled using the data of 21 observation wells distributed on the salt flat. Landsat images were processed to obtain NDVI values of the forest. The study revealed that NDVI values decreased as the WTD increased. The tamarugos of the Pintados salt flat reached a minimum NDVI (NDVI = 0.1) at 10 m WTD. This reduction in NDVI was associated with diminishment of foliar biomass as observed by Ortiz (2010) in the Llamara salt flat, P. tamarugo trees responded to WTD by reducing the green canopy fraction.
Through the use of remote sensing, Chávez et al. (2013b) monitored vegetation indexes of the tamarugo forest (NDVI or NDVI derivatives) along with the WT depletion in different salt flats of the Pampa del Tamarugal. The time series that illustrated WTD and NDVI values of the Pampa del Tamarugal indicated that as a consequence of the WT depletion in the Pampa del Tamarugal aquifer during 1988–2013, water condition of tamarugos presented a decline. During that period of time, Landsat NDVIWinter (indicator of green biomass) values diminished in 19 % and Landsat ΔNDVIWinter-Summer (an indicator of the available water in trees needed to perform leaf pulvinus movement) values decreased 51 % (Chávez et al. 2013b). Investigators identified the range up to 10–12 m as an optimal range for tamarugo survival, considering historical distribution and water status retrieved from NDVI metrics. Taking into account the historical distribution of P. tamarugo at depths less than 18–20 m, that trees getting close to this WTD presented a green canopy fraction (GCF) of 0.25 (established by Chávez et al. 2013b as a threshold for tamarugo conservation), and that beyond this threshold trees can no longer perform pulvinar movements to avoid direct high solar radiation accelerating the drying process, the conclusions of this research suggested a groundwater depth of 20 m as a threshold for tamarugo’s survival.
Sequence of physiological events triggered by GW depletion
Water deficit affects all the physiological processes of a plant. The temporal sequences in which these processes are affected are relevant at the moment of establishing thresholds according to the behavior of physiological variables. Rood et al. (2000) described an eco-physiological model of tree responses toward water stress, in plant species that regulate water loss through surface reduction, similar to what is observed in P. tamarugo who sheds foliage facing WTD increase. According to the model, water deficit affects two main physiological processes, stomatal closure and diminishment of twig growth. The sequential events when water deficit increases steadily induces xylem cavitation leading to early senescence and ultimately ending with twig death.
The researches revisited in this review provide an opportunity to adapt this model for the study of P. tamarugo trees. The behavior of variables associated with plant Ψ, stomatal resistance, vigor, and biomass faced with WTD increase has been described (Table 1).
Integrated variables such as: δ18O, NDVI, twig growth, and GCF are far more reactive toward WTD increase and presented lower error values. These variables respond to mechanisms that P. tamarugo perform to maintain a stable water condition when water supply (WTD) is deficient regarding water demand (transpiration).
Under this scenario, P. tamarugo responds with strategies that lead toward a reduction in water demand. Considering that tamarugo would behave as a relatively anisohydric plant, facing water stress, tamarugo would face a decrease in water conditions with partial stomatal closure and reduction of green canopy fraction would precede permanent stomatal closure, most likely performed toward final stages of the response sequence. Using available information, defoliation would be one of the first elements observed at our scale of work of the tamarugo water stress response sequence (TWSRS) along with twig growth diminishment (as growth is particularly sensitive to changes in cell turgor associated with water condition) (Hsiao and Acevedo 1974). Later in the sequence, partial stomatal closure would generate extreme plant Ψ values that would lead to xylem cavitation and twig death in the TWSRS. Twig death, in conjunction with an ongoing and constant defoliation, would contribute to green biomass reduction strategy up to a point, aggravated by the inability of P. tamarugo to perform pulvinary movements to avoid direct radiation, and would lead to hydraulic failure and complete drying of the P. tamarugo.
Therefore, Rood’s model is a good starting point, with the difference that defoliation occurs as one of the first responses of P. tamarugo to lowered WTD and stomatal closure has low sensitivity to WTD. All the information point to an anisohydric behavior of P. tamarugo. What appears to cause twig death is xylem cavitation immediately after P. tamarugo cease to perform pulvinary movements (Fig. 2).
Physiological events triggered at various water table depths in Prosopis tamarugo Phil. Tamarugo maintains its water status to a groundwater depth of approximately 10 m by decreasing water demand through partial defoliation and decreased twig growth. Beyond this depth, the trees start to decrease their predawn water potentials. A drop in water offer negatively affects tamarugo water status and triggers the tamarugo water stress response sequence (TWSRS). Tamarugo faces drought with partial stomatal closure. Early senescence and twig growth diminishment are the first mechanisms in the sequence, followed by twig death caused by xylem cavitation. This biomass reduction strategy contributes to reduce water demand. Tamarugo drawing taken from Sudzuki (1969)
Conclusions
Thresholds for conservation
Can we identify the thresholds of WTD related to the triggering of each step of the TWSRS? Thresholds presented by Squella, Ortiz, and Chavez may be related to different physiological processes in the TWSRS.
Ortiz’s use of the NDVI to monitor foliage loss in response to GW depletion proposes a threshold at which NDVI values tend to zero and tamarugo activity is almost null (10 m). Squella’s threshold holds relation with its analog process in the sequence, twig growth diminishment, and describes GW table depth at which growth rates tend to zero (11.7 m). Chavez proposes a threshold of 20 m, facing which, trees are exposed to drying out and have the reflectance properties of a dead tree most adequately describing the last stage of the sequence, prior to death (20 m) (Fig. 2).
In regard to the information recollected and aiming a better understanding of the survival and mortality mechanism of P. tamarugo Phil., integrated variables proved to be more assertive and sensitive toward WTD. Environmental monitoring of the tamarugo forest could aim efforts toward perfecting and implementing methodologies that include measurements of the variables presented that are related to physiological responses located at each WTD threshold.
Efforts have been made and documented toward a better understanding about why and how tamarugos die due to water depletion. Although current researches may not be entirely conclusive, they shed light toward understanding that WTD is directly related to tamarugo water relations and, ultimately, to its conservation. The current scenario is a groundwater over-exploitation; therefore, if economic efforts are addressed to conserve and/or restore P. tamarugo, habitat groundwater extraction is a key element for an effective management. Reaching of the thresholds presented above depends on adequate authority management of groundwater extraction rate; this is an especially sensitive subject regarding the Chilean Water Code and may require political efforts.
Abbreviations
- DGA:
-
Dirección General de Aguas
- GCF:
-
green canopy fraction
- GW:
-
groundwater
- MMA:
-
Ministerio del Medio Ambiente
- NDVI:
-
normalized difference vegetation index
- TWSRS:
-
tamarugo water stress response sequence
- VPD:
-
vapor pressure demand
- WTD:
-
water table depth
References
Acevedo E, Pastenes J (1983) Distribución de Prosopis tamarugo Phil. En la Pampa del Tamarugal (Desierto de Atacama). Terra Aridae 2:317–335
Acevedo E, Sotomayor D, Zenteno V (1985) Parámetros ambientales y comportamiento hídrico de Prosopis tamarugo Phil. en la localidad de Refresco (Pampa del Tamarugal). In: Habit M (ed) Estado Actual Sobre el Conocimiento de Prosopis tamarugo. FAO, Arica, p 483
Acevedo E, Ortiz M, Franck N, Sanguineti P (2007) Relaciones hídricas de Prosopis tamarugo Phil. Uso de isótopos estables (Water Relations of Prosopis Tamarugo Phil. Use of Stable Isotopes)., Universidad de Chile, Agronomical Science issue N°14. The University of Chile, Santiago, Chile, p 82
Aravena R, Acevedo E (1985) Estudio de la relación hídrica de Prosopis tamarugo Phil. mediante isótopos estables, oxígeno-18 y deuterio. (Study of the water relations of Prosopis Tamarugo Phil using stable isotopes, oxigen-18 and deuterium). In: Habit M (ed) Estado Actual Sobre el Conocimiento de Prosopis tamarugo. FAO, Arica, p 483
Barbour MM, Fischer RA, Sayre KD, Farquhar GD (2000) Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain yield in irrigated wheat. Aust J Plant Physiol 27:625–637
Barbour M (2007) Stable oxygen isotope composition of plant tissue: a review. Funct Plant Biol 34:83–94
Campillo UR, Hojas A (1975) Hidrogeología de la Pampa del Tamarugal. Instituto de Investigación de Recursos Naturales. Corporación de Fomento de la Producción, Santiago, p 61
Chávez RO (2014) Assessing water stress of desert vegetation using remote sensing: the case of the tamarugo forest in the Atacama Desert (Northern Chile), PhD thesis Wageningen University for the degree of doctor in the year 2014., p 174
Chávez RO, Clevers JG, Herold M, Acevedo E, Ortiz M (2013a) Assessing water stress of desert tamarugo trees using in situ data and very high spatial resolution remote sensing. Remote Sens 5(10):5064–5088
Chávez RO, Clevers JG, Herold M, Acevedo E, Ortiz M (2013b) Modelling the spectral response of the desert tree Prosopis tamarugo to water stress. Int J Appl Earth Obs Geoinf 21:53–65
DGA (2011) Actualización de la oferta y la demanda de recursos hídricos subterráneos del sector hidrogeológico de aprovechamiento común Pampa del Tamarugal. Ministerio de Obras Públicas, Dirección General de Aguas, Santiago, p 92
DICTUC (2006) Estudio hidrológico para actualización de la estimación de la recarga de los acuíferos de la Pampa del Tamarugal, Sur Viejo y Llamara. Pontificia Universidad Católica de Chile, Escuela de Ingeniería, Departamento de Ingeniería Hidráulica y Ambiental, Santiago, SOQUIMICH
Deshayes M, Guyon D, Jean H, Stach N, Jolly A, Hagolle O (2006) The contribution of remote sensing to the assessment of drought effects in forest ecosystems. Ann For Sci 63(6):579–595
Elmore AJ, Manning SJ, Mustard JF, Craine JM (2006) Decline in alkali meadow vegetation cover in California: the effects of groundwater extraction and drought. J Appl Ecol 43(4):770–779
Ezcurra E (2006) Global deserts outlook. UNEP/Earthprint, San Diego, p p154
Farquhar GD, O’Leary M, Berry J (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Habit M (1985) Estado Actual del Conocimiento sobre Prosopis tamarugo Phil. FAO, Santiago, p 483, Oficina Regional para América Latina y El Caribe
Hsiao TC, Acevedo, E (1974) Plant responses to water deficits, water use efficiency, and drought resistance. Agricultural Meteorology 14:59–84.
IREN (Instituto de Investigación de Recursos Naturales), Chile (1976) Inventario de los recursos naturales de la Primera Región, Tarpacá., Convenio IREN-SERPLAC, Primera Región. Informe No 36, Vol. I
JICA-DGA-PCI (1995) Study on the development of water resources in northern Chile. Gobierno de Chile, Santiago
Kahn D (1987) Physiological survey of Pakistan coast with special reference to pasture and forest development through biosaline technique., p 543, PhD Thesis, University of Karachi, Department of Botany
Lhener G, Delatorre J, Lütz C, Cardemil L (2001) Field studies on the photosynthesis of two desert Chilean plants: Prosopis chilensis and Prosopis tamarugo. Journal of Photochemistry and Photobiology B: Biology 64:36–44.
Liu CC, Welham CVJ, Zhang XQ, Wang RQ (2007) Leaflet movement of Robinia pseudoacacia in response to a changing light environment. Journal of Integrative Plant Biology 49:419–424
Mandre M (2002) Stress concepts and plants. Forestry Studies 36:9–17
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Ministry of Environment (Ministerio del medio ambiente), Gobierno de Chile (2012) Clasificación de especies, fichas de proceso, Prosopis tamarugo. p 6.
Mooney H, Gulmon S, Rudel P, Ehleringer J (1980) Further observations on the water relations of Prosopis tamarugo of the northern Atacama Desert. Oecologia 44:177–180
Ortiz M (2010) Nivel Freático en la Pampa del Tamarugal y crecimiento de Prosopis tamarugo Phil. Tesis doctoral programa de doctorado en ciencias silvoagropecuarias y veterinarias, Universidad de Chile., p 92
Ortiz M, Morales L, Silva P, Acevedo E (2012) Estimación del nivel freático a partir del NDVI Landsat en La Pampa del Tamarugal (Chile). Teledetección 37:42–50
Pallardy SG (2008) Physiology of woody plants. Elsevier, Amsterdam/Boston/Heidelberg, p 454
Pastenes C, Porter V, Baginsky C, Norton P, González J (2004) Paraheliotropism can protect water stressed bean (Phaseolus vulgaris L.) plants against photoinhibition. J Plant Physiol 161:1315–1323
Pliscoff P, Leubert F (2006) Sinopsis bioclimática y vegetacional de Chile. Santiago. Editorial Universitaria, Santiago, p 316
PRAMAR (2007). Existencias y estado vital de Tamarugos y Algarrobos Blancos en la Pampa del Tamarugal y Salar de Llamara 58° Congreso de la Sociedad Agronómica de Chile, Arica, Chile, PRAMAR Ambiental Consultores.
Ramoliya P, Patel H, Joshi J, Pandey A (2006) Effect of salinization of soil on growth and nutrient accumulation in seedling of Prosopis cineraria. J Plant Nutr 29(2):283–303
Reinoso H, Sosa L, Ramírez L, Luna V (2004) Salt-induced changes in the vegetative anatomy of Prosopis strombulifera (Leguminoseae). Can J Bot 82(5):618–628
Rood SB, Patiño S, Coombs, K, Tyree MT (2000) Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14:248–257.
Rood SB, Braatne JH, Hughes FMR (2003) Ecophysiology of riparian cottonwoods: stream flow dependency, water relations and restoration. Tree Physiol 23:1113–1124
Rojas R, Dassargues A (2007) Groundwater flow modelling of the regional aquifer of the Pampa del Tamarugal, northern Chile. Hydrogeology Journal 15:537–551.
Rojas R, Batelaan O, Feyen L, Dessargues A (2013) Assesment of conecptual model uncertainty for the regional acquifer Pampa del Tamarugal- North Chile. Hrydrology and Earth Systems Sciences 14:171–192.
Santibañez F, Luzio W, Verw W, Etienne M, Lailhacar S (1982) Análisis de los Ecosistemas de la I Región. CORFO, Santiago, p p195
Sperry JS (2000) Hydraulic constraints on plant gas exchange. Agric For Meteorol 104:13–23
Squella C (2013) Respuestas de Tamarugo (Prosopis tamarugo Phil.) a un estado hídrico decreciente en el Salar de Llamara, Msc thesis Universidad de Chile for the degree on Master in the year 2013., p 94
Sudzuki F (1969). Absorción foliar de humedad atmosferica en tamarugo, Prosopis tamarugo Phil. Universidad de Chile, Facultad de Agronomia, Boletín Tecnico. Boletin Tecnico de la Estación Experimental Agronómica de la Universidad de Chile 30:1–23
Sudzuki F (1985) Utilización de humedad ambiental por Prosopis tamarugo Phil. In: Mario H (ed) Estado actual sobre el conocimiento de Prosopis tamarugo. FAO, Arica, Chile, 11-15 de Junio de 1984. p 483
Thomas PA, Packham JR (2007) Ecology of woodlands and forests. Description, dynamics and diversity. Cambridge University Press, Cambridge, p 528
Wilson BF (2000) Apical control of branch growth and angle in woody plants. J Botany 87(5):601–607
Acknowledgements
Financial support for this review was provided by the FONDECYT Project No. 1150799.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The Soil-Plant-Water Relations Laboratory of the Faculty of Agronomy of the University of Chile has an agreement with SQM, a Chilean mining industry, to follow up on the water relations of Prosopis tamarugo Phil. This review and proposed thresholds for conservation will help SQM to manage the water table depth in their water extraction wells of the Pampa del Tamarugal.
Authors’ contributions
GC contributed toward conception and design of the review article. Analyzing and acquiring data relevant to the review purpose and drafting the publication. MG contributed data, and state of the art knowledge regarding environmental monitoring undergone in the Pampa del Tamarugal; he also contributed critical review and inputs. EA contributed with experience to the design of the article and maturation of concepts. Along with this, Acevedo contributed to the systematic review of the document and gave final approval for the publication of this release. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Calderon, G., Garrido, M. & Acevedo, E. Prosopis tamarugo Phil.: a native tree from the Atacama Desert groundwater table depth thresholds for conservation. Rev. Chil. de Hist. Nat. 88, 18 (2015). https://doi.org/10.1186/s40693-015-0048-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40693-015-0048-0