Abstract
This study was conducted to provide evidence, using in vitro and in silico testing methods, regarding the adverse effects of iprodione, a representative dichlorophenyl dicarboxamide fungicide, on the endocrine system. In the present study, we used the HeLa9903 stably transfected transactivation assay (OECD TG 455), 22Rv1/MMTV_GR‒KO androgen receptor transcriptional activation assay (OECD TG 458), and toxicity prediction using VEGA QSAR. Our results showed that iprodione had no estrogen receptor antagonistic or androgen receptor agonistic effects; however, iprodione was determined to be an estrogen receptor agonist (log PC10 value is less than − 9) and androgen receptor antagonist (log IC30 value is − 4.58) without intrinsic toxicity against the human cell lines used in this study. VEGA QSAR was used to evaluate five substances with structures similar to that of iprodione. Among them, four chemicals were found to have positive androgen receptor and aromatase activities and have been observed to be developmental toxicants. These results suggest that iprodione regulates steroid hormone receptor interactions and is a potential reproductive toxicant.
Similar content being viewed by others
Introduction
Pesticides have been extensively used worldwide to achieve adequate volumes of food crops that are of an acceptable quality [1]. Pesticides comprise a variety of compound classes, including herbicides, fungicides, and insecticides, for controlling pests. The use of pesticides has not only contributed to a significant increase in agricultural yield but has also helped to fight vector- and/or food-borne diseases [2]. However, the repeated and extensive use of pesticides has caused serious environmental pollution of the atmosphere, soil, and water [3]; moreover, exposure to certain pesticides is associated with various adverse effects, such as asthma, allergy, cancer, endocrine system disruption, and hypersensitivity [4].
Iprodione is a dichlorophenyl dicarboxamide fungicide used to control a broad range of root and stem rots, molds, and mildew in a variety of fields, fruits, and vegetable crops, including grapes, peaches, tomatoes, potatoes, berries, and onions [5, 6]. Iprodione has relatively low toxicity compared to organochlorine and organophosphate fungicides; however, environmental residues of iprodione have been a concern because of its extensive use and environmental persistence [7]. In a previous study regarding the adverse effects of iprodione pesticide products on the endocrine system, iprodione weakly promoted aromatase activity and increased estrogen production [8] and the acute toxicity of iprodione was low in zebrafish [9]. Additionally, iprodione inhibits steroid hormone synthesis and causes atrophy of the liver, ovaries, and kidneys, leading to changes in body weight [10]. However, studies on the endocrine-disrupting potential of iprodione-mediated hormone receptors are lacking.
As the risk of endocrine disruptors are presented, the Organization for Economic Cooperation and Development (OECD) has published a guidance document (GD150) providing details regarding the endocrine disrupting potential of several chemicals that humans could be exposed to through the environment and food stuffs [11]. The OECD offered a conceptual framework for the testing and assessment of endocrine-disrupting chemicals (EDCs), comprising five different levels. Level two is used to ascertain the endocrine mechanism affected by chemicals via data collection from in vitro assays [11]. According to the OECD endocrine disrupters testing assessment (EDTA), if a chemical does not affect the estrogen, androgen, and thyroid hormone‒mediated reaction and steroid hormone biosynthesis process, it is judged to be a substance that does not disrupt the endocrine system [12]. The stably transfected transactivation (STTA) assay using the HeLa9903 cell line was performed, adopting the performance-based test guideline (PBTG) No. 455 [13], and the androgen receptor transactivation (ARTA) method involving the 22Rv1/MMTV_GR‒KO cell line was described as an OECD test guideline (TG) No. 458 [14], including in vitro OECD conceptual framework level 2. These assays involve several mechanically similar in vitro assays for identifying androgen or estrogen receptor agonists and antagonists.
Pesticides are a representative group of endocrine disruptors (EDs), defined by the World Health Organization (WHO) as “an exogenous substance or mixture that alters the function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations” [15]. With regard to the endocrine-disrupting potential of pesticides, several reports have suggested that pesticide products can directly interact with hormone receptors such as estrogen or androgen receptors [16,17,18]. Aldrin and atrazine, which are organochlorine pesticides, may disrupt the endocrine system by exerting estrogen receptor agonist and androgen receptor antagonist effects [19, 20]. The organophosphorus pesticide diazinon induces the proliferation of a rat pituitary tumor cell line via estrogen receptor agonistic effects [21]. In the case of pyrimidine fungicides, fenarinol acts as an estrogen receptor agonist by inhibiting aromatase [22, 23]. Azole fungicides including hexaconazole and prochloraz influence the endocrine system by interacting with several cytochrome P450 enzymes [24]. However, there is insufficient evidence confirming the endocrine-disrupting potential of dichlorophenyl dicarboximide pesticides.
Therefore, the aim of the present study was to conduct research on iprodione (IFD), estrogen receptor reference (17β-estradiol and 4-hydroxytamoxifen) and androgen receptor reference (5α-dihydrotestosterone and bicalutamide) to provide mechanistic insights into its endocrine-disrupting activity (agonist and antagonist) using sex hormone receptor assays and toxicity prediction results.
Materials and methods
Test substances
Iprodione (IFD) was purchased from Sigma-Aldrich (St. Louis, MO, USA) for STTA and ARTA. Reference substances of estrogen receptor (ER), 17β-estradiol (E2) and 4-hydroxytamoxifen (OHT), and androgen receptor (AR), 5α-dihydrotestosterone (DHT) and bicalutamide, were obtained from Sigma-Aldrich (St. Louis, MO, USA). Reagents for cell culture, such as media, fetal bovine serum (FBS), and antibiotics, were commercially obtained.
Cell culture
HeLa9903 human uterine cervix cells transfected with human estrogen receptor α (hERα) reporter gene was obtained from the JCRB cell bank (Osaka, Japan) and cultured in Eagle’s Minimum Essential Media (EMEM) without phenol red containing 10% dextran-coated charcoal-treated FBS (DCC-FBS) and 60 mg/L of kanamycin. For the androgen receptor transactivation assay, 22Rv1/MMTV_GR‒KO human prostate cancer cells were obtained from KCTC (Jeongeup, Korea) and cultured in RPMI 1640 medium supplemented with 10% FBS, 1% antibiotics (penicillin, streptomycin, and amphotericin B), and 2 mM GlutaMax™ (Gibco, USA). Before chemical treatment, the medium was replaced with phenol-free RPMI 1640 medium supplemented with 5% DCC-FBS, antibiotics, and 2 mM GlutaMax™. Cells were incubated in a humidified atmosphere of 5% CO2 at 37 ℃. Cells were sub-cultured every 2–3 days at 80–85% confluence for a maximum of 20 passages.
Cell viability assay
HeLa9903 cells (1 × 104 cells/well) and 22Rv1/MMTV_GR‒KO cells (3 × 104 cells/well) were seeded into 96-well plates. The cytotoxicity of ER and AR antagonists, which are detected by comprehensive tests, was determined in the test concentration range of IFD using the Cell Count Kit-8 (CCK-8, CK04, Dojindo, Japan) and CellTiter-Flour ™ assay reagent (G6081, Promega, USA), following the manufacturer’s instructions. The absorbance of each well was measured at 450 nm.
Stably transfected transactivation assay using HeLa9903 cell line
To evaluate the potential endocrine-disrupting of IFD, E2 and OHT against estrogen receptor, the STTA assay using the HeLa9903 cell line was conducted following the OECD test guideline 455 [13]. Briefly, the cells (1 × 104 cells/well) were pre-incubated in 5% CO2 at 37 ℃ for 3 h before exposure to the test chemicals. After adding the chemical without (agonist assay) or with (antagonist assay) of E2, the plates for testing were incubated in 5% CO2 at 37 ℃ for 24 h. The media were removed from the test plates and 50 μL/well of luciferase assay reagent (Steady-Glo® Luciferase Assay System, E2510, Promega, USA) was added, and the plate was shaken for 10 min. The luminescence intensity of luciferase activity was assessed using a luminometer (Thermo, USA). The test results were divided into positive and negative according to the classification criteria of the OECD test guidelines [13].
Androgen receptor transactivation assay (ARTA) using 22Rv1/MMTV_GR‒KO cell line
To evaluate the potential endocrine-disrupting of IFD, DHT and bicalutamide against androgen receptor, the ARTA assay using the 22Rv1/MMTV_GR‒KO cell line was performed following the OECD test guideline 458 protocol [14]. The cells (3 × 104 cells/well) were pre-incubated in 5% CO2 at 37 ℃. After 48 h, the media were replaced with new media treated with various concentrations of the test chemicals and incubated for 20–24 h. The luciferase activity of test chemical was assessed using Steady-Glo® luciferase assay reagent (E2510, Promega, USA) at a concentration of 50 μL/well and luminometer (Thermo, USA). The test results were divided into positive and negative according to the classification criteria of the OECD test guidelines [14].
In silico modeling using VEGA QSAR
To adequately describe the IFD molecules, the simplified molecular-input line-entry system (SMILES) was used to extract the chemical formula as a linear string of atoms. IFD was screened to predict estrogen receptor, androgen receptor, aromatase activity, and developmental toxicity using VEGA quantitative structure activity relationship (QSAR). The VEGA platform is an in silico program containing dozens of QSAR models for various endpoints. In silico techniques have been used to predict various toxicological endpoints of chemicals based on their physicochemical properties and structures.
Statistical analysis
In this study, data were described as the means ± standard deviation. We compared each group using one-way analysis of variance (ANOVA) of GraphPad PRISM software (GraphPad Software Inc., La Jolla, CA, USA). Differences between groups were considered significant at a P value of < 0.05.
Results
Proficiency test for the STTA and ARTA assays using human cell line
A proficiency test was conducted using reference standards: 17β-estradiol (E2) and 4-hydroxytamoxifen (OHT) for STTA assay and 5α-dihydrotestosterone and bicalutamide for ARTA assay, before testing with iprodione (IFD). For the STTA assay, the log PC10 and log PC50 values of 17β-estradiol were < − 14 and − 10.71 (Fig. 1A and Table 1), respectively, against agonist effects and the log IC30 and log IC50 values of 4-hydroxytamoxifen were − 8.45 and − 9.29 (Fig. 1B and Table 1), respectively, against antagonist effects. For the ARTA assay, the log PC10 and log PC50 values of 5α-dihydrotestosterone were − 9.91 and − 9.21 (Fig. 2A and Table 1), respectively, against agonist effects and the log IC30 and log IC50 values of bicalutamide were − 6.54 and − 6.18 (Fig. 2B and Table 1), respectively, against antagonist effects.
Results for Stably transfected transactivation assay (STTA) using HeLa9903 cell line. Luciferase activities of (A) 17β‒estradiol as agonist reference and (B) 4‒hydroxytamoxifen as antagonist reference. Estrogen receptor transactivation Result of agonist (C) and antagonist (D) activities for STTA assay in HeLa9903 cells treated with IFD. Bar graph showing cell viability (%) obtained from CCK‒8 assay. The dots represented the luciferase activity (%) compared to the vehicle control group. Vehicle control is 0.1% dimethyl sulfoxide (DMSO). Data were expressed as mean ± standard deviations (n = 3). Asterisks denote statistical significance compared to respective control; *P < 0.05
Results for Androgen receptor transactivation assay (ARTA) using 22Rv1/MMTV_GR‒KO cell line. Luciferase activities of (A) 5α‒dihydrotestosterone as agonist reference and (B) bicalutamide as antagonist reference. Androgen receptor transactivation Result of agonist (C) and antagonist (D) activities for ARTA assay in 22Rv1/MMTV_GR‒KO cells treated with IFD. Bar graph showing cell viability (%) obtained from CellTiter‒Flour ™ assay. The dots represented the luciferase activity (%) compared to the vehicle control group. Vehicle control is 0.1% dimethyl sulfoxide (DMSO). Data were expressed as mean ± standard deviations (n = 3). Asterisks denote statistical significance compared to respective control; *P < 0.05
Agonistic and antagonistic effects of IFD on estrogen receptor
An STTA assay using HeLa9903 cells was performed to estimate the agonist and antagonist effects of IFD on estrogen receptor. In the agonist assay, IFD was confirmed as an estrogen receptor agonist, with log PC10 and log PC50 values of < − 9 and − 3.21, respectively (Fig. 1C and Table 1). In contrast, IFD did not exert an estrogen-antagonistic effect (Figs. 1A, 3D, and Table 1). The mean luciferase activity of the positive control (1 nM 17β-estradiol) was 4.8-fold higher in the agonist assay and 4.5-fold higher in the antagonist assay compared with that of the mean vehicle control (VC) on each test plate. The mean luciferase activity of the positive control should be at least fourfold greater than the mean VC for the agonist and antagonist assay. Therefore, these results satisfy the validation criterion.
Effect of IFD on cell cytotoxicity test. Result of cell viability (%) of HeLa9903 (A) and 22Rv1/MMTV_GR‒KO (B) cell line. Cells were treated with different concentrations of IFD for 24 h. Cell viability was measured using the (A) CCK‒8 and (B) CellTiterFlour™ assays. Vehicle control is 0.1% dimethyl sulfoxide (DMSO). Data were expressed as mean ± standard deviations (n = 3). Asterisks denote statistical significance compared to respective control; *P < 0.05
Agonistic and antagonistic effects of IFD on androgen receptor
The results showed that IFD did not exert an androgen receptor agonist effect (Fig. 2C and Table 1) but had an androgen receptor antagonist effect without intrinsic cytotoxicity (above 70% cell viability) on the 22Rv1/MMTV_GR‒KO cell line (Figs. 2B, 3D and Table 1). In the antagonist assay, the log IC30 and log IC50 values of IFD were − 4.58 and − 3.88, respectively. The mean luciferase activity of the positive control (800 pM 5α-dihydrotestosterone) was 14.8-fold higher in the agonist assay and 26.2-fold higher in the antagonist assay compared with that of the mean VC on each test plate. The mean luciferase activity of the positive control should be at least 13-fold greater than the mean VC for the agonist assay, and at least tenfold greater than the mean VC for the antagonist assay. Therefore, these results satisfy the validation criterion.
Acceptability criteria on STTA and ARTA assay
The relative transcriptional activity (RTA) value of IFD for the estrogen receptor agonist assay was 20.5 ± 1.9, which is presented as a percentage of the PC10 value of 10 nM E2. If the maximum response of the test substance was more than 10% of the activity of 10 nM E2, that is, if the RTA value was more than 10, it was judged as positive. In the androgen receptor antagonist assay, the RTA of IFD was − 24.4 ± 3.2, which was presented as a response to the IC30 value of 800 pM DHT. If the test substance inhibited the activity of 800 pM DHT by more than 30%, that is if the RTA values were less than 70 and the cell viability was more than 80%, it was judged positive. Therefore, IFD affect estrogen and androgen receptor activity, which can lead to problems (or toxicity) related to excessive activity or inhibition of hormones (estrogen and androgen).
Toxicity prediction using VEGA QSAR
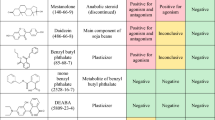
VEGA QSAR was used to evaluate the five substances with structures similar to that of IFD for potential toxicities, including estrogen receptor (ER)- and androgen receptor (AR)-mediated effects, aromatase activity, and developmental toxicity (Table 2). Overall, four chemicals with structures similar to that of IFD had positive AR and aromatase activities and were observed to be developmental toxicants.
Discussion
Estrogen receptor alpha (ERα) is present mainly in mammary glands, uterus, ovary (theca cell), and male reproductive organs including testes, epididymis, and prostate [25]. Androgen receptor (AR) is activated by binding of any of the androgenic hormones, such as testosterone and dihydrotestosterone [26].
Agonistic or antagonistic activities of receptors cause different conformational changes in human reproductive tissues, such as the uterus, breast, and prostate glands. The risk of endometrial proliferation, endometrial hyperplasia, uterine sarcomas, and vaginal bleeding upon exposure to tamoxifen, a representative ERα agonist, has been reported [27, 28]. Antiandrogens also have serious adverse effects, including prostate cancer, enlarged prostate, and early puberty in males [29]. The STTA assay (OECD PBTG 455) and ARTA assay (OECD TG 458) were established to detect the agonist/antagonist activities of endocrine-disrupting chemicals because ERα and AR are essential in the maturation of the female and male reproductive systems, respectively [13, 14, 30].
In the current study, the evaluation of IFD toxicity using the VEGA QSAR platform, which is a representative in silico model, did not predict any adverse outcomes, including ER, AR, or aromatase activity, except for developmental toxicity. However, the evaluation of chemicals with structures similar to that of IFD positively predicted AR and aromatase activity. Because VEGA QSAR is based on compound structure, some models may produce different results. Therefore, an integrated assessment that carefully considers the availability of sufficient information is necessary.
To demonstrate proficiency, the reference chemicals (17β-estradiol, 4-hydroxytamoxifen, 5α-dihydrotestosterone, and bicalutamide) were tested with each test method. In our previous study, Hong et al. (2023) acquired proficiency for STTA and ARTA assays [31]. According to acceptable criteria for STTA assays, the log PC10 value of 17β-estradiol is < − 11 and the log IC50 value of 4-hydroxytamoxifen is − 8.4. In addition, for ARTA assay, the log PC10 value of 5α-dihydrotestosterone is − 12.2 to − 9.7 and the log IC50 value of bicalutamide is − 7.0 to − 5.8. Therefore, the proficiency of the STTA and ARTA assays fell within acceptable criteria.
Our results demonstrated that IFD had no ER antagonistic or AR agonistic effects; however, it was determined to be an ER agonist and AR antagonist without intrinsic toxicity against the studied human cell lines. Therefore, IFD exposure causes endocrine-disrupting effects by interacting with human estrogen and androgen receptors. From these data, IFD did not display ER antagonistic and AR agonistic effects in test ranges (0.001 to 1000 μM) by both in vitro assays. In contrast, IFD exhibited ER agonistic and AR antagonistic activities, as confirmed by STTA and ARTA assays. In addition, IFD was also found to have no cytotoxicity against the 22Rv1/MMTV_GR‒KO cell line. The intrinsic toxicity of test chemicals against cell lines is an important aspect, because the cytotoxicity of test chemicals can interfere with the detection of antagonistic activity [32]. If the cell viability is reduced by 30% or more at exposure concentrations of test chemicals, this concentration is regarded as exhibiting a cytotoxic effect, and the concentrations at or above the cytotoxic concentration should be excluded from the data analysis [14].
These findings are consistent with those of previous studies. Blystone et al. (2007) suggested that iprodione delayed pubertal development in male rats and reduced serum and testicular testosterone production [10]. Additionally, Hassan et al. reported that iprodione and chlorpyrifos induced testicular damage, oxidative stress, apoptosis, and the suppression of steroidogenic-related genes in male rats [33]. However, the association between IFD and ER has not been investigated in previous studies. The findings of our study indicate the need for further research on reproductive toxicity of IFD and effects on the female reproductive system.
In this study, we attempted to confirm the potential endocrine disruption cause by agonist and antagonist effects of the representative dichlorophenyl dicarboxamide fungicide, IFD, using in vitro and in silico assays. Thus, the evidence of ER agonistic and AR antagonistic activity obtained using OECD in vitro PBTG 455 and TG 458 in this study will be a valuable reference for the human health-based guidance value of IFD. Furthermore, the present study has some limitations. First, steroid hormone–mediated reactions need to be investigated. Further studies on steroidogenesis will reveal the endocrine disrupting effects of IFD. Second, the effect of IFD on ERβ could not be determined.
Availability of data and materials
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Abbreviations
- EDTA:
-
Endocrine disrupters testing assessment
- EDC:
-
Endocrine-disrupting chemicals
- STTA:
-
Stably transfected transactivation
- ARTA:
-
Androgen receptor transactivation
- PBTG:
-
Performance-based test guideline
- IFD:
-
Iprodione
- E2:
-
17β-Estradiol
- OHT:
-
4-Hydroxytamoxifen
- DHT:
-
5α-Dihydrotestosterone
- RTA:
-
Relative transcriptional activity
- QSAR:
-
Quantitative structure activity relationship
References
Kim KH, Kabir E, Jahan SA (2017) Exposure to pesticides and the associated human health effects. Sci Total Environ 575(1):525–535. https://doi.org/10.1016/j.scitotenv.2016.09.009
Tudi M, Ruan HD, Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung D (2021) Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 18(3):1112. https://doi.org/10.3390/ijerph18031112
Chawla P, Kaushik R, Swaraj VJ, Kumar N (2018) Organophosphorus pesticides residues in food and their colorimetric detection. Environ Nanotechnol Monit Manag 10:292–307. https://doi.org/10.1016/j.enmm.2018.07.013
Dang VD, Kroll KJ, Supowit SD, Halden RU, Denslow ND (2016) Tissue distribution of organochlorine pesticides in largemouth bass (Micropterus salmoides) from laboratory exposure and a contaminated lake. Environ Pollut 216:877–883. https://doi.org/10.1016/j.envpol.2016.06.061
Bian Y, Wang J, Liu F, Mao B, Huang H, Xu J, Li X, Guo Y (2020) Residue behavior and removal of iprodione in garlic, green garlic, and garlic shoot. J Sci Food Agric 100(13):4705–4713. https://doi.org/10.1002/jsfa.10527
Wang YS, Wen CY, Chiu TC, Yen JH (2004) Effect of fungicide iprodione on soil bacterial community. Ecotoxicol Environ Saf 59(1):127–132. https://doi.org/10.1016/j.ecoenv.2004.01.008
Park W, An G, Lim W, Song G (2022) Exposure to iprodione induces ROS production and mithchondrial dysfunction in porcine trophectoderm and uterine luminal epithelial cells, leading to implantation defects during early pregnancy. Chemosphere 307(2):135894. https://doi.org/10.1016/j.chemosphere.2022.135894
Andersen HR, Cook SJ, Waldbillig D (2022) Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol 179(1):1–12. https://doi.org/10.1006/taap.2001.9347
Lai Q, Sun X, Li L, Li D, Wang M, Shi H (2021) Toxicity effects of procymidone, iprodione and their metabolite of 3,5-dichloroaniline to zebrafish. Chemosphere 272:129577. https://doi.org/10.1016/j.chemosphere.2021.129577
Blystone CR, Lambright CS, Furr J, Wilson VS, Gray LE Jr (2007) Iprodione delays male rat pubertal development, reduces serum testosterone levels, and decreases ex vivo testicular testosterone production. Toxicol Lett 174:74–81. https://doi.org/10.1016/j.toxlet.2007.08.010
OECD (2018) Revised guidance document 150 on standardized test guidelines for evaluating chemicals for endocrine disruption. In: OECD Series on Testing and Assessment. OECD. https://doi.org/10.1787/9789264304741-en
Gelbke HP, Kayser M, Poole A (2004) OECD test strategies and methods for endocrine disruptors. Toxicology 205(1–2):17–25. https://doi.org/10.1016/j.tox.2004.06.034
OECD (2021) Test Guideline No. 455: Performance-based Test guideline for Stably Transfected Transactivation in Vitro Assay to Detection Estrogen Receptor Agonist and Antagonist. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. https://doi.org/10.1787/9789264265295-en
OECD (2023) Test Guideline No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals. OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. https://doi.org/10.1787/9789264264366-en
WHO/IPCS (WHO, International Programme on Chemical Safety) (2002) Global Assessment of the State‒of‒the‒Science of Endocrine Disruptors. WHO/PCS/EDC/02.2. Available: http://www.who.int/publications/i/item/WHO-PCS-EDC-02.2. Accessed 13 July 2002
Kjeldsen IS, Ghisari M, Bonefeld-Jørgensen EC (2013) Currently used pesticides and their mixtures affect the function of sex hormone receptors and aromatase enzyme activity. Toxicol Appl Pharmacol 272(2):453–464. https://doi.org/10.1016/j.taap.2013.06.028
Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K (2004) Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 112(5):524–531. https://doi.org/10.1289/ehp.6649
Li J, Li N, Ma M, Giesy JP, Wang Z (2008) In vitro profiling of the endocrine disrupting potency of organochlorine pesticides. Toxicol Lett 183(1–3):65–71. https://doi.org/10.1016/j.toxlet.2008.10.002
Cocco P (2002) On the rumors about the silent spring: review of the scientific evidence linking occupational and environmental pesticide exposure to endocrine disruption health effects. Cad Saúde Pública 18(2):379–402. https://doi.org/10.1590/S0102-311X2002000200003
Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Tyrey LE, Stoker TE (2007) Atrazine and reproductive function: mode and mechanism of action studies. Dev Reprod Toxicol 80(2):98–112. https://doi.org/10.1002/bdrb.20110
Manabe M, Kanda S, Fukunaga K, Tsubura A, Nishiyam T (2006) Evaluation of the estrogenic activities of some pesticides and their combinations using MtT/Se cell proliferation. Int J Hyg Environ Health 209(5):413–421. https://doi.org/10.1016/j.ijheh.2006.04.004
Andersen HR, Bonefeld-Jørgensen EC, Nielsen F, Jarfeldt K, Jayatissa MN, Vinggaard AM (2006) Estrogenic effects in vitro and in vivo of the fungicide fenarimol. Toxicol Lett 163(2):142–152. https://doi.org/10.1016/j.toxlet.2005.10.004
Vinggaard AM, Hnida C, Breinholt V, Larsen JC (2000) Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol Vitro 14(3):227–234. https://doi.org/10.1016/S0887-2333(00)00018-7
Kahle M, Buerge IJ, Hauser A, Muller MD, Poiger T (2008) Azole fungicides: occurrence and fate in wastewater and surface waters. Environ Sci Technol 42(19):7193–7200. https://doi.org/10.1021/es8009309
Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA (2006) International union of pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58:773–781. https://doi.org/10.1124/pr.58.4.8
Davey RA, Grossmann M (2016) Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev 37(1):3–15
Mourits MJ, De Vries EG, Willemse PH, Ten Hoor KA, Hollema H, Van der Zee AG (2001) Tamoxifen treatment and gynecologic side effects: a review. Obstet Gynecol 97(5 Pt 2):855–866. https://doi.org/10.1016/s0029-7844(00)01196-0
Jordan VC (2004) Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 5(3):207–213. https://doi.org/10.1016/S1535-6108(04)00059-5
Student S, Hejmo T, Poterala-Hejmo A, Lesniak A, Buldak R (2020) Anti-androgen hormonal therapy for cancer and other disease. Eur J Pharmacol 866:172783. https://doi.org/10.1016/j.ejphar.2019.172783
Sun S, Park EJ, Choi YH, Lee HS, Ahn BY, Dong MS (2016) Development and pre-validation of an in vitro transactivation assay for detection of (anti)androgenic potential compounds using 22Rv1/MMTV cells. Reprod Toxicol 60:156–166. https://doi.org/10.1016/j.reprotox.2016.02.0006
Hong SH, Yang JY, Lim JH, Park SJ, Shin JY, Yang SY, Gil GH (2023) Toxicological evaluation of endocrine disruption pesticides applying steroid hormone receptor transcriptional activation methods. Korean J Pestic Sci 27(4):334–343. https://doi.org/10.7585/kjps.2023.27.4.1
Satoh K, Ohyma K, Aoki N, Iida M, Nagai F (2004) Study on anti-androgenic effects of bisphenol a diglycidyl ether (BADGE), bisphenol F diglycidyl ether (BFDGE) and their derivatives using cells stably transfected with human androgen receptor. AR-EcoScreen Food Chem Toxicol 42(6):983–993. https://doi.org/10.1016/j.fct.2004.02.011
Hassan MA, El Bohy KM, El Sharkawy NI, Imam TS, El-Metwally AE, Arisha AH, Mohammed HA, Abd-Elhakim YM (2021) Iprodione and chlorpyrifos induce testicular damage, oxidative stress, apoptosis and suppression of steroidogenic- and spermatogenic- related genes in immature male albino rats. Andrologia 53(4):1–14. https://doi.org/10.1111/and.13978
Acknowledgements
Not applicable.
Funding
This research was supported by a grant from the Research Program for Agriculture Science and Technology Development (Project No. PJ016745), National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Writing original draft preparation, J.-Y.Y. and S.-H.H.; writing review and editing, S.-H.H.; Conceptualization, S.-H.H. and J.-H.L.; Formal analysis and methodology, J.-Y.Y.; Validation, S.-J.P. and M.-K.P.; Data analysis, Y.M.J. and S.Y.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of the National Institute of Agricultural Sciences (approval no. IRB 2022-04-02 and IRB 2023-03-02).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, JY., Lim, JH., Park, SJ. et al. Potential endocrine-disrupting effects of iprodione via estrogen and androgen receptors: evaluation using in vitro assay and an in silico model. Appl Biol Chem 67, 76 (2024). https://doi.org/10.1186/s13765-024-00932-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00932-4