Abstract
Background
Documentation on water mites in Spain is scarce, as is information on the parasite-host relationship between certain water mite species and representatives of the dipteran family Simuliidae. The discomfort caused to humans and animals by black flies seems to be increasing in recent years. In this context, an investigation of parasitic water mites is of great importance, not only from the point of view of biodiversity, but also in terms of their potential to control black fly populations.
Methods
Rivers across a wide region of eastern Spain were sampled to determine the specific richness of simuliid dipterans and to investigate their possible parasites, such as water mites, mermithid nematodes and microsporidia (fungal microbes). Data on environmental variables, abundance, prevalence and intensity of parasitism on the collected specimens were analyzed.
Results
In 10 streams, 15,396 simuliid pupae were collected and checked for the presence of water mite larvae; 426 pupae in seven streams were found to be associated with water mite larvae. Of the 21 simuliid species identified based on morphological characters, eight were found to be associated with water mite larvae. Water mite infection was not equally distributed among black fly species. Also, the prevalence of parasitism was low and differed among simuliid species, ranging from one to 13 water mites per black fly pupa. Variation at the intra- and interspecific levels was detected in terms of the number of water mites inside the black fly cocoons. Free-living deutonymphal and adult water mites representing 15 different species of six genera and five families were morphologically identified. The taxonomic identity of the parasitic mite larvae is unclear at present. Morphologically, they fit descriptions of larval Sperchon (Hispidosperchon) algeriensis Lundblad, 1942, but the possibility cannot be excluded that they represent Sperchon algeriensis, the most abundant species at the adult stage in this study and unknown at the larval stage, or even another species of the genus. A molecular analysis produced for the first time cytochrome oxidase I gene sequences for S. algeriensis.
Conclusions
Our results contribute to current knowledge on Spanish Hydrachnidia and their relationships with simuliids as hosts. However, further research is needed to evaluate the diversity, distribution, bioecology and prevalence of this parasitism.
Graphical Abstract

Similar content being viewed by others
Background
Mites are a taxonomic group that has achieved great success at adapting to almost any kind of habitat. High numbers of species are reported both from terrestrial and aquatic environments, from freshwater to seawater. Mites are able to distribute in many different ways, with some species even having developed structures that allow them to glide through the air [1]. Adaptation has also resulted in a wide radiation of feeding types, ranging from the ancestral predatory mode to phytophagy, saprophagy, mycophagy, necrophagy or parasitism [1, 2].
Parasitic water mites may play an important role in the natural control of insect populations [1, 3]. In this context, these arthropods may function as biological controllers of harmful organisms [1], as is the case of black flies, and could be considered as a potential tool in the control of various insect populations [4, 5]. Currently, there is an increasing interest in this type of biological control [6, 7]. However, it is important to take into account that the immense majority of water mites are found only in streams with year-round flow; consequently, simuliids are safe from water mites in intermittent streams, where many species are adapted to a seasonal water shortage. The eggs of some species can remain viable in a state of diapause for up to 2 years after oviposition in the dry beds of temporary water courses, which are typical of markedly seasonal climates in semi-arid zones. A precondition for the long-term survival of these eggs is shelter in the deepest and most humid strata of the interstices of the sediment [8, 9].
The life-cycle of water mites consists of seven stages: egg, prelarva, larva, protonymph, deutonymph, tritonymph and adult. Of these, only the larval, deutonymph and adult stages are active [10,11,12]; that is, they swim and crawl to locate appropriate hosts or prey—larvae as parasites, deutonymphs and adults as predators. Among the preferred hosts of larval water mites are the imaginal stages of the diptera [12,13,14,15], of which a special case are the black fly larvae parasitized by some species of the water mite genus Sperchon (most published data refer to Sperchon (Hispidosperchon) setiger Thor, 1898). While the larvae of most Sperchon species are known to use exclusively, or additionally, chironomids as their hosts [16,17,18], most records refer to S. setiger larvae parasitizing adult black flies [13]. The typical life-cycle of water mites has larvae present inside the cocoon harboring the black fly pupa during metamorphosis [19, 20]. These larvae wait until emergence of the black fly imago and then anchor to it, emerging into the open air with their host. After the adult black fly emerges into the air, the mite larvae feed by sucking the host’s body liquids. As parasites, water mites have an additional benefit in attaching to hosts since this attachment is also a means to increase dispersion [13]. When adult black flies approach the water, for example, to lay eggs, mite larvae return to the water [12, 21, 22] and continue progressing through their own life-cycle over several molts to the adult stage.
As many Sperchon larvae have been found to be remarkably engorged during their stay in the cocoon, Renz et al. [20] suggest that they are also able to feed before the host emerges. In this case, Sperchon larvae parasitizing simuliid pupae are a rare exception in the typical mode of water mite parasitism that predominantly targets insect adults. It is currently unclear how this food uptake takes place (lesions in the hosts integument are found only in exceptional cases). Should Sperchon larvae be able to complete their life-cycle without parasitism on adult hosts, then they would leave the black fly cocoon for further development—in no case would protonymphal Sperchon then be found inside cocoon. In the same study, Renz et al. [20] also tried to attract Sperchon larvae to simuliid larvae, but the latter were ignored completely by mites. Deutonymphal and adult water mites of most species feed by sucking the body fluids of small invertebrates or their eggs following their injection of digestive enzymes [23,24,25]. A special prey preference for black fly larvae was reported by Ullrich [26] and Martin [25] for water mites of the species S. setiger.
There are still many gaps in our knowledge of the relationship between water mites and black flies. A main limitation is that most research on black fly parasitism by water mite larvae has been attributed to one species of water mite only, namely Sperchon setiger. However, the larval morphology and hosts of many other species of the genus remain as yet unknown, and it cannot be excluded that a wider range of sperchontids have developed a host preference for simuliids. In this context, recent molecular studies (Stur and Gerecke, unpublished) suggest that other related Sperchon species coexisting with S. setiger might be simuliid parasites as well, and that even S. setiger in the classic sense might represent a mix of two or more cryptic species.
In the present study, simuliid-parasitic water mite larvae were found in habitats from which S. setiger was collected in low numbers, in association with strong populations of Sperchon (Hispidosperchon) algeriensis Lundblad, 1942, a related species as yet relatively unknown regarding its larval morphology and host preference. Unfortunately, our attempt to use molecular methods to determine if the simuliid-parasitic mite larvae might belong to the latter species was unsuccessful. However, with the support of Vladimir Pešić (Podgorica), in the present study we were able to obtain for the first time S. algeriensis cytochrome oxidase I gene (COI) sequence data. The results of the present study may contribute towards elucidating this complex question in the near future. At the present time, we can state that the identification of the mite larvae treated in our study seems to agree with that by Ullrich [27] for S. setiger larvae; however, given the general uncertainty of the taxonomic situation, we use “Sperchon sp.” in the study. Nearly all Sperchon species known worldwide are reported from running waters, the habitat to which simuliid larvae are perfectly adapted.
In the study reported here, we specifically address the following questions: (i) How prevalent is water mite parasitism on pupae of black flies in the field? (ii) Do water mites display any preference for parasitizing certain black fly species? (iii) Does the prevalence (i.e. fraction of parasitized hosts) and intensity (i.e. the mite load in hosts) of water mite parasitism vary geographically or in association to local ecological conditions of streams and rivers? Finally (iv), in order to better know the potential sources of black fly parasites: what is the composition of water mite assemblages in the field?
Methods
Study area and sampling design
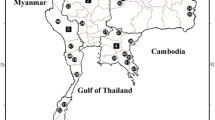
In order to address the issues outlined above, a study area was chosen in eastern Spain, comprising mainly the three provinces that form the Autonomous Region of Valencia, but also the adjacent provinces of Albacete and Cuenca (Castilla-La Mancha) and Teruel (Aragón) (see Fig. 1). It should be noted that the prevalence and intensity of water mite parasitism on black flies will reflect the conditions in the study area, but we expect that, with due caution, the patterns that will emerge could be extended to other geographic areas and inspire future work to support our findings. The study area has already been the subject of an intensive investigation on the abundance and ecological preferences of black fly species, especially those of most concern at the biomedical and veterinary levels [28]. Based on data from this earlier study, we investigated 14 rivers and their tributaries in the present study. During the study period, two of these 14 rivers were completely dry (Girona and Jalón rivers) and the Segura and Vinalopó rivers were negative for the presence of both simuliids and water mites. Sampling sites ranged in latitude from the Senia river in the north (N 40º40′17.6″; E 0º14′20.1″) to the Algar river in the south (N 38º39′35,5"; W 0º5′52,5"), and in elevation from 88 to 664 m a.s.l. Samplings took place between 27 June 2013 and 7 August 2015.
At each sampling site, the following physico-chemical variables of the water were measured in situ: temperature (ºC), pH, dissolved oxygen concentration (mg//l) and its percentage of saturation (%), conductivity (μS/cm), turbidity (nephelometric turbidity units [NTU]), salinity (g/l), total dissolved solutes (TDS; mg/l) and redox potential (mV). Other local environmental variables, such as elevation, air temperature, mean particle size of streambed or the riparian vegetation, were also recorded since such factors have been shown to be useful in predicting aquatic insect distribution in streams [29, 30]. All of these collected data were used to construct an environmental variables’ dataset (Table 1), to be used in subsequent data analyses.
Pupae of black flies and water mite larvae found in the pupal cocoons were sampled following a protocol based on the recommendations of previous researchers [31, 32]. At each sampling point, we chose a river section of 5–10 m in length where black fly pupae were collected from the substrate (i.e. cobbles, pebbles, tree branches, tree leaves, helophytes or submerged macrophytes) during a 15-min period while walking from one bank to the other. The sampling time invested in each single substrate type was proportional to its relative abundance in the river section. No net was used to specifically collect water mites, but the same sort of methodology was implemented to collect both the host and the parasite. The samples were brought to the laboratory where each sample (1 sample being the set of all specimens collected at a sampling point) was processed by first detaching the black fly pupae from the different substrates to which they were fixed. During the identification of pupae (for details, see López-Peña et al. [33]), water mite larvae were preserved in 80% ethanol, together with the black fly pupae that they were parasitizing, for later identification. Free-living adults and deutonymphs of water mites were also collected from all types of substrates in order to characterize the natural assemblages from which parasites could arise.
Identification of water mite and black fly species
Water mite species identification was based on morphological descriptions in several taxonomic keys and the bibliography cited therein (for adults, see references [11, 34, 35]; for larvae, see references [26, 36,37,38]). Once identified, the specimens were stored in pure ethanol (collection Gerecke, Tübingen). In total, 922 mites were examined, among which were 80 free-living adults and deutonymphs; the remaining samples were larvae parasitizing the pupae of the black fly species collected. Selected water mite larvae (n = 5) and deutonymphs or adults (Atractides (Atractides) nodipalpis Thor, 1899 [n = 1]; Aturus gallicus Viets, 1939 [n = 2]; Hygrobates (Hygrobates) fluviatilis gr. (Ström, 1768) [ n = 1] Lebertia (Pilolebertia) porosa gr. Thor, 1900 [n = 1]; Sperchon algeriensis [n = 4]; S. (Hispidosperchon) compactilis Koenike, 1911 [n = 4]; S. setiger [n = 2], Torrenticola (Torrenticola) barsica (Szalay, 1933) [n = 1]) from the samples were sent for CO1 barcoding to the laboratory of Vladimir Pešić (University of Montenegro, Podgorica). Probably due to the sample age, only one of these specimens could be successfully sequenced, a male of S. algeriensis.
Identification of the black fly pupae at the species level was carried out using morphological taxonomic keys [39,40,41]. The samples are deposited in labeled vials in the Colección de Entomología de la Universitat de València (Estudi General). In total, 15,396 pupae were examined, of which 426 were parasitized by water mites.
Data analysis
The prevalence of water mite parasitism on pupae of black fly species was investigated by describing, for each sample site and species, the fraction of the host population infected with parasites per sample site. The proportion of pupae of each black fly species infested with water mites was compared using generalized linear models (GLMs) with binomial responses that took into account the different rivers studied as grouping factor to search for eventual geographic patterns. The intensity of parasitism (i.e. mite load per host) was analyzed for parasitized black fly species using GLMs with Poisson distribution of errors and again considering the different rivers as a grouping variable. Because the GLMs were run in a species-by-species fashion, significance was corrected for multiple comparisons by applying Bonferroni’s method [42]. The correlation between prevalence and intensity of water mite parasitism was also explored, as well as their respective correlations with black fly abundance at each sampling point. The prevalence and intensity of water mite parasitism in each black fly species parasitized were also studied in relation to physico-chemical parameters describing local ecological conditions. Prior to analyses, the correlation among physico-chemical variables was explored to remove highly correlated variables (R2 > 0.8). The remaining variables were used as predictors in multiple logistic regression analyses for each parasitized black fly species, using binomial or Poisson responses for parasitism prevalence and intensity, respectively.
Finally, we assessed the similarity between species assemblages of free-living water mites according to the environmental variables of the sites where they were found. For this purpose, we followed a canonical ordination analysis approach [43]. As a first step, we used detrended canonical correspondence analysis (DCCA) to determine water mite species gradient lengths with respect to the same environmental variables as used in the principal component analysis (PCA) and, therefore, to assess whether unimodal (for further canonical correspondence analysis [CCA]) or linear-based (for further redundancy analysis [RDA]) models underlie the response of water mite species to environmental variables [44]. DCCA was performed on log-transformed data of water mite abundances at the adult and deutonymph stage per site and revealed a dominance of linear gradients (all maximal lengths < 3 standard deviations [SD] [45]), which enabled further analyses on taxonomic turnover in free-living water mites across ecological gradients to be based on RDA. Thus, RDA with forward selection was run to detect the main environmental variables that best explained the variability in water mite abundance. The significance of the variables introduced at each step was inferred from Monte Carlo permutation tests (999 permutations; P-value < 0.05), and model performance was assessed through the adjusted-R2 value.
All statistical analyses were carried out using the free software R version 3.3.3 from The R Foundation for Statistical Computing ([46] https://cran.r-project.org]. GLMs (including multiple logistic regression) and correlation analyses on the prevalence and intensity of water mite parasitism on black fly pupae were performed using the glm and cor functions, respectively, both available from the “stats” package [46]. DCCA and RDA on the assemblages of free-living water mites were performed using the decorana, rda and ordistep functions from package “vegan” [47].
DNA extraction, amplification and sequencing
The barcode region of the COI of one male of S. algeriensis was sequenced using standard invertebrate DNA extraction [48], amplification [49] and sequencing protocols [50]. The COI data are deposited in the reference library of DNA barcodes of BOLD (The Barcode of Life Data System; https://www.boldsystems.org/), which provides a basis for building DNA barcode libraries at the regional and/or national level that contributes to the expansion of information on taxonomic, geographical and molecular species diversity, as well as on their distribution patterns. The voucher specimen is deposited in the collection Gerecke, Tübingen.
Results
In total, 137 samples were collected from 94 sites distributed along 10 watercourses. Of these 137 samples, simuliids were found in 116 samples from 81 sites, and water mites were found in 37 samples from 25 sites. Likewise, water mite larvae were identified in pupal case pupae of Simuliidae in 18 samples from 12 sites of five rivers (Cenia, Mijares, Serpis and Algar rivers and Cabriel a tributary of Júcar river), free-living water mites were found in 15 samples from eight sites of four rivers (Mijares, Cabriel and Magro rivers and the tributaries of Júcar and Serpis rivers) and both water mites parasitizing pupae of Simuliidae and free-living water mites were found in four samples from four sites of three rivers (Cenia and Mijares rivers, and the Cabriel and Magro rivers, tributaries of Júcar river).
Black fly—water mite relationship
Of a total of 15,396 black fly pupae isolated from the samples, 426 were parasitized (2.8% of total black fly pupae isolated). Water mite infection was not equally distributed among black fly species (Table 2): only eight of the 21 black fly species identified were parasitized by water mites, including Simulium (Nevermannia) angustitarse (Lundström, 1911), S. (Wilhelmia) equinum (Linnaeus, 1758), S. (Simulium) intermedium Roubaud, 1906, S. (Wilhelmia) lineatum (Meigen, 1804), S. (Simulium) ornatum Meigen, 1818, S. (Wilhelmia) pseudequinum Séguy, 1921, S. (Wilhelmia) sergenti and S. (Simulium) trifasciatum Curtis, 1839. Water mites were found mainly in the space between the pupa and the pupal case, although some were also recorded on the pupal cases (Fig. 2). A relatively low percentage (< 30%) of these larvae was freshly hatched and unengorged, often co-existing in the same pupal cocoon with slightly to distinctly engorged specimens. In no case was attachment of larvae to the pupal skin observed. All mite larvae extracted from simuliid cocoons were found to be morphologically homogeneous, in agreement with the description given for S. setiger by Ullrich [26] and Martin [51] (lacking seta C3 on coxal plate II; small dimension of the dorsal plate; Fig. 3). However, in view of the above-described unclear taxonomic situation, water mite larvae will be addressed here generally as “Sperchon sp.” as further research is needed in order to clarify if these larvae do in fact represent S. setiger (a species in this study found in a few specimens only), or another Sperchon species (eventually S. algeriensis, the dominant Sperchon in this study and still unknown at the larval stage). Since the very most larval specimens were supposed to be in a pre-parasitic stage awaiting hatching of the adult black fly hosts, in the following text the mite larvae are termed as parasites—regardless of whether they may have engorged while attached to black fly pupae.
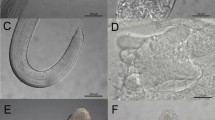
a, b Adults of Sperchon setiger, specimens from Germany: a fronto-lateral, b dorsal (note the setal pair on palp segment 3, typically present in S. setiger as well as in S. algeriensis). c–e Larvae of Sperchon setiger: c dorsal idiosoma, d ventral idiosoma and gnathosoma (c, d from [51]), e total, dorsally. Scale bars: 100 µm. a, b provided by R Gerecke, c, d provided by P Martin, e provided by A Renz
Prevalence of parasitism differed among black fly species (Binomial GLM: deviance = 411.03, df = 20, P-value < 0.001) and was generally low (Fig. 4), with the highest values (± SD) being around 3% in S. trifasciatum (3.5 ± 1.6%), S. equinum (3.3 ± 2.5%) and S. pseudequinum (3.1 ± 1.2%). Even for these three Simulium species, the infection rate was unevenly distributed, and in many samples no parasites were found at all. The prevalence of water mite parasitism differed significantly among river basins in all the eight species parasitized (P-value < 0.001 for the “basin” factor in all cases after binomial GLM analyses). Table 3 shows the prevalence data according to river basins, with the Cenia and Mijares rivers being the most affected by: (i) the total number of pupae of black fly species parasitized; (ii) the incidence of sampling sites where parasites were detected; and (iii) the number of black fly species parasitized. Regarding the geographical distribution of parasitism by Simulium species, S. intermedium was found parasitized in three rivers (Cenia, Mijares and Algar rivers), while S. equinum, S. lineatum and S. sergenti were each found in only one river (Mijares, Júcar and Serpis rivers, respectively). The other four affected Simulium species were found in only two rivers each: S. angustitarse was found parasitized in the Cenia and Serpis rivers, S. ornatum and S. trifasciatum in the Cenia and Mijares rivers and S. pseudequinum in the Mijares and Júcar rivers.
In terms of parasitism intensity, the load of water mites per pupae ranged from 1 to 13. However, this range was not homogeneous, and there was both intra- and interspecific variation in the number of water mite parasites present in black fly cocoons (Fig. 5). Significant differences were observed among species (Poisson GLM: deviance = 32.95, df = 7, P-value = 0.001). Simulium pseudequinum was the most affected of the eight black fly species. No significant correlation was found between the prevalence and intensity of parasitism for any of the infected black fly species, nor was there any relationship between these two variables and the abundance of black fly pupae at the sampling points.
Multiple logistic regression analysis revealed subsets of environmental variables explaining the prevalence of water mite parasitism for the different black fly species found (Table 4). The analyses were reduced to testing the effects of elevation, pH, conductivity, turbidity and oxygen concentration, since other variables measured in the field were highly correlated (R2 > 0.8) with other variables of this subset. The prevalence of water mite parasitism was significantly affected by any of these environmental variables only in four of the parasitized black fly species. Absence of significant relationships occurred in S. angustitarse and S. sergenti (both with very low values of parasite prevalence) and in S. equinum, for which the environmental dataset was limited (some variables could not be measured in the field due to logistic reasons and the number of sampling points where the species was present was scarce). Parasitism prevalence in S. lineatum, S. ornatum, S. intermedium and S. trifasciatum was significantly affected by pH. Notably, the coefficient estimates of the variable pH were negative in all cases, indicating that, in general, an increase in pH will be associated with a decreased probability of black fly species being parasitized by Sperchon sp. water mites. Further, for S. lineatum, S. pseudequinum and S. trifasciatum, pH was again the most significant environmental variable explaining water mite load per individual (Table 5). The sign of other environmental variables with significant effects on prevalence or intensity of parasitism was variable depending on each species, and no general pattern could be derived.
Free-living water mite assemblage study
Free-living deutonymphs and adults of water mites were recovered from only 15 of the 94 sampling sites (Table 6) and included a total of 78 specimens of true water mites, representing 15 different species of six genera and five families; two unidentified specimens of Oribatida were also collected (Table 7). The order Trombidiformes was represented by five families (indet. specimens are counted as separate species when obviously not belonging to one of the listed taxa): Hygrobatidae with four species (Atractides nodipalpis Thor, 1899, Hygrobates calliger Piersig, 1896, H. fluviatilis (Ström, 1768), and H. sp.); Aturidae with two species (Aturus gallicus Viets, 1939 and A. sp.); Lebertiidae with two species (Lebertia porosa Thor, 1900 and L. sp.); Sperchontidae with four species (all of the subgenus Hispidosperchon: Sperchon algeriensis Lundblad, 1942, S. compactilis Koenike, 1911), S. denticulatus gr. and S. setiger Thor, 1898) and Torrenticolidae with two species (Torrenticola barsica (Szalay, 1933) and Torrenticola sp.). In this study, the abundance of aquatic mites is referred to the immediate space where their (potential) hosts are present, above all the pupa stage, and the space between their body and the cocoon, but not to the whole river section in question. As a consequence, the representativeness of water mite data presented here is biased by the collection methodology used. A greater diversity of water mite species as well as a precise relative abundance would be expected applying a specific methodology for the collection of mites.
For most of these species, data on larval morphology and host preference are completely lacking (Aturus gallicus, Sperchon algeriensis, S. compactilis, Torrenticola barsica), or the data are not trustworthy due to unclear taxonomic state (Atractides nodipalpis, Hygrobates calliger and Lebertia porosa are representatives of groups of sibling species in the course of revision (V Pešić and R Gerecke, unpublished). As the taxonomic attribution of populations from the Iberian Peninsula is unclear in many cases, the host range also needs to be reconsidered at a lower geographic scale.
Free-living stages of water mite species were not homogeneously distributed. The vast majority of the identified water mite species were singletons (i.e. species reported just from one sample), as was the case for Atractides nodipalpis, Aturus sp., A. gallicus, Hygrobates calliger, H. fluviatilis gr., Lebertia sp., L. porosa and Torrenticola sp. The most diverse and abundant share of free-living individuals belonged to the family Sperchontidae, with S. algeriensis as the most abundant species (30 specimens) and present in the highest number of samples (7), followed by S. compactilis (15 specimens from 5 different samples). Postlarval specimens of S. setiger, the only species previously reported parasitizing black fly pupae, were also present in some samples, although it was neither remarkably abundant nor common in the assemblages of free-living adult water mites.
Sequencing and genetic distance
The most frequent Sperchon species in the study area, S. algeriensis, was originally described from northern Africa (Sperchon (Hispidosperchon) algeriensis Lundblad, 1942) and subsequently recorded from many sites in the central and western Mediterranean Palaearctic area [34]. Here we present for the first time molecular data for a population of this species, collected in the proximity of Villalonga, a town located on the banks of the Serpis river. This analysis was made possible due to the “DNA-Eco” project coordinated by Vladimir Pešić (University of Montenegro, Podgorica), and sequencing was done at the Canadian Centre for DNA Barcoding (CCBD, Guelph, ONT, Canada; http://ccdb.ca/; sample ID: CCDB 41824 E04; BOLD ID: HYDBH052-22). The results of this analysis confirm that S. algeriensis is a distinct species, clearly separable from S. compactilis and S. setiger (with the latter probably being an aggregate of 2 or more species). Furthermore, the high genetic distance of 15.4% between the Spanish material and a specimen from Iran, attributed to S. algeriensis by Pešić et al. [52], suggests that the latter belongs to a further distinct species. It likely represents S. beneckei Bader & Sepasgosarian, 1982, a species proposed to be a synonym of S. algeriensis by Asadi et al. [53].
Discussion
The ecological importance of water mites in freshwater lotic ecosystems has often been inappropriately underestimated in ecological studies [21]. Far beyond their diversity and abundance, water mites can exert important effects on lotic community structure as predators, parasites or both [25, 54]. Black fly species may constitute a biomedical and veterinary problem of growing concern [33] as potential hosts. They can be parasitized mostly as imagos following the hatch, to which water lice larvae are attached following parasitism during the pupal stage of the black fly [19]. Therefore, in this study we provide information on the prevalence and intensity of water mite parasitism on black fly pupae in the field for a broad span of territory in eastern Spain where the abundance of black fly species is already beginning to be treated as a public health issue [33, 55,56,57,58].
Overall, we report a relatively low prevalence of water mite parasitism on black fly pupae, with the highest values being approximately 3%, which agrees with other observations, such as in chironomids [59,60,61]. Most of the species of black flies were not parasitized at all, and in the parasitized species, water mite larvae were found only on a low proportion of pupae. Notwithstanding, several aspects are remarkable. First, some species of black flies showed a significantly higher level of parasitism than others, as also reported previously by other authors (see [62]). In our study, this was the case for Simulium trifasciatum, S. equinum and S. pseudequinum, the latter two species characterized by their hematophagic habits and veterinary importance. Showing a lower prevalence of parasitism were two other species of biosanitary concern, namely S. ornatum (approx. 2%) and S. lineatum (< 1%). Second, a single water mite species of the genus Sperchon was probably responsible for all the black flies specimens parasitized in our study. Larvae of Sperchon species typically suck hemolymph from their adult hosts [19, 62, 63]. Interestingly, mite larvae in the black fly pupal cocoons were found in states ranging from the unfed to engorged, suggesting that they used black fly pupae to obtain food. Regarding the specificity of the host-parasite relationship, our results are qualitatively consistent with previous findings by Gledhill et al. [19], who also reported cases of S. equinum and S. ornatum parasitized by water mites attributed to S. setiger in southern England, although with a higher prevalence in S. ornatum. Regarding water mite load per pupae, the highest number of Sperchon larvae found in the present study was described for a S. pseudequinum pupa with 13 mites, while Gledhill et al. [19] reported an approximately twofold higher maximum number of mites in S. ornatum. For adult simuliids, Ullrich [27] reported an average of about four larvae attached to simuliid imago, reaching a maximum of 21 larvae per host. Intensity rates were highest in emergence peaks of the preferred host species. Since a high intensity of parasitism probably deeply affects the vitality of the host individuals, food intake in the pupal simuliids (visible in the engorged water mite larvae) might be an alternative way of survival for the water mite larvae in periods when too many mite larvae and too few simuliids are present in the field. Insect larvae and/or pupae are generally seldom used as water mite hosts. For example, for Arrenurus (Megaluracarus) globator (Müller, 1776), a species with an unusually broad host spectrum, both larvae and adults of nematocerans were found as host [64]. Larvae of the water mite genus Unionicola, which are typical parasites of adult chironomids, were found additionally found attached to caddisfly larvae [65].
Several factors may affect the prevalence and intensity of parasitism of water mites on black fly pupae. On one hand, it has been suggested that the morphology of the pupae is a determining factor for some black fly species to be potential hosts of S. setiger [19]. Such specificity may be related to the thickness of the pupal respiratory filaments. In this context, Gledhill et al. [19] suggested that thinner respiratory filaments facilitate the infestation by water mite larvae. This notion holds true in our study for black fly species with thin respiratory filaments, such as S. ornatum, S. intermedium, S. pseudequinum and S. trifasciatum (also heavily affected in the study by Gledhill et al. [19]), but not for S. equinum (whose respiratory filaments are quite thick and short). Davies [62] suggested that the prevalence of parasitism may be related to both factors, namely the ease of access into the pupal chamber and the amount of stored nutrients in fat bodies or maturing eggs. This observation infers the possibility that Sperchon water mites tend to pre-select their hosts on the basis of some kind of chemical attraction, a matter for further research ([19], but see also [27]).
On the other hand, prevalence and intensity of parasitism by water mite larvae may be differentially affected by ecological factors. Most water mites are known to be very vulnerable to modifications in substrata, water quality or discharge [66] and also to be affected by the different ecological demands of their developmental stages. Therefore, a detailed study of the relative abundance of the specimens of each species collected in the different substrates of the river frequented, together with the ecological factors of the habitats, such as the physical–chemical nature of the water, could shed more light on these mites. However, according to Renz et al. [20], levels of infestation seem to be linked to water pollution, and these authors reported that infestation levels were lowest in rivers with a low organic load. In Spain, we observed that parasitism prevalence in S. lineatum, S. ornatum, S. intermedium and S. trifasciatum was negatively affected by pH, and this same environmental variable explained water mite load per individual in S. lineatum, S. pseudequinum and S. trifasciatum. Roughly, our results suggest a trend to lower prevalence of water mite parasitism with increasing values of pH, conductivity and turbidity, factors which ultimately impair water quality. Specifically free-living stages of water mite species were also negatively correlated with pH. Further, our results suggest a positive relationship between black fly pupae abundance and prevalence. Thus, we conjecture that the higher prevalence of parasitism by water mites is due to a density-dependent effect, by which greater overcrowding would favor transmission, but it is generally not well understood how water mite larvae search for their potential hosts in lotic environments [12].
Natural species assemblages of free-living stages of water mites are the potential sources from where black fly parasites could emerge. Typically, different assemblages are associated to varying ecological features of the stream habitats and ultimately depend on the ecological preferences of each species. Water mites are known to be among the most diverse of freshwater organisms [67], with more than 370 species described in Spain (Iberian Peninsula, Balearic Islands and Canary Islands) [68] and, for example, more than 450 species described in Central Europe [69]. However, species richness is generally reduced in running waters with strong seasonal changes [70]. The species that parasitized black fly pupae in our study is a representative of the genus Sperchon. Sperchon setiger, the species that so far has attracted the most interest as a parasite of black flies, is present in the study area, but the species most frequently encountered in the assemblages of free-living stages of water mites is S. algeriensis. The latter is reported from Sicily as a character species of summer-warm Mediterranean streams with temporaneous surface flow [70], but its larval morphology and life-cycle are still unknown. In fact, the hydrography of the present study area is characterized by summer drought. Therefore, the high abundance of this species in the present study is not surprising because of the presence of intermittent habitats. Along with some other species of the subgenus Hispidosperchon, adults and deutonymphs of S. algeriensis, S. setiger and S. compactilis, also species recorded in our study, share a series of morphological similarities. Further research on the life-cycles and larval morphology is required in order to better understand the parasite-host relationship of all these species. Questions that naturally arise concern the general host preference of the involved species. Also, we cannot exclude that we have to deal with a set of cryptic species differing in host preference. To the contrary, we may be dealing with a single species that parasitizes a much wider range of dipteran taxa and is able to switch, following ecological conditions, from one host to another. It is likely that in the natural habitats where free living stages of water mites are found, a wider choice of flying-host items may be available in varying proportions for these parasites; for example, several species in the genus Sperchon are known to use chironomids as their hosts as well [16, 17], which Martin [18] showed for S. setiger.
Conclusions
The main aim of this study was to get insight into the parasitic relationship of water mites (Hydrachnidia) with black flies. The results show that these Hydrachnidia affect the pupal stage of different species of simuliids. A variation in the load of water mites per pupae was detected at both intra- and interspecific level. The data reported here contribute to a better understanding of the water mite–black fly relationship, the prevalence of parasitism, bioecology and geographical distribution of water mites.
Availability of data and materials
All data analyzed during this study are included in this published article. Simuliids and most water mite voucher specimens are stored in the Colección de Entomología de la Universitat de València (Estudi General); selected water mite specimens are stored in the collection Reinhard Gerecke (Tübingen), all in properly labeled vials.
Abbreviations
- BOLD System:
-
Barcode of Life Data System
- CCA:
-
Canonical correspondence analysis
- COI:
-
Cytochrome oxidase I gene
- DCCA:
-
Detrended canonical correspondence analysis
- GLMs:
-
Generalized linear models
- RDA:
-
Redundancy analysis
References
Iraola V. Introducción a los ácaros (II): Hábitats e importancia para el hombre. Boletín SEA. 2001;28:141–6.
Iraola V. Introducción a los ácaros (I): Descripción general y principales grupos. Boletín SEA. 1998;23:13–9.
Sánchez MI, Coccia C, Valdecasas AG, Boyero L, Green AJ. Parasitism by water mites in native and exotic Corixidae: Are mites limiting the invasion of the water boatman Trichocorixa verticalis (Fieber, 1851)? J Insect Conserv. 2015;19:433–47.
Guillet P. The control of human onchocerciasis and the prospects for biological agents. Entomophaga. 1948;29:121–32.
Guillet P. Endotoxin of B. thuringiensis H-14: Mode of action, formulation and aplication against mosquito and blackfly larvae. Proceedings of the 6th European Symposium on animal, plant and microbial toxin. Basle; 1984. p. 21–31. https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_5/b_fdi_18-19/24596.pdf. Accessed 22 Feb 2022.
Lacey LA, Undeen AH. The biological control potential of pathogens and parasites of blackflies. In: Kim KC, Menitt RW, editors. Blackf1ies. Philadelphia: University Press; 1987. p. 327–40.
Smith BP. The potential of mites as biological control agents of mosquitos. In: Hoy MA, Cunningham GL, Knutson L, editors. Biological control of pests by mites. University of California Special Publications No. 3304; 1988. p. 79–85.
Colbo MH, Moorhose DE. The survival of the eggs of Astrosimulium pestilens Mack and Mack (Diptera, Simuliidae). Bull Entomol Res. 1974;64:629–32.
Crosskey RW. Blackflies (Simuliidae). In: Lane RP, Crosskey RW, editors. Medical insects and arachnids. London: Chapman & Hall; 1993. p 241–287.
Böttger K. The general life cycle of fresh water mites (Hydrachnellae, Acari). Acarologia. 1977;18:496–502.
Davids C, Di Sabatino A, Gerecke R, Gledhill T, Smit H. Acari, Hydrachnidia I. In: Gerecke R, editor. Süßwasserfauna von Mitteleuropa, vol 7(2-1). Munich: Spektrum Akademischer Verlag/Elsevier GmbH; 2007. p. 241–388.
Proctor HC, Smith IM, Cook DR, Smith BP. Chapter 25 subphylum Chelicerata, class Arachnida. In: Thorp JH, Rodgers DC, editors. Ecology and general biology: Thorp and Covich’s freshwater invertebrates. 4th ed. London: Academic Press; 2015. p. 599–660.
Smith IM, Oliver DR. Review of parasitic associations of larval water mites (Acari: Parasitengona: Hydrachnida) with insect hosts. Can Entomol. 1986;118:407–72.
Zawal A. The role of insects in the dispersion of water mites. Acta Biol Univ Daugavp. 2003;3:9–14.
Martin P, Gerecke R. Diptera as hosts of water mite larvae—an interesting relationship with many open questions. Lauterbornia. 2009;68:95–103.
Jones RKH. Descriptions of the larvae of Aturus scaber Kramer, Protzia eximia Protz, and Piona uncata Koenike with notes on the life histories of the latter two. Ann Limnol. 1967;3:231–47.
Smith IM, Oliver DR. The parasitic associations of larval water mites with imaginal aquatic insects, especially Chironomidae. Can Entomol. 1976;108:1427–42.
Martin P. Larval morphology and host-parasite associations of some stream living water mites (Hydrachnidia, Acari). Arch Hydrobiol Suppl Monogr Beitr. 2000;121:269–320.
Gledhill T, Cowley J, Gunn RJM. Some aspects of the host: parasite relationships between adult blackflies (Diptera: Simuliidae) of the water-mite Sperchon setiger (Acari; Hydrachnellae) in a small chalk stream in southern England. Freshw Biol. 1982;12:345–57.
Renz A, Gerecke R, Martin P. Parasitic mites (Acari: Hydrachnidia) on pupae and adults of Simuliidae (Insecta: Diptera). DGaaE-Nachrichten. 2004;18:121–2.
Di Sabatino A, Gerecke R, Martin P. The biology and ecology of lotic water mites (Hydrachnidia). Freshw Biol. 2000;44:47–62.
Rolff J. Better host dive: detachment of ectoparasitic water mites (Hydrachnellae: Arrenuridae) from damselflies (Odonata: Coenagrionidae). J Insect Behav. 1997;10:819–27.
Mwango J, Williams T, Wiles R. A preliminary study of the predator-prey relationships of watermites (Acari: Hydrachnidia) and blackfly larvae (Diptera: Simuliidae). Entomologist. 1995;114:107–17.
Proctor H, Pritchard G. Neglected predators: water mites (Acari: Parasitengona: Hydrachnellae) in freshwater communities. J North Am Benthol Soc. 1989;8:100–11.
Martin P. Water mites (Hydrachnidia, Acari) as predators in lotic environments. Phytophaga. 2004;2005:307–21.
Ullrich F. Biologisch-ökologische Studien an rheophilen Wassermilben (Hydrachnellae, Acari), unterbesonderer Berücksichtigung von Sperchon setiger (Thor 1898). PhD thesis. Kiel: University of Kiel; 1976.
Ullrich F. Biologisch-ökologische Studien an den Larven rheophiler Wassermilben (Hydrachnellae, Acari) Schlitzer Produktionsbiologische Studien (29). Arch Hydrobiol. 1978;54:189–255.
López-Peña D. Simúlidos (Diptera: Simuliidae) de los ríos de la Comunidad Valenciana: Implicaciones en la salud pública y su control. PhD thesis. Valencia: Universitat de València; 2018.
McCreadie JW, Colbo MH. Larval and Pupal Microhabitat Selection by Simulium truncatum Lundström, S. rostratum Lundström and S. verecundum AA (Diptera: Simuliidae). Can J Zool. 1993;71:358–67.
Vinson MR, Hawkins CP. Biodiversity of stream insects: variation at local, basin, and regional scales. Annu Rev Entomol. 1998;43:271–93.
Wright JF, Moss D, Armitage PD, Furse MT. A preliminary classification of running- water sites in Great Britain based on macro-invertebrate species and the prediction of community type using environmental data. Freshw Biol. 1984;14:221–56.
McCreadie JW, Colbo MH. Spatial distribution patterns of larval cytotypes of the Simulium venustum/verecundum complex (Diptera: Simuliidae) on the Avalon Peninsula, Newfoundland: factors associated with occurrence. Can J Zool. 1991;69:2651–9.
López-Peña D, García-Roger EM, Jiménez-Peydró R. Pre-imaginal black fly assemblages in streams of Eastern Spain: environmental and substrate requirements. Hydrobiologia. 2020;847:1521–38.
Di Sabatino A, Gerecke R, Gledhill T, Smit H. Acari: Hydrachnidia II. In: Gerecke R, editor. Chelicerata: Acari II. Süßwasserfauna von Mitteleuropa, vol 7(2-2). Heidelberg: Elsevier Spektrum Akademischer Verlag; 2010. p. 1–234.
Gerecke R, Gledhill T, Pešić V, Smit H. Chelicerata: Acari III. Süßwasserfauna von Mitteleuropa, Bd. 7/2-3. Gerecke R, editor. Heidelberg Dordrecht London New York: Springer; 2016.
Prasad V, Cook DR. The taxonomy of water mite larvae. Mem Am Entomol Inst. 1972;18:1–326.
Smith IM, Cook DR, Smith BP. Water mites (Hydrachnida) and other arachnids. In: Thorp JH, Covich AP, editors. Ecology and classification of North American freshwater invertebrates. 2nd ed. San Diego: Academic Press; 2001. p. 551–659.
Smith IM, Cook DR, Smith BP. Water mites (Hydrachnida) and other arachnids. In: Thorp JH, Covich AP, editors. Ecology and classification of North American freshwater invertebrates. 3rd ed. San Diego: Academic Press; 2009. p. 485–586.
González G. Claves para la identificación de las larvas y pupas de los simúlidos (Diptera) de la Península Ibérica. Asoc Española Limnol. 1997;6:1–77.
Crosskey RW, Crosskey ME. An investigation of the blackly fauna of Andalusia, southern Spain (Diptera: Simuliidae). J Nat Hist. 2000;34:895–951.
Rivosecchi L, Addonisio M, Maiolini B. I ditteri simulidi. Nuove chiavi dicotomiche per l’identificazione delle specie italiane con brevi note bio-tassonomiche. Trento:Quaderni del Museo Tridentino di Scienze Naturali, vol 2; 2007. p. 149.
Bonferroni C. Teoria statistica delle classi e calcolo delle probailita. Pubblizazioni del R Istituto Superiore di Scienze Economiche e Commericiali di Firenze. 1936;8:3–62.
Borcard D, Gillet F, Legendre P. Numerical ecology with R. New York: Springer; 2011.
Birks HJB. Chapter 6 quantitative palaeoenvironmental reconstructions. In: Maddy D, Brew JS, editors. Statistical modelling of quaternary science data, technical guide 5. Cambridge: Quaternary Research Association; 1995.
Lepš J, Šmilauer P. Multivariate analysis of ecological data using CANOCO. Cambridge: Cambridge University Press; 2003.
R development core team. Contributed packages. 2017. https://cran.r-project.org. Accessed 08 Mar 2022.
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara B., Simpson G, et al. Vegan: Community Ecology Package. R Package Version. 2019. https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 16 Mar 2022.
Ivanova NV, deWaard J, Hebert PDN. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes. 2006;6:998–1002.
Ivanova NV, Grainger CM. COI amplification. CCDB protocols. 2007a. https://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_Amplification.pdf. Accessed 23 Aug 2022.
Ivanova NV, Grainger CM. Sequencing. CCDB protocols. 2007b. https://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_Sequencing.pdf. Accessed 27 Sept 2022.
Martin P. On the morphology and classification of larval water mites (Hydrachnidia, Acari) from springs in Luxembourg. Zootaxa. 2006;1138:1–44.
Pešić V, Esen Y, Gülle P, Zawal A, Saboori A, Jovanović M, et al. New records of water mites from Turkey and Iran revealed by DNA barcoding, with the description of a new species (Acari, Hydrachnidia). Syst Appl Acarol. 2022;27:1393–407.
Asadi M, Pešić V, Etemadi I. A revised survey of water mites (Acari: Hydrachnidia) from Iran: new synonyms and descriptions of three new species. Zootaxa. 2010;2628:43–55.
Di Sabatino A, Cicolani B, Gerecke R. Biodiversity and distribution of water mites (Acari, Hydrachnidia) in spring habitats. Freshw Biol. 2003;48:2163–73.
López-Peña D, Jiménez-Peydró R. Biodiversity of blackflies (Diptera, Simuliidae) in the basins of the Algar, Amadorio, Monnegre and Serpis rivers (Alicante and Valencia Provinces, East of the Iberian Peninsula). Bol Asoc Esp Entomol. 2020;44:379–400.
López-Peña D, Jiménez-Peydró R. Factores desencadenantes del perjuicio de los simúlidos. Revista Salud Ambiental. 2021;21:132–6.
López-Peña D, Lis-Cantí Á, Jiménez-Peydró R. Health risks of Simulium (Boophthora) erythrocephalum (De Geer, 1776) in the Valencian Autonomous Region, eastern Spain. Biomed J Sci Tech Res. 2021;39:31761–9.
López-Peña D, García-Roger EM, Lis-Cantín Á, Jiménez-Peydró R. Environmental drivers of breeding sites in blackfly species of medical and veterinary importance in eastern Spain. Med Vet Entomol. 2022;36:97–112.
Paterson CG. Water mites (Hydracarina) as predators of chironomid larvae (Insecta: Diptera). Can J Zool. 1970;48:610–4.
Martin P, Stur E. Parasite-host associations and life cycles of spring-living water mites (Hydrachnidia, Acari) from Luxembourg. Hydrobiologia. 2006;573:17–37.
Martin P, Stur E, Wiedenbrug S. Larval parasitism of spring-dwelling alpine water mites (Hydrachnidia, Acari): a study with particular reference to chironomid hosts. Aquat Ecol. 2010;44:431–48.
Davies DM. The parasitism of black flies (Diptera, Simuliidae) by larval water mites mainly of the genus Sperchon. Can J Zool. 1959;37:353–69.
Böttger K. Types of parasitism by larvae of water mites (Acari: Hydrachnellae). Freshw Biol. 1976;6:497–500.
Böttger K, Martin P. On the morphology and parasitism of Arrenurus globator (O.F. Müller, 1776) (Hydrachnidia, Acari) a water mite with an unusually extensive host spectrum. Acarologia. 2003;43:49–57.
Martin P, Tempelman D. An unusual association between water mite larvae (Hydrachnidia, Acari) and a larval caddis fly host (Trichoptera). Lauterbornia. 2014;77:15–21.
Gerecke R, Di Sabatino A. Water mites (Hydrachnidia and Halacaridae) in spring habitats: a taxonomical and ecological perspective. In: Cantonati M, Bertuzzi E, Spitale D, editors. The spring habitat: biota and sampling methods. Trento: Museo Tridentino di Scienze Naturali (Monografie del Museo Tridentino di Scienze Naturali, 4); 2007. p. 193–216.
Di Sabatino A, Smit H, Gerecke R, Goldschmidt T, Matsumoto N, Cicolani B. Global diversity of water mites (Acari, Hydrachnidia; Arachnida) in freshwater. In: Balian EV, Lévêque C, Segers H, Martens K, editors. Fresh water animal diversity assessment, vol. 595. Dordrecht: Springer, Hydrobiologia; 2008. p. 303–15.
Valdecasas AG. Hydrachnidia España. Ministerio de Transición Ecológica (datos no publicados). 2019.
Blattner L, Gerecke R, Von Fumetti S. Hidden biodiversity revealed by integrated morphology and genetic species delimitation of spring dwelling water mite species (Acari, Parasitengona: Hydrachnidia). Parasit Vectors. 2019;12:1–13.
Gerecke R. Taxonomische, faunistische und ökologische Untersuchungen an Wassermilben (Acari, Actinedida) aus Sizilien unter Berücksichtigung anderer aquatischer Invertebraten. Lauterbornia. 1991;7:1–304.
Acknowledgements
The authors express their gratitude to Álvaro Lis Cantín for his contribution in making some figures appearing in this article, Andrew Duncan for checking the English and improving its grammatical and vocabulary quality, as well as to Vladimir Pešić (Podgorica), who kindly supported us with the molecular analysis of 21 water mite specimens from this study within the framework of the scientific project “DNA-Eco” (University of Montenegro), supported by a grant of the Montenegrin Ministry of Science.
Funding
This study was made possible by partial funding from the Conselleria de Sanitat Universal i Salut Pública de la Generalitat Valenciana within the project: “Servicio de vigilancia y control de determinados vectores (insectos) que suponen un riesgo para la salud”.
Author information
Authors and Affiliations
Contributions
RJP and DLP equally contributed to both the study conception and design, as well as to the sample collection. DLP carried out the identification of the Simuliidae specimens and was a major contributor in writing the manuscript. RG carried out the identification of the Hydrachnidia specimens. EMGR conducted bioinformatics, and together with DLP performed the analysis and interpreted the data. DLP, EMGR, RG and PM jointly drafted the manuscript. All authors have read, revised, modified and approved the final writing version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
López-Peña, D., Gerecke, R., García-Roger, E.M. et al. Parasite-host relationships of water mites (Acari: Hydrachnidia) and black flies (Diptera: Simuliidae) in southeastern Spain. Parasites Vectors 15, 474 (2022). https://doi.org/10.1186/s13071-022-05610-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05610-2