Abstract
Background
While the high haemoglobin glycation index (HGI) has been extensively investigated in diabetic populations, its impact on patients with diabetic kidney disease (DKD) remains unclear.
Methods
We examined data from the National Health and Nutrition Examination Surveys (NHANES) conducted between 1999 and 2018. HGI was determined using the formula recommended by Hempe et al., which calculates the difference between measured and predicted HbA1c. Predicted HbA1c was derived from the equation: 0.024 FPG + 3.1. National death index records up to December 31, 2019, were utilized to assess mortality outcomes. To estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for both all-cause and cardiovascular disease (CVD) mortality, we utilized Cox proportional hazard models. A restricted cubic spline analysis was performed to explore the potential nonlinear relationship between HGI levels and mortality.
Results
Our cohort study comprised data from 1,057 participants with DKD (mean [SE] age, 61.61 [0.57] years; 48.24% female). The mean HGI level was 0.44 (SE 0.04). Over a median follow-up period of 6.67 years, we observed 381 deaths, including 140 due to CVD. Compared with participants in the second tertile of HGI levels (0.03–0.74), those in the lowest tertile of HGI (-5.29–0.02) exhibited an all-cause mortality hazard ratio of 1.39 (95% CI, 1.02–1.88) and a CVD mortality hazard ratio of 1.10 (95% CI, 0.67–1.81). Conversely, participants in the highest tertile (0.75–9.60) demonstrated an all-cause mortality hazard ratio of 1.48 (95% CI, 1.05–2.08) and a CVD mortality hazard ratio of 2.06 (95% CI, 1.13–3.77) after further adjusting for HbA1c and other important variables. Additionally, a restricted cubic spline analysis revealed a U-shaped relationship between HGI and all-cause mortality (P < 0.001 for nonlinearity) and a J-shaped relationship between HGI and CVD mortality (P = 0.044 for nonlinearity).
Conclusions
Our cohort study suggests that HGI in DKD populations exhibits a U-shaped association with all-cause mortality and a J-shaped association with CVD mortality, independent of HbA1c levels.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD), a major complication of diabetes mellitus, is characterized by proteinuria, glomerulosclerosis, and progressive renal failure [1]. DKD affects approximately 20–40% of all diabetic patients and is a leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide [2]. Aggressive glycemic intervention is crucial for this high-risk population, significantly improving disease outcomes [3]. However, traditional glycemic monitoring metrics, such as glycated hemoglobin (HbA1c), have limitations. While HbA1c is widely used to measure glycemic control, its levels are influenced by factors beyond blood glucose [4]. HbA1c reflects only 60–80% of average blood glucose levels [5, 6], with the remaining 20–40% variation attributed to factors such as age [7], genetic variation [8], red blood cell longevity [9], and race [10].
In patients with DKD, the reliability of HbA1c is further compromised due to CKD-related abnormalities that affect red blood cell turnover [11], such as suppressed erythropoiesis or a shortened red blood cell lifespan. Uremia-related changes in hemoglobin conversion and carbamylation also interfere with HbA1c measurements [12]. These limitations suggest that HbA1c may not be the optimal therapeutic target or risk predictor in DKD patients.
The haemoglobin glycation index (HGI), proposed by Hempe et al. in 2002 [13], offers an alternative measure. HGI is calculated by applying fasting plasma glucose (FPG) to a linear regression equation that quantifies individual differences in glycated hemoglobin independent of blood glucose levels [14, 15]. Unlike HbA1c, HGI is not affected by red blood cell longevity [13]. Previous studies have associated high HGI with increased risks of both macrovascular and microvascular complications in diabetics [16]. High HGI also correlates with an elevated risk of CKD in healthy adults [17] and worsened renal function in type 2 diabetes patients [18]. Moreover, the benefits of intensive glucose-lowering therapy in diabetes are primarily seen in patients with low to intermediate HGI, while those with high HGI exhibit higher overall mortality rates [19].
Despite these findings, there is limited research on HGI in DKD patients. This study aims to explore the association between HGI and all-cause and cardiovascular disease (CVD) mortality in DKD patients using National Health and Nutrition Examination Survey (NHANES) data. Our goal is to identify high-risk populations and provide a scientific basis for setting individualized glycemic control targets in the future.
Methods
Study population
The NHANES is an ongoing program designed to assess the health and nutritional status of the U.S. population. Ethical oversight for all surveys is provided by the Ethics Review Committee of the National Center for Health Statistics, with participants giving written informed consent. The survey employs multistage, stratified sampling to ensure representativeness.
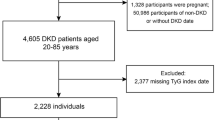
This study utilized data from the NHANES database, covering the years 1999 to 2018. Patients were selectively excluded based on predefined criteria. Exclusions included individuals without DKD (n = 97,651), those under the age of 20 (n = 28), individuals with a baseline cancer diagnosis (n = 596), and those lacking data on HbA1c (n = 1), FPG (n = 1,240), mortality status (n = 2), fasting weights (n = 194), or covariates (n = 283). Additionally, individuals treated for anemia within the previous three months were excluded (n = 264) due to potential influences on erythrocyte longevity and HbA1c measurements. After applying these criteria, the analysis included 1,057 individuals with DKD (Fig. 1).
Diagnostic criteria for diabetic kidney disease
The diagnostic criteria for DKD were established based on diagnostic standards for diabetes and aligned with the CKD guidelines set forth by the Kidney Disease Improving Global Outcomes (KDIGO) working group [20]. These criteria encompassed either an elevated urinary albumin-to-creatinine ratio (ACR) exceeding 30 mg/g or a reduced estimated glomerular filtration rate (eGFR) falling below 60 ml/min/1.73 m² [3].
For the diagnosis of diabetes, several parameters were considered: a clinical diagnosis of diabetes by a healthcare professional, a HbA1c level exceeding 6.5%, a fasting blood glucose level of ≥ 7.0 mmol/L, a random blood glucose level of ≥ 11.1 mmol/L, or a random blood glucose level exceeding 11.1 mmol/L after a two-hour oral glucose tolerance test (OGTT). Meeting any of these criteria confirmed the diagnosis of diabetes [21].
Calculation of haemoglobin glycation index
We employed the standardized HGI formula, developed by Hempe et al., a pioneering figure in HGI research [14]. This formula is grounded in data extracted from the 2005–2016 NHANES. The derivation of this formula involved a cohort of 18,675 adults who were either untreated for diabetes or did not self-report diabetes.
The projected HbA1c value (projected HbA1c = 0.024 FPG + 3.1) is determined by entering the FPG value into a regression equation describing the linear correlation between FPG and HbA1c in the reference population. HGI is computed as the disparity between the measured HbA1c and the predicted HbA1c.
Outcome assessment
All-cause mortality, representing death resulting from any cause, was designated as the primary outcome. CVD mortality was delineated utilizing International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes I00 to I09, I11, I13, I20 to I51, and I60 to I69. Mortality data, acquired through linkage of the cohort database with the National Death Index up to December 31, 2019, underwent thorough analysis.
Covariates
Several covariates potentially influencing the outcomes were incorporated, covering age (years), sex (male and female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other races including multi-racial), education level (< 9 years, 9–13 years, or ≥ 13 years), household poverty-to-income ratio (PIR), smoking status (never, current, and former), alcohol intake (never, moderate, and heavy), use of diabetes medications (other, insulin, oral medications, and unknown), diabetes duration (< 10 years, ≥ 10 years, and unknown). Hypertension was characterized by systolic and diastolic blood pressure readings of at least 140 mmHg and 90 mmHg, respectively, self-reported hypertension, physician-diagnosed hypertension, or the requirement for medication to regulate elevated blood pressure [22]. CVD was self-reported as a diagnosis of congestive heart failure, angina pectoris, myocardial infarction, or coronary heart disease. Cancer diagnoses were self-reported by a physician. Hyperlipidemia was delineated by triglyceride (TG) levels ≥ 150 mg/dL (1.7 mmol/L) or total cholesterol (TC) levels ≥ 200 mg/dL (5.18 mmol/L), low-density lipoprotein (LDL) levels ≥ 130 mg/dL (3.37 mmol/L), or, in men, high-density lipoprotein (HDL) levels < 40 mg/dL (1.04 mmol/L), or, in women, HDL levels < 50 mg/dL (1.30 mmol/L) [23]. The healthy eating index (HEI) score was computed in accordance with HEI-2015 guidelines [24]. Physical activity was defined as engaging in moderate to vigorous exercise, fitness programs, or recreational activities for more than 10 min per week; otherwise, participants were classified as inactive. The eGFR was calculated using the CKD-EPI 2009 (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation.
Statistical analyses
This study rigorously adhered to NHANES guidelines, meticulously adjusting for complex sampling designs and weights. Weighted averages for continuous variables and percentages for categorical ones were reported, ensuring accuracy and reliability. Statistical comparisons were conducted using appropriate methods: continuous variables were analyzed using ANOVA or the Kruskal-Wallis test, while categorical data differences were assessed using the chi-square test. To assess the impact of HGI on both all-cause mortality and CVD mortality, survey-weighted Cox regression analysis was employed. HGI was categorized into three tertiles, with the second tertile serving as the reference for exploratory analysis. To mitigate potential confounding factors, four multivariate models were constructed: Model I adjusted for age and sex. Model II expanded adjustments to include race/ethnicity, education level, PIR, body mass index (BMI), smoking status, comorbidities (hypertension, CVD, hyperlipidemia), physical activity, and alcohol intake. Model III further included variables such as diabetes duration, use of diabetes medications, hemoglobin, eGFR, and HEI. Model IV introduced HbA1C. To explore the nonlinear relationship between HGI and mortality outcomes, restricted cubic spline analysis with 4 knots was conducted. The likelihood ratio test was utilized to assess nonlinearity, ensuring robustness in the analysis. Additionally, analyses were stratified by various factors including age (< 60 or ≥ 60 years), sex (male or female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other races including multi-racial), PIR (< 1, 1–3, ≥ 3), smoking status (never, ever, or current), BMI (< 30 or ≥ 30), HbA1c (< 7% or ≥ 7%), hypertension (yes or no), hyperlipidemia (yes or no), CVD (yes or no), GFR category (G1, G2, G3a + G3b, G4 + G5), use of diabetes medications (other, insulin, oral medications, and unknown), and diabetes duration (< 10 years, ≥ 10 years, and unknown). Interactions between HGI and these factors were rigorously assessed, enhancing the depth of analysis and interpretation.
Sensitivity analyses were also conducted to ensure the robustness of our findings: (1) Variation in HGI calculation: The existing literature shows slight variations in the calculation of HGI, primarily due to differences in the populations used to derive the formula for predicting HbA1c. To address this, we developed distinct predictive formulas for HbA1c using both the entire cohort and a subset specifically focused on DKD. These formulas were then used to calculate HGI, validating its association with mortality outcomes. (2) Mitigating Reverse Causality Bias: To mitigate potential reverse causality bias, we excluded participants who experienced mortality within a 2-year follow-up period from the analysis.
Results
Study participants and baseline characteristics
The study enrolled a total of 1,057 participants, with a mean age of 61.61 years (standard error [SE] = 0.57), and 48.24% (n = 497) were females. The mean HGI level was 0.44 (SE 0.04). During a median follow-up period of 6.67 years, 381 deaths occurred, including 140 from CVD. Baseline characteristics across HGI tertiles—Q1 (-5.29–0.02), Q2 (0.03–0.74), and Q3 (0.75–9.60)—are detailed in Table 1. Elevated HGI levels were associated with younger age, Mexican-American ethnicity, current smoking, physical activity, insulin use, longer diabetes duration, and higher levels of HbA1c, and FPG among individuals.
HGI and all-cause and CVD mortality
In model IV of the weighted multivariate Cox regression analysis, a non-linear relationship between HGI levels and both all-cause and CVD mortality was observed. Compared with Q2 (0.03–0.74), the hazard ratios (HRs) for Q1 (-5.29–0.02) and Q3 (0.75–9.60) for all-cause mortality were 1.39 (95% CI: 1.02–1.88) and 1.48 (95% CI: 1.05–2.08), respectively. Regarding CVD mortality, compared with Q2, the HRs for Q1 and Q3 were 1.10 (95% CI: 0.67–1.81) and 2.06 (95% CI: 1.13–3.77), respectively (Table 2). Additionally, a smoothed curve fit depicted a U-shaped association between HGI and both all-cause mortality (P < 0.001 for nonlinearity) and a J-shaped association between HGI and CVD mortality (P = 0.044 for nonlinearity) (Fig. 2).
Associations between haemoglobin glycation index with all-cause (A) and CVD mortality (B) among participants with diabetic kidney disease in NHANES 1999–2018. HRs were adjusted for age (continuous), sex (male or female), race (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or other races including multi-racial), education (<9, 9-13, ≥13), BMI (continuous), poverty income ratio (continuous), smoking status (never, former, current), hypertension (no, yes), CVD (no, yes), hyperlipidemia (no, yes), physical activity (inactive, active), alcohol intake (none, moderate, heavy), diabetes duration (<10 years, ≥10 years, unknow), use of diabetes medications (other, insulin, oral medications unknow), HEI (continuous), eGFR (continuous), hemoglobin (continuous), HbA1c (continuous)
Stratified and sensitivity analyses
Stratified analysis across various demographic and clinical factors, including age, sex, race/ethnicity, smoking status, BMI, HbA1c, hypertension, hyperlipidemia, CVD, GFR category, use of diabetes medications, and diabetes duration, revealed no evidence suggesting alterations in the U-shaped relationship between HGI and all-cause mortality (Table 3). Additionally, no significant interactions were detected among any of the strata of variables.
Furthermore, after deriving the predicted HbA1c using a distinct population and recalculating the HGI (Additional file 1: Figure S1), the main findings of the current study were replicated, with no significant alterations observed in the results (Additional file 1: Table S1). Moreover, our sensitivity analyses, excluding participants who died within 2 years of follow-up, demonstrated overall robustness in the results (Additional file 1: Table S2).
Discussion
In a comprehensive prospective cohort study of adults with DKD in the United States, we observed U- and J-shaped associations of HGI with all-cause mortality and CVD mortality, respectively. Notably, these associations were independent of HbA1c. Stratified analyses across various demographic and clinical factors, as well as sensitivity analyses, confirmed the stability and robustness of our findings.
High HGI was confirmed to be an independent risk factor for all-cause mortality and CVD mortality in the diabetic population in a meta-analysis that included six prospective cohort studies and one nested case-control study [25]. The current study further confirmed the U- and J-shaped associations of HGI with all-cause mortality and CVD mortality, respectively, in a DKD population. Our conclusions differ from those in previous studies in diabetic populations in that we found that not only did high HGI increase the risk of all-cause mortality, but low HGI also increased the risk of all-cause mortality. Differences in the study populations may account for the inconsistent findings. In the DKD population, glucose metabolism becomes more complex due to CKD, and multiple factors mediate insulin resistance, including decreased β-cell response to blood glucose, decreased renal insulin clearance, and increased hepatocellular gluconeogenesis [26]. Additionally, the suppression of erythropoiesis due to CKD directly affects the measurement of HbA1c, and the combination of these factors results in a mismatch between HbA1c and blood glucose (as quantified by HGI) that may become more pronounced in patients with DKD. These factors may complicate the association of HGI with clinical outcomes in patients with DKD.
In the current study, high HGI increased the risk of all-cause mortality and CVD mortality. The possible mechanism for this is that high HGI reflects long-term glucose fluctuations and glycation patterns [15], which are associated with poor prognosis. The literature confirms that glycemic fluctuations in diabetic patients lead to more severe diabetic complications [27]. Hypoglycemia and poor postprandial glycemic control are more prevalent in diabetic patients with high HGI [16], and the resulting dysregulation of glucose homeostasis can exacerbate vascular endothelial dysfunction and promote atherosclerosis [28]. Additionally, higher HGI values are associated with higher glycation [29], which has been shown to cause damage to neuronal and vascular tissues and promote early atherogenesis [30]. This mechanism also corroborates with previous studies in which high HGI was reported to be associated with coronary artery calcification [31] and carotid atherosclerosis [32]. Moreover, in the current study, we found that patients with high HGI were younger, had a longer duration of diabetes, and had a higher rate of insulin use, which is consistent with findings from the ACCORD Trial [19] and the ADVANCE trial [33]. These combined factors may suggest that patients with high HGI may have a type of diabetes that is more difficult to treat; however, these findings may not be applicable to explain the increased risk of all-cause mortality and CVD mortality in patients with DKD due to the fact that the association of high HGI with all-cause and CVD mortality remained stable and did not interact across diabetes duration and diabetic medication use in stratified analyses.
Another important finding of our study is that low HGI also leads to increased all-cause mortality in patients with DKD, but the association with CVD mortality was not significant. The association of low HGI with adverse clinical outcomes has been reported previously in the literature. In a study that also used NHANES data, it was reported that low HGI increased all-cause mortality in the prediabetic and diabetic population, but this association was only found in men [34]. Low HGI was also found to increase the risk of death at 365 days in another study of ICU patients with critical coronary artery disease [35], and an increased risk of all-cause mortality was also found in another population from China undergoing percutaneous coronary intervention [36]. It may increase the risk of all-cause mortality in patients with DKD through the following mechanisms. First, HGI reflects the disparity between laboratory-measured HbA1c and projected HbA1c levels. Individuals exhibiting an HGI notably below zero are deemed to possess measured HbA1c levels significantly lower than the average HbA1c level observed in the population at corresponding FPG levels. Thus, erroneous HbA1c levels may be accepted as evidence of good glycemic control in patients in the low HGI subgroup, which may lead to further exacerbation of the condition in patients who would otherwise be required to receive glycemic therapy but who are not receiving effective glycemic control. Secondly, A low HGI (indicative of low HbA1c) might serve as an indicator of frequent hypoglycemia, a recognized risk factor for cardiovascular morbidity (such as myocardial infarction, stroke, and sudden cardiac death) and mortality [37]. CKD increases the risk of hypoglycemia due to several factors, including impaired renal regeneration, reduced renal insulin clearance, compromised insulin degradation from uremia, heightened erythrocyte glucose uptake during hemodialysis, impaired counter-regulatory hormonal responses (such as cortisol and growth hormone), nutritional deficiencies, and fluctuations in exposure to oral hypoglycemic agents and exogenous insulin [38]. In addition, we failed to find an association between low HGI and CVD mortality, which may be due to a lack of statistical certainty because of fewer CVD events. However, the above are only our speculations, and the exact mechanisms of HGI associated with all-cause and CVD mortality still need to be further investigated.
Previous studies have debated the clinical value of HGI, with some suggesting that HGI is merely a surrogate for HbA1c. Studies have shown that the association of high HGI with the risk of complications in patients with type 2 diabetes was mediated by HbA1c [39], and that HGI did not predict adverse CVD events and all-cause mortality in patients with type 2 diabetes when HbA1c was included in statistical models [40]. However, Mee Kyoung Kim et al. found that HGI was independently associated with long-term CVD events in type 2 diabetic patients without CVD at baseline [41]. The current study confirmed the association of HGI with all-cause mortality and CVD mortality independently of HbA1c in patients with DKD. The reason for this discrepancy is not clear at this time, but we hypothesize that HGI should play a role independently of HbA1c because the literature confirms that HGI is an indicator reflecting genetic variation [33], which is the main cause of interpersonal differences in HbA1c and has also been shown to be independent of blood glucose concentration [13]. Meanwhile, in a further stratified analysis, we found that the association between HGI and all-cause mortality remained stable across HbA1c strata (< 7.0%, ≥ 7.0%), further supporting the independent role of HGI. It should be noted that there are differences in the way HGI is calculated in the existing literature, which may also be one of the reasons for the differences in the conclusions. In the current study, HGI was calculated using the standardized hemoglobin glycemic index recommended by Hempe et al. (the discoverers of HGI), which was extrapolated using FPG from adults without diabetes or pre-diabetes at baseline of the NHANES and HbA1c [14]. This facilitates the standardization of HGI. Other literature mostly uses the whole population recruited in the study [34] or a screened study population [33, 42] to calculate HGI, thus potentially contributing to the uncertainty of the conclusions. To further validate the stability of the conclusions of the current study, we used different HGI calculations from previous studies and repeated the main study in sensitivity analyses, respectively, and obtained consistent conclusions.
The U-shaped and J-shaped relationships between HGI and all-cause mortality, as well as CVD mortality, respectively, have profound clinical implications for DKD patients. These findings indicate that both extremely low and high HGI values are associated with increased mortality risks, emphasizing the critical need for clinicians to target an optimal HGI range rather than the lowest possible value. The risk at low HGI levels may be attributed to frequent hypoglycemia or malnutrition, while high HGI levels are indicative of poor glycemic control and a higher glycation burden. This highlights the necessity for personalized glycemic targets that consider individual factors influencing HGI, thereby advocating for a tailored approach to glycemic management in DKD patients. Our results underscore the potential benefits of integrating HGI into clinical practice for risk assessment and treatment decision-making, as previous studies have shown that intensified glucose-lowering interventions have yielded diverse outcomes across HGI subgroups in type 2 diabetes mellitus [19]. Specifically, while interventions have been favorable for those with low to medium HGI levels, patients with high HGI have demonstrated increased all-cause mortality and limited CVD event reduction, reinforcing the importance of HGI in optimizing patient outcomes. Future research should concentrate on defining the optimal HGI range for DKD patients and creating interventions to maintain HGI within this range, ensuring a more personalized and effective clinical approach.
Several strengths characterize our study, beginning with the utilization of a nationally representative sample. This approach facilitates the extrapolation of findings to the broader population of the United States. In addition, with the comprehensive data collected in NHANES, we were able to control for potential confounding effects from a variety of demographics, socioeconomics, lifestyles, dietary factors, and medication use. Finally, we performed stratification and sensitivity analyses to verify the reliability of the findings. However, the current study has some limitations. First, given the observational nature of our investigation, only causal relationships can be proposed, and residual confounding or confounding by unmeasured factors cannot be completely excluded. Second, the predicted HbA1c used to calculate HGI was based on a single FPG measurement. While this is a common practice in previous studies, it has limitations in accurately calculating HGI, as the mean glucose of the participants may have varied from the recommended value. Ideally, more glucose measurements, including postprandial levels, should be used to calculate HGI to improve accuracy. Third, it is important to note that a significant number of patients were excluded from our analysis due to the lack of FPG measurements. While this exclusion was necessary for the calculation of HGI, it may have introduced some selection bias. The characteristics of the excluded patients might differ from those included in the study, potentially affecting the generalizability of our results. Future studies with more complete data on FPG measurements could help address this limitation.
Conclusion
Our study provides important insights into the association between the HGI and mortality outcomes in patients with DKD. We observed significant U- and J-shaped associations of HGI with all-cause and CVD mortality, respectively, in this population, independent of traditional glycemic markers such as HbA1c.
Data availability
The datasets generated and analyzed during the current study are available on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
References
11. Chronic kidney Disease and Risk Management: standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S219–30.
de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, et al. Diabetes management in chronic kidney disease: a Consensus Report by the American Diabetes Association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–90.
KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102(5s):S1–127.
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Reviews Disease Primers. 2015;1:15019.
Gonzalez-Covarrubias V, Sánchez-Ibarra H, Lozano-Gonzalez K, Villicaña S, Texis T, Rodríguez-Dorantes M, Transporters, et al. TBC1D4, and ARID5B variants to explain Glycated Hemoglobin variability in patients with type 2 diabetes. Pharmacology. 2021;106(11–12):588–96.
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–8.
Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham offspring study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31(10):1991–6.
Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes. 2001;50(12):2858–63.
McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(6):1259–64.
Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97(4):1067–72.
Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE. 2014;9(1):e84943.
Tang M, Berg A, Rhee EP, Allegretti AS, Nigwekar S, Karumanchi SA, et al. The impact of Carbamylation and Anemia on HbA1c’s Association with renal outcomes in patients with diabetes and chronic kidney disease. Diabetes Care. 2023;46(1):130–7.
Hempe JM, Gomez R, McCarter RJ Jr., Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complicat. 2002;16(5):313–20.
Hempe JM, Yang S, Liu S, Hsia DS. Standardizing the haemoglobin glycation index. Endocrinol Diabetes Metabolism. 2021;4(4):e00299.
Hempe JM, Hsia DS. Variation in the hemoglobin glycation index. J Diabetes Complicat. 2022;36(7):108223.
van Steen SC, Woodward M, Chalmers J, Li Q, Marre M, Cooper ME, et al. Haemoglobin glycation index and risk for diabetes-related complications in the action in diabetes and vascular disease: Preterax and Diamicron Modified Release Controlled evaluation (ADVANCE) trial. Diabetologia. 2018;61(4):780–9.
Nakasone Y, Miyakoshi T, Sakuma T, Toda S, Yamada Y, Oguchi T, et al. Hemoglobin glycation index: a novel risk factor for incident chronic kidney disease in an apparently healthy Population. J Clin Endocrinol Metab. 2024;109(3):e1055–60.
Lin CH, Lai YC, Chang TJ, Jiang YD, Chang YC, Chuang LM. Hemoglobin glycation index predicts renal function deterioration in patients with type 2 diabetes and a low risk of chronic kidney disease. Diabetes Res Clin Pract. 2022;186:109834.
Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care. 2015;38(6):1067–74.
de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–9.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–40.
Zhang X, Wei R, Wang X, Zhang W, Li M, Ni T, et al. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc Diabetol. 2024;23(1):117.
Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Dietetics. 2018;118(9):1591–602.
Zhang L, Wang M, Zhang R, Zhong Y, Fan H, Wang M, et al. Hemoglobin glycation index in relationship to the risk of cardiovascular complication in patients with type 2 diabetes: a systematic review and meta-analysis. J Diabetes Complicat. 2020;34(10):107673.
Rahhal MN, Gharaibeh NE, Rahimi L, Ismail-Beigi F. Disturbances in insulin-glucose metabolism in patients with Advanced Renal Disease with and without diabetes. J Clin Endocrinol Metab. 2019;104(11):4949–66.
Shah MS, Brownlee M. Molecular and Cellular mechanisms of Cardiovascular disorders in Diabetes. Circul Res. 2016;118(11):1808–29.
Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1.
Chalew SA, McCarter RJ, Thomas J, Thomson JL, Hempe JM. A comparison of the glycosylation gap and Hemoglobin Glycation Index in patients with diabetes. J Diabetes Complicat. 2005;19(4):218–22.
Cosson E, Banu I, Cussac-Pillegand C, Chen Q, Chiheb S, Jaber Y, et al. Glycation gap is associated with macroproteinuria but not with other complications in patients with type 2 diabetes. Diabetes Care. 2013;36(7):2070–6.
Rhee EJ, Cho JH, Kwon H, Park SE, Park CY, Oh KW, et al. Association between coronary artery calcification and the Hemoglobin Glycation Index: the Kangbuk Samsung Health Study. J Clin Endocrinol Metab. 2017;102(12):4634–41.
Marini MA, Fiorentino TV, Succurro E, Pedace E, Andreozzi F, Sciacqua A, et al. Association between hemoglobin glycation index with insulin resistance and carotid atherosclerosis in non-diabetic individuals. PLoS ONE. 2017;12(4):e0175547.
van Steen SC, Schrieks IC, Hoekstra JB, Lincoff AM, Tardif JC, Mellbin LG, et al. The haemoglobin glycation index as predictor of diabetes-related complications in the AleCardio trial. Eur J Prev Cardiol. 2017;24(8):858–66.
Yang J, Shangguan Q, Xie G, Yang M, Sheng G. Sex-specific associations between haemoglobin glycation index and the risk of cardiovascular and all-cause mortality in individuals with pre-diabetes and diabetes: a large prospective cohort study. Diabetes, obesity & metabolism; 2024.
Wei X, Chen X, Zhang Z, Wei J, Hu B, Long N, et al. Risk analysis of the association between different hemoglobin glycation index and poor prognosis in critical patients with coronary heart disease-A study based on the MIMIC-IV database. Cardiovasc Diabetol. 2024;23(1):113.
Cheng MD, Tang JN, Liu ZY, Guo QQ, Zhang JC, Zhang ZL, et al. Association of hemoglobin glycation index with prognosis of coronary artery disease after percutaneous coronary intervention: a retrospective cohort study. Diabetes Vasc Dis Res. 2023;20(4):14791641231193306.
Yu TM, Lin CL, Chang SN, Sung FC, Kao CH. Increased risk of stroke in patients with chronic kidney disease after recurrent hypoglycemia. Neurology. 2014;83(8):686–94.
Rhee CM, Kovesdy CP, Kalantar-Zadeh K. Glucose homeostasis, hypoglycemia, and the burnt-out diabetes phenomenon in kidney disease. Semin Nephrol. 2021;41(2):96–103.
Wu JD, Liang DL, Xie Y, Chen MY, Chen HH, Sun D, et al. Association between Hemoglobin Glycation Index and Risk of Cardiovascular Disease and all cause mortality in type 2 Diabetic patients: a Meta-analysis. Front Cardiovasc Med. 2021;8:690689.
Østergaard HB, Mandrup-Poulsen T, Berkelmans GFN, van der Graaf Y, Visseren FLJ, Westerink J. Limited benefit of haemoglobin glycation index as risk factor for cardiovascular disease in type 2 diabetes patients. Diabetes Metab. 2019;45(3):254–60.
Kim MK, Jeong JS, Yun JS, Kwon HS, Baek KH, Song KH, et al. Hemoglobin glycation index predicts cardiovascular disease in people with type 2 diabetes mellitus: a 10-year longitudinal cohort study. J Diabetes Complicat. 2018;32(10):906–10.
Basu S, Raghavan S, Wexler DJ, Berkowitz SA. Characteristics Associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and High Cardiovascular Risk: machine learning analysis of the ACCORD Trial. Diabetes Care. 2018;41(3):604–12.
Acknowledgements
We are grateful to all the patients for their participation.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
XYL conducted analyses. LHH and LLH wrote the first draft of the article. XQZ conceived the study design. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The survey protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/irba98.htm), and NHANES has obtained written informed consent from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, L., He, L., Luo, X. et al. Association of haemoglobin glycation index with all-cause and cardiovascular disease mortality in diabetic kidney disease: a cohort study. Diabetol Metab Syndr 16, 221 (2024). https://doi.org/10.1186/s13098-024-01462-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01462-1