Abstract
As an essential marker of cancer treatment, PARP-1 inhibitors could effectively kill tumor cells through a mechanism known as synthetic lethality and are used to treat a variety of cancers. In order to explore novel PARP-1 inhibitors, a series of 22 novel erythrina derivatives were reported and preliminarily explored their mechanism of action. The antitumor activities against four human cancer cell lines including A549, OVCAR-3, HCT-116, and MCF-7 were evaluated, and the preliminary SARs were summarized. Among them, compound 11b exhibited better anti-proliferative effects against A549 cells (IC50 = 1.95 µM). The SI results showed that compound 11b had low toxicity. Moreover, compound 11b displayed excellent PARP-1 inhibitory activities with IC50 values of 19.24 nM. In addition, molecular docking studies provided the rational binding modes of compound 11b in complexes with PARP-1. The flow cytometry assays revealed that compound 11b could induce apoptosis of A549 cells (P < 0.001). Simultaneously, compound 11b could effectively reduce the formation of PAR (P < 0.001). The ADMET prediction results indicated compound 11b had similar properties to rucaparib. Collectively, compound 11b has potential research value for further investigation.
Similar content being viewed by others
Introduction
Cancer is a large group of diseases characterized by the uncontrollable growth of abnormal cells [1]. To date, the family of poly (ADP-ribose) polymerase (PARP) proteins has 18 members that share structural and functional similarities while constituting a diverse and remarkable group of proteins [2]. Among this family, PARP-1 is the most abundant and specific isoform, exerting more than 90% of the PARP enzyme activity [3, 4]. Therefore, PARP-1 is considered an ideal target for cancer chemotherapy.
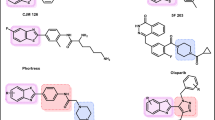
Natural products are now explored as new alternatives for cancer treatment [5]. As a member of the Fabaceae family, erythrina has been reported to have potential antitumor activities (Fig. 1) [6,7,8,9,10]. In addition, we are very interested in the unique structure of the benzo-spiral ring. Rucaparib is a PARP-1 inhibitor approved by the Food and Drug Administration (FDA) for cancer treatment in 2016 [11, 12]. 5 H-dibenzo[b,e]azepine-6,11-dione derivatives are the novel PARP-1 inhibitors we reported previously (Fig. 1) [13]. Furthermore, we found that they have structural similarities (red parts in Fig. 1). Therefore, we attempted to explore novel antitumor drugs targeting PARP-1 using erythrina and 5 H-dibenzo[b,e]azepine-6,11-dione derivatives as the lead compounds.
As a key enzyme involved in DeoxyriboNucleic Acid (DNA) damage repair and recombination, PARP-1 shows excellent potential in developing antitumor drugs [14,15,16]. In addition, several novel PARP-1 inhibitors have been approved by the FDA in recent years [17, 18]. Among them, talazoparib and fluzoparib were approved for cancer treatment in 2018 and 2020, respectively (Fig. 1) [19,20,21,22]. We found that the structure of talazoparib and fluzoparib both contain a triazole group (brown parts in Fig. 1). Triazole groups are not merely passive linkers; they readily bind to biological targets through hydrogen bonding and dipole interactions. Simultaneously, the triazole group is a privileged building block in the discovery of novel antitumor agents, and some of its derivatives have already been applied in clinics to treat cancer [23,24,25]. So, a triazole group was designed in the target structure to improve the compounds’ activity further.
Herein, we designed and synthesized a series of erythrina derivatives as potential PARP-1 inhibitors (Fig. 2). First, we performed 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay of target compounds, and found that compound 11b had better inhibitory activity on A549 (IC50 = 1.95 µM). Compound 11b was then investigated for selectivity index (HPAEpiC: SI = 15.38) and PARP-1/2 inhibition (PARP-1: IC50 = 19.24 nM; PARP-2: IC50 = 32.58 nM). Flow cytometry and western blot indicated that compound 11b could effectively induce the apoptosis of A549 cells, and reduce the biosynthesis of poly (ADP-ribose) (PAR). Next, the molecular docking and molecular dynamics results further elucidated the binding mode and stability of compound 11b to PARP-1. The prediction results of ADMET indicated that compound 11b and rucaparib had similar pharmacokinetic characteristics. All these findings indicated that compound 11b could provide new scaffolds for developing novel PARP-1 inhibitors applicable to cancer therapy.
Results and discussion
Chemistry
The synthetic routes adopted for preparing target compounds (11a-11v) were depicted in Scheme 1.
Compound 9 was obtained according to the method reported by our previous study [26]. Next, compound 10 was obtained by the reduction reaction with compound 9. Finally, target compounds were obtained by the click chemistry of compound 10 with the corresponding azidebenzene. The triazole ring is a crucial five-membered heterocycle structure for designing novel bioactive molecules. This electron-rich heterocycle easily binds to various types of enzymes and receptors. Therefore, triazole compounds have a broad spectrum of biological activities [27, 28]. Meanwhile, we used the microwave reaction generator to accelerate the reaction speed and increase the reaction yield in the reaction process. 1 H NMR, 13 C NMR and HRMS spectroscopy confirmed the structures of target compounds.
Reactions and conditions: (i) Mg, cyclohexanone, ethoxyethane, 35℃, 3 h; (ii) NaN3, 20℃, 12 h; (iii) LiAlH4, 40℃, 12 h; (iv) Methyl-acrylate, no solvent required, 40℃, 12 h; (v) NaOH, water, 50℃, 2 h; (vi) SOCl2, 35℃, 2 h; (vii) AlCl3, 25℃, 24 h; (viii) 3-bromoprop-1-yne, 70℃, 12 h; (ix) LiAlH4, 60℃, 12 h; (x) Corresponding azidebenzene, 35℃, 9 min, 71.91-84.00%
Biological evaluation
MTT assay and structure-activity relationship (SAR) analysis
The anti-proliferative effects on A549, OVCAR-3, HCT-116 and MCF-7 cells were evaluated by MTT assay, and rucaparib as a positive control. (Table 1)
These results showed that compound 11b had excellent anti-proliferative activity against A549 cells (IC50 = 1.95 ± 0.33 µM). The compounds 11t-11v (CN substitution) showed the worst anti-proliferative activity. We believed that it was related to the massive steric hindrance of the CN. Then, we found that compound 11b-11d (F substitution) exhibited better proliferative inhibitory activity than compound 11 h-11j (Cl substitution) and compound 11n-11p (Br substitution) when R was replaced by halogen, especially against A549 cells.
Simultaneously, when R was replaced by CH3 and OCH3, we found that the proliferative inhibitory activity of the para-substituted compounds (11 g, 11 m) was better than that of the ortho-substituted (11e, 11k) and meso-substituted compounds (11f, 11 L). When R was OH (11q-11s), the proliferation inhibition activity of the compounds was weaker than that of CH3 (11e-11 g) and OCH3 (11k-11 m). In conclusion, these findings provide new insight for the future design of novel PARP-1 inhibitors.
The SI of compound 11b
MTT results showed compound 11b had the most prominent effect among the target compounds. We then further evaluated the low toxicity of compound 11b by the SI, and the results were indicated in Table 2. Compound 11b had a better effect for HPAEpiC (SI = 15.38), and the value was better than rucaparib.
In vitro inhibitory activity against PARP-1
Considering that the compound 11b had excellent anti-proliferative activities and low toxicity at the cellular level, we evaluated the ability of compound 11b to inhibit PARP-1/2 enzyme activity in vitro. In addition, rucaparib served as a control.
The results indicated that compound 11b had a slightly better inhibitory effect on PARP-1 than rucaparib, and compound 11b had a selective inhibitory effect between PARP-1/2 (Table 3).
Molecular docking and molecular dynamics of compound 11b
In order to more directly observe the vital structural characteristics and binding stability of compound 11b and PARP-1, molecular docking studies (PDB code: 4BJC) and molecular dynamics were carried out.
The molecular docking studies showed that compound 11b could enter the active pocket, and fully occupy the active pocket (Fig. 3A). The molecular dynamics results indicated that compound 11b binds stably at the active site. The Root Mean Square Deviation (RMSD) value of compound 11b fluctuates less than rucaparib (Fig. 3B).
Effects of compound 11b on the apoptosis in A549 cells
PARP-1 inhibitors have been reported to induce further apoptosis of cancer cells [29, 30], which was also confirmed by our previous studies [26, 31, 32]. An annexin-V/PI binding assay was conducted in A549 cells to evaluate the capacity of compound 11b to induce apoptosis in cancer cells. Flow cytometry analysis showed that the apoptosis rates of A549 cells treated with different concentrations of compound 11b were 4.6%, 8.0%, 10.9% and 19.0%, respectively. (Fig. 4).
This suggested that compound 11b could induce apoptosis in a dose-dependent manner. And we found that the effect of late apoptosis was better. The result is significantly different (P < 0.001).
Effects of compound 11b on the apoptosis in A549 cells. Cell apoptosis analysis was assessed in A549 cells during incubation with different concentrations for 48 h. Q1: Necrotic cell; Q2: Late apoptotic cell; Q3: Early apoptotic cell; Q4: Normal cell. Data were expressed as an average ≥ 3 determinations with standard deviation reported (**P < 0.01 and ***P < 0.001. Compound 11b-treated group VS control)
Effects of compound 11b on PAR and the expression of apoptosis-related protein in A549 cells
PARylation is a critical post-translational modification in which ADP-ribose units are added to a wide array of target proteins by PARP-1 [33,34,35]. PAR is the active product of PARP-1, and the biosynthesis of PAR will be reduced while PARP-1 is inhibited. Here, we used the western blot to evaluate PAR levels and PARP-1 expression. As shown in Fig. 5A, compound 11b could reduce the biosynthesis of PAR, and had the best effect at 4.0 µM.
Meanwhile, we also detected the expression of Cleaved-Caspase 3 and Caspase 3 after different treatment concentrations to explore the mechanism of compound 11b inducing cell apoptosis. The results showed that the ratio of Cleaved-Caspase 3/Caspase 3 was best at 4.0 µM. The expression of Caspase 3 decreased with the increase of compound 11b concentration (Fig. 5B, C).
These results demonstrated that compound 11b could reduce the biosynthesis of PAR, and induce apoptosis in A549 cells. The result is significantly different (P < 0.001).
Western blot analysis: A: Effects of compound 11b on PAR in A549 cells. B, C: Effect of compound 11b on Caspase 3 and Cleaved-Caspase 3 in A549 cells. Data were expressed as an average ≥ 3 determinations with standard deviation reported (*P < 0.05; **P < 0.01 and ***P < 0.001. Compound 11b -treated group VS control)
Prediction of ADMET bioactivity of compound 11b
With the progress of science and technology, the application of computers makes the related research of drug metabolism more convenient and efficient [36]. So, we evaluated the ADMET properties of compound 11b using relevant websites, such as AdmetSAR and SwissADME, and summarized the results in Table 4.
The prediction results indicated compound 11b and rucaparib had similar pharmacokinetic characteristics. The reference values of corresponding indexes were relatively close. Therefore, the above results also provide theoretical support for further study of the activity of compound 11b in vivo.
Conclusion
This study designed and identified a series of novel 2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol derivatives as potent PARP-1 inhibitors. We performed the MTT assay on 22 target compounds and found that compound 11b had better anti-proliferation activity and SI value on lung cancer cells. In addition, compound 11b had better PARP-1/2 enzyme activity than rucaparib. Moreover, molecular docking and molecular dynamics studies showed that compound 11b could fully occupy the active pocket and bind stably at the active site. Mechanistically, flow cytometry indicated that compound 11b could effectively induce apoptosis of A549 cells. Western blot analysis demonstrated that compound 11b could reduce the biosynthesis of PAR, and up-regulate the ratio of Cleaved-Caspase 3/Caspase 3. Afterward, the results of pharmacokinetic prediction showed that compound 11b had similar properties to rucaparib, which provided theoretical support for further study.
In conclusion, compound 11b could offer a significant guiding effect for further research on novel PARP-1 inhibitors and might open new horizons for discovering more potent PARP-1 inhibitors.
Experimental procedures
General methods
All solvents and reagents were commercially available, and were used without further purification unless stated. The progress of the reactions was monitored by thin-layer chromatography on a glass plate coated with silica gel with a fluorescent indicator (GF254, Qingdao Ocean Chemicals, China). The melting point of target compounds were detected on the RD-1 melting apparatus (Tianjin Guoming Medical Equipment Co., LTD, China). The 1 H and 13 C nuclear magnetic resonance (NMR) spectra were recorded on a model 600 Bruker Avance spectrometer (Bruker, Germany) at 600 and 150 MHz, respectively. Chemical shifts are given in parts per million (δ) referenced to DMSO-d6 at δ 2.50 for 1 H and δ 39.5 for 13 C. High-resolution mass spectra (HRMS) of target compounds were performed by a Waters Q-TOF Premier spectrometer (Waters, USA).
The synthesis of 2-(prop-2-yn-1-yl)-3,4-dihydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5(2 H)-one (compound 9 )
The preparation conditions of compound 9 had been reported in our previous study [26]. At the same time, we also gave a detailed description of it in the supplementary materials 1.
The synthesis of 2-(prop-2-yn-1-yl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (compound 10 )
Compound 9 (2.00 g, 0.007 mol) and lithium aluminum hydride (0.57 g, 0.012 mol) were added to tetrahydrofuran at -5℃, and then heated to 60℃ for 12 h to obtain compound 10. The preparation process mainly refers to the treatment method of compound 4 [26]. Detailed information was presented in the supplementary materials 1.
The synthesis of target compounds 11a-11v
The preparation process of the target compounds 11a-11v was similar to the method reported in our previous study [26, 31]. And they were also explained in detail in the supplementary materials 1.
2-((1-phenyl-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11a)
A white solid, yield: 75.91%. Mp: 164.0-164.7℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.95 (s, 1 H), 7.89 (dd, J = 8.6, 1.0 Hz, 1 H), 7.61–7.53 (m, 3 H), 7.46 (m, 3 H), 7.38–7.32 (m, 1 H), 7.25–7.17 (m, 1 H), 3.33 (s, 1 H), 2.89 (s, 2 H), 2.73 (s, 2 H), 1.64 (d, J = 11.5 Hz, 2 H), 1.45 (d, J = 10.9 Hz, 2 H), 1.22 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 147.62, 142.27, 138.46, 137.23, 134.55, 130.26, 128.81, 126.53, 123.60, 121.77, 121.21, 120.31, 69.94, 65.25, 62.45, 60.23, 40.40, 40.26, 40.12, 39.98, 39.84, 39.70, 39.56, 36.24, 31.22, 26.43, 14.55. ESI-HRMS calcd. for C24H29N4O [M + H]+ 389.2263, found: 389.2324.
2-((1-(2-fluorophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11b)
A brown solid, yield: 77.93%. Mp: 166.6-166.9℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.82–7.79 (m, 3 H), 7.42 (td, J = 7.9, 1.2 Hz, 3 H), 7.36–7.34 (m, 3 H), 2.88 (s, 1 H), 2.73–2.72 (m, 2 H), 1.98 (s, 2 H), 1.63 (d, J = 12.1 Hz, 2 H), 1.48 (m, 3 H), 1.29–1.15 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 161.22 (d, J = 238.9 Hz), 155.05, 153.39, 147.18, 146.20, 143.14, 126.53, 126.35, 125.96, 125.94, 125.11, 125.09, 117.62, 117.49, 71.00, 66.30, 62.45, 60.23, 29.70, 26.41, 22.25, 14.55. ESI-HRMS calcd. for C24H28FN4O [M + H]+ 407.2149, found: 407.2254.
2-((1-(3-fluorophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11c)
A brown solid, yield: 79.81%. Mp: 167.1-167.8℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.88–7.75 (m, 2 H), 7.63 (td, J = 8.3, 6.4 Hz, 2 H), 7.39–7.27 (m, 2 H), 7.22 (s, 3 H), 5.33 (s, 1 H), 3.34 (s, 2 H), 1.99 (s, 2 H), 1.64 (dd, J = 16.8, 9.4 Hz, 3 H), 1.48 (m, 4 H), 1.32–1.13 (m, 5 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.91 (d, J = 244.8 Hz), 147.81, 138.50, 138.43, 132.25, 132.19, 126.55, 121.99, 116.18, 116.16, 115.57, 115.43, 107.82, 107.65, 62.46, 60.23, 26.43, 22.26, 21.92, 21.23, 19.13, 14.56. ESI-HRMS calcd. for C24H28FN4O [M + H]+ 407.2149, found: 407.2247.
2-((1-(4-fluorophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11d)
A brown solid, yield: 80.10%. Mp: 166.2-166.8℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.97–7.91 (m, 3 H), 7.42–7.37 (m, 3 H), 7.32 (d, J = 8.3 Hz, 1 H), 7.19 (d, J = 6.4 Hz, 2 H), 2.87 (s, 1 H), 2.72 (s, 2 H), 1.97 (s, 2 H), 1.62 (d, J = 11.5 Hz, 2 H), 1.45 (m, 3 H), 1.28–1.12 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 161.91 (d, J = 245.7 Hz), 147.66, 145.23, 143.12, 133.81, 133.79, 126.52, 122.56, 122.50, 121.94, 117.08, 116.92, 62.46, 60.21, 36.20, 31.18, 26.43, 22.27, 21.15, 14.49. ESI-HRMS calcd. for C24H28FN4O [M + H]+ 407.2149, found: 407.2234.
2-((1-(o-tolyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11e)
An off-white solid, yield: 76.19%. Mp: 165.2–166.0℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.48–7.43 (m, 3 H), 7.38 (m, 4 H), 7.21 (s, 2 H), 2.89 (s, 1 H), 2.73 (s, 2 H), 2.13 (s, 2 H), 1.99 (s, 3 H), 1.63 (d, J = 11.8 Hz, 2 H), 1.45 (s, 2 H), 1.29–1.15 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.77, 154.71, 146.63, 143.37, 140.10, 136.95, 135.52, 133.43, 131.74, 130.03, 128.89, 127.37, 126.43, 125.27, 73.04, 69.93, 62.46, 60.23, 36.25, 31.23, 26.43, 17.89, 14.55. ESI-HRMS calcd. for C25H31N4O [M + H]+ 403.2420, found: 403.2481.
2-((1-(m-tolyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11f)
An off-white solid, yield: 78.23%. Mp: 167.3-168.2℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.74–7.67 (m, 3 H), 7.41 (t, J = 7.8 Hz, 2 H), 7.33 (d, J = 7.9 Hz, 1 H), 7.22 (m, 3 H), 2.87 (s, 1 H), 2.73 (s, 2 H), 2.38 (s, 2 H), 1.97 (s, 3 H), 1.63 (d, J = 10.9 Hz, 2 H), 1.46 (m, 2 H), 1.30–1.12 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 161.75, 154.93, 146.57, 144.03, 142.22, 138.98, 136.25, 132.61, 129.01, 128.36, 125.53, 120.65, 119.70, 116.39, 68.95, 64.05, 61.48, 59.25, 35.24, 30.22, 25.49, 20.38, 13.54. ESI-HRMS calcd. for C25H31N4O [M + H]+ 403.2420, found: 403.2480.
2-((1-(p-tolyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11 g)
An off-white solid, yield: 77.22%. Mp: 164.3-165.2℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.76 (d, J = 8.4 Hz, 1 H), 7.34 (t, J = 9.0 Hz, 4 H), 7.20 (s, 4 H), 2.87 (s, 1 H), 2.74–2.71 (m, 2 H), 2.35 (s, 3 H), 1.98 (s, 2 H), 1.63 (d, J = 11.6 Hz, 2 H), 1.46 (m, 3 H), 1.29–1.13 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 161.72, 154.63, 151.99, 146.46, 143.42, 141.39, 137.30, 133.99, 129.54, 125.48, 120.56, 119.14, 69.13, 64.76, 61.40, 59.19, 35.20, 30.19, 25.41, 19.98, 13.51. ESI-HRMS calcd. for C25H31N4O [M + H]+ 403.2420, found: 403.2492.
2-((1-(2-chlorophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11 h)
A yellow solid, yield: 80.17%. Mp: 167.9-168.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.37–7.26 (m, 5 H), 7.20 (s, 2 H), 6.84 (dd, J = 8.1, 1.4 Hz, 2 H), 2.88 (s, 1 H), 2.72 (s, 2 H), 1.98 (s, 2 H), 1.64 (d, J = 11.7 Hz, 2 H), 1.47 (m, 2 H), 1.20 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.77, 158.88, 147.48, 146.06, 143.18, 138.22, 131.11, 126.52, 121.73, 119.03, 115.76, 110.71, 107.24, 103.45, 70.17, 65.78, 62.44, 60.23, 36.25, 31.23, 26.43, 14.55. ESI-HRMS calcd. for C24H28ClN4O [M + H]+ 423.1873, found: 423.1952.
2-((1-(3-chlorophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11i)
A yellow solid, yield: 81.23%. Mp: 168.1-168.8℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.97–7.90 (m, 3 H), 7.61 (t, J = 8.1 Hz, 2 H), 7.54 (m, 2 H), 7.39–7.33 (m, 1 H), 7.22 (m, 1 H), 2.89 (s, 1 H), 2.75–2.71 (m, 2 H), 1.99 (s, 2 H), 1.66 (d, J = 12.0 Hz, 2 H), 1.48 (s, 3 H), 1.30–1.15 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.78, 158.75, 154.61, 151.73, 147.81, 143.71, 138.26, 134.62, 132.03, 128.62, 126.55, 122.02, 120.04, 118.87, 70.17, 65.26, 62.46, 60.23, 36.26, 31.24, 26.43, 14.56. ESI-HRMS calcd. for C24H28ClN4O [M + H]+ 423.1873, found: 423.1960.
2-((1-(4-chlorophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11j)
A yellow solid, yield: 81.91%. Mp: 165.6-166.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.98–7.91 (m, 3 H), 7.64–7.58 (m, 3 H), 7.36–7.30 (m, 1 H), 7.24–7.17 (m, 2 H), 2.87 (s, 1 H), 2.73 (s, 2 H), 1.97 (s, 2 H), 1.62 (d, J = 11.9 Hz, 2 H), 1.43 (m, 4 H), 1.30–1.12 (m, 6 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.71, 159.30, 153.54, 147.82, 145.01, 142.96, 136.02, 133.03, 130.15, 126.50, 121.87, 121.77, 62.43, 60.20, 54.89, 50.19, 36.20, 31.17, 26.44, 14.49. ESI-HRMS calcd. for C24H28ClN4O [M + H]+ 423.1873, found: 423.1948.
2-((1-(2-methoxyphenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11k)
A light yellow solid, yield: 82.38%. Mp: 171.1-171.9℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.61–7.57 (m, 2 H), 7.53–7.49 (m, 2 H), 7.37–7.33 (m, 1 H), 7.29 (dd, J = 8.4, 0.9 Hz, 1 H), 7.21 (d, J = 3.3 Hz, 1 H), 7.13 (dd, J = 7.7, 1.1 Hz, 2 H), 3.83–3.80 (m, 2 H), 2.89 (s, 1 H), 2.73 (d, J = 0.4 Hz, 2 H), 1.99 (s, 3 H), 1.68–1.61 (m, 3 H), 1.48 (m, 4 H), 1.31–1.15 (m, 5 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.76, 151.96, 146.31, 143.41, 139.02, 134.86, 130.89, 126.50, 126.39, 126.05, 125.49, 121.30, 116.92, 113.43, 63.56, 62.43, 60.23, 56.54, 36.24, 26.43, 22.25, 21.22, 14.55. ESI-HRMS calcd. for C25H31N4O2 [M + H]+ 419.2369, found: 419.2440.
2-((1-(3-methoxyphenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11 L)
A light yellow solid, yield: 83.44%. Mp: 172.3-174.1℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.50–7.42 (m, 3 H), 7.34 (d, J = 8.7 Hz, 2 H), 7.20 (d, J = 4.3 Hz, 2 H), 7.01 (m, 2 H), 3.83 (s, 3 H), 2.87 (s, 1 H), 2.72 (s, 2 H), 1.97 (s, 2 H), 1.63 (d, J = 12.2 Hz, 2 H), 1.46 (m, 2 H), 1.28–1.13 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 161.75, 159.64, 153.62, 146.55, 142.73, 137.32, 133.64, 130.16, 125.53, 120.86, 116.78, 113.43, 111.29, 104.94, 63.74, 61.48, 59.23, 55.03, 35.23, 30.21, 25.45, 20.20, 13.53. ESI-HRMS calcd. for C25H31N4O2 [M + H]+ 419.2369, found: 419.2435.
2-((1-(4-methoxyphenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11 m)
A light yellow solid, yield: 83.66%. Mp: 170.2-172.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.79 (d, J = 9.0 Hz, 1 H), 7.36–7.33 (m, 3 H), 7.21 (d, J = 4.0 Hz, 4 H), 7.10 (d, J = 9.0 Hz, 1 H), 3.82 (s, 3 H), 2.89 (s, 2 H), 2.73 (s, 2 H), 1.98 (s, 1 H), 1.68–1.61 (m, 2 H), 1.46 (s, 2 H), 1.30–1.14 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.76, 159.49, 152.60, 150.11, 141.50, 138.31, 130.71, 129.13, 124.73, 121.96, 117.57, 115.22, 65.49, 62.43, 60.23, 55.99, 36.24, 31.22, 19.13, 14.55, 14.02. ESI-HRMS calcd. for C25H31N4O2 [M + H]+ 419.2369, found: 419.2410.
2-((1-(2-bromophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11n)
A brown solid, yield: 79.22%. Mp: 168.2-169.8℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.87 (d, J = 7.7 Hz, 1 H), 7.64–7.49 (m, 4 H), 7.40–7.33 (m, 2 H), 7.27–7.18 (m, 2 H), 3.47 (s, 1 H), 3.37 (s, 2 H), 1.98 (s, 2 H), 1.64 (d, J = 11.7 Hz, 2 H), 1.47 (s, 2 H), 1.22 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 146.61, 144.75, 142.43, 139.05, 136.91, 134.02, 132.10, 129.30, 129.05, 126.50, 125.74, 122.79, 120.45, 119.23, 70.09, 67.01, 62.48, 60.21, 26.47, 22.29, 21.18, 14.52. ESI-HRMS calcd. for C24H28BrN4O [M + H]+ 467.1368, found: 467.1435.
2-((1-(3-bromophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11o)
A brown solid, yield: 80.17%. Mp: 169.3-170.2℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.80–7.75 (m, 2 H), 7.69–7.63 (m, 3 H), 7.39–7.32 (m, 2 H), 7.26–7.17 (m, 2 H), 2.89 (s, 1 H), 2.73 (s, 2 H), 1.99 (s, 2 H), 1.65 (d, J = 9.6 Hz, 2 H), 1.47 (s, 2 H), 1.30–1.15 (m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.76, 147.81, 138.34, 136.02, 133.14, 132.23, 131.53, 130.22, 126.54, 122.89, 122.75, 121.97, 119.24, 118.84, 69.64, 65.78, 62.45, 60.23, 36.25, 31.23, 21.23, 14.55. ESI-HRMS calcd. for C24H28BrN4O [M + H]+ 467.1368, found: 467.1442.
2-((1-(4-bromophenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11p)
A brown solid, yield: 79.86%. Mp: 167.1-166.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.95 (s, 1 H), 7.88 (d, J = 8.8 Hz, 2 H), 7.76 (d, J = 8.8 Hz, 2 H), 7.37–7.30 (m, 2 H), 7.25–7.16 (m, 2 H), 2.88 (s, 1 H), 2.72 (s, 2 H), 1.98 (s, 2 H), 1.63 (d, J = 12.5 Hz, 2 H), 1.45 (s, 3 H), 1.28–1.12 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.75, 147.83, 143.00, 138.28, 136.42, 133.13, 132.20, 130.21, 126.53, 122.19, 121.80, 121.38, 69.95, 65.54, 62.44, 60.22, 36.24, 31.22, 26.44, 14.54. ESI-HRMS calcd. for C24H28BrN4O [M + H]+ 467.1368, found: 467.1447.
2-((1-(2-hydroxyphenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11q)
A brown solid, yield: 83.42%. Mp: 156.8-157.2℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.51 (m, 2 H), 7.37–7.34 (m, 2 H), 7.30 (dd, J = 8.4, 0.9 Hz, 2 H), 7.21 (s, 1 H), 7.13 (m, 2 H), 3.83 (s, 1 H), 2.69 (s, 2 H), 1.99 (s, 2 H), 1.64 (d, J = 12.3 Hz, 2 H), 1.48 (m, 3 H), 1.22 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.78, 158.46, 151.99, 146.30, 140.84, 135.62, 130.92, 126.51, 126.38, 126.09, 125.53, 121.31, 118.21, 113.45, 66.32, 62.43, 60.23, 56.56, 36.26, 31.24, 26.42, 14.56. ESI-HRMS calcd. for C24H29N4O2 [M + H]+ 405.2212, found: 405.2276.
2-((1-(3-hydroxyphenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11r)
A brown solid, yield: 82.94%. Mp: 157.4-158.6℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.37–7.23 (m, 5 H), 7.23–7.10 (m, 4 H), 2.89 (s, 1 H), 2.73 (s, 2 H), 1.99 (s, 2 H), 1.65 (m, 2 H), 1.27–1.16 (m, 6 H), 0.93–0.83 (m, 4 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.77, 150.59, 143.14, 140.09, 138.43, 132.00, 129.14, 127.23, 121.37, 121.35, 115.93, 113.53, 113.47, 109.09, 74.12, 65.51, 65.49, 56.61, 36.25, 31.23, 30.47, 19.13. ESI-HRMS calcd. for C24H29N4O2 [M + H]+ 405.2212, found: 405.2278.
2-((1-(4-hydroxyphenyl)-1 H-1,2,3-triazol-4-yl)methyl)-2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol (11s)
A brown solid, yield: 84.00%. Mp: 171.1-172.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.68–7.64 (m, 3 H), 7.38–7.32 (m, 3 H), 7.21 (s, 3 H), 6.95–6.89 (m, 3 H), 2.89 (s, 1 H), 2.75–2.71 (m, 2 H), 1.99 (s, 2 H), 1.64 (d, J = 11.9 Hz, 2 H), 1.47 (m, 5 H), 1.27 (m, 5 H); 13 C NMR (150 MHz, DMSO-d6) δ 157.95, 153.51, 147.22, 143.41, 138.28, 133.26, 129.44, 126.51, 122.17, 121.64, 119.26, 116.40, 66.00, 62.42, 60.23, 54.05, 36.25, 26.43, 22.26, 14.56. ESI-HRMS calcd. for C24H29N4O2 [M + H]+ 405.2212, found: 405.2290.
2-(4-((5-hydroxy-4,5-dihydrospiro[benzo[c]azepine-1,1’-cyclohexan]-2(3 H)-yl)methyl)-1 H-1,2,3-triazol-1-yl)benzonitrile (11t)
A brown solid, yield: 71.91%. Mp: 161.1-162.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 8.10 (dd, J = 7.8, 1.0 Hz, 2 H), 7.96–7.91 (m, 4 H), 7.86 (d, J = 7.9 Hz, 1 H), 7.73 (t, J = 7.6 Hz, 2 H), 2.88 (s, 1 H), 2.72 (s, 2 H), 1.97 (s, 2 H), 1.63 (d, J = 11.6 Hz, 2 H), 1.47 (m, 4 H), 1.22 (m, 4 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.75, 154.38, 147.59, 143.18, 138.48, 135.19, 135.11, 132.81, 130.41, 126.54, 125.99, 124.66, 119.80, 116.41, 107.29, 66.30, 62.47, 60.22, 56.18, 36.23, 31.21, 26.43, 14.53. ESI-HRMS calcd. for C26H27N5O [M + H]+ 424.2216, found: 414.2278.
3-(4-((5-hydroxy-4,5-dihydrospiro[benzo[c]azepine-1,1’-cyclohexan]-2(3 H)-yl)methyl)-1 H-1,2,3-triazol-1-yl)benzonitrile (11u)
A brown solid, yield: 72.72%. Mp: 165.4-166.3℃. 1 H NMR (600 MHz, DMSO-d6) δ 7.95 (s, 1 H), 7.88 (d, J = 7.7 Hz, 3 H), 7.74 (t, J = 8.0 Hz, 3 H), 7.31 (d, J = 7.8 Hz, 2 H), 2.87 (s, 2 H), 2.72 (s, 2 H), 1.96 (s, 1 H), 1.63 (d, J = 11.1 Hz, 2 H), 1.46 (m, 3 H), 1.20 (m, 7 H); 13 C NMR (150 MHz, DMSO-d6) δ 162.68, 155.89, 148.03, 145.22, 143.17, 141.06, 137.61, 132.20, 131.54, 126.50, 124.62, 123.38, 121.88, 118.28, 113.20, 62.43, 60.19, 56.40, 52.75, 26.45, 22.28, 21.10, 14.45. ESI-HRMS calcd. for C26H27N5O [M + H]+ 424.2216, found: 414.2270.
4-(4-((5-hydroxy-4,5-dihydrospiro[benzo[c]azepine-1,1’-cyclohexan]-2(3 H)-yl)methyl)-1 H-1,2,3-triazol-1-yl)benzonitrile (11v)
A brown solid, yield: 75.06%. Mp: 172.6-173.2℃. 1 H NMR (600 MHz, DMSO-d6) δ 8.15 (d, J = 8.7 Hz, 3 H), 8.07 (d, J = 8.8 Hz, 3 H), 7.36–7.32 (m, 3 H), 7.21 (s, 3 H), 2.89 (s, 1 H), 2.72 (d, J = 12.7 Hz, 2 H), 1.98 (s, 2 H), 1.64 (d, J = 11.7 Hz, 2 H), 1.47 (m, 2 H), 1.21 m, 8 H); 13 C NMR (150 MHz, DMSO-d6) δ 153.33, 147.12, 142.66, 139.06, 136.15, 133.63, 129.94, 125.51, 121.00, 119.61, 117.60, 114.07, 110.09, 61.42, 59.18, 54.06, 47.85, 30.15, 25.38, 21.22, 13.50. ESI-HRMS calcd. for C26H27N5O [M + H]+ 424.2216, found: 414.2289.
Cell lines and cell culture
A549, OVCAR-3, HCT-116, and MCF-7 were purchased from American type culture collection (ATCC, USA). All cell lines were cultured in RPMI-1640 (Beijing Thermo Fisher Scientific Company, China), supplemented with 10% FBS at 37 °C and 5% CO2. And all cell lines used in the experiment were tested for mycoplasma contamination every two weeks.
MTT assay
A549, OVCAR-3, HCT-116, and MCF-7 cells were seeded in 96-well plates, and then treated with target compounds for 72 h. Then, 20 µL MTT (5 mg/mL, in PBS) was added into each well, and dissolved in 150 µL DMSO after incubation for 4 h. Finally, the absorbance was measured with microplate reader (490 nm).
Enzyme inhibitory activity assay
The PARP-1 and PARP-2 inhibition assays were performed by the colorimetric 96-well PARP assay kits provided by BPS Bioscience, USA. (Catalog No. 80,580, 80,581)
Molecular docking and molecular dynamics study
Molecular docking studies of compound 11b were carried out as previously reported [31, 32]. The three-dimensional structure of PARP-1 was retrieved from the Protein Data Bank (PDB code: 4BJC).
In the docking results, the binding conformations from the molecular docking results were extracted, and preserved for further molecular dynamics (MD) simulation. We then analyzed the binding affinity and stability in Groningen Machine for Chemicals Simulations (GROMACS) and VMD (visual molecular dynamics, version 1.9.3) [36].
Apoptosis analysis
A549 cells were cultured in 6-well plates (3.0 × 105/well), and treated with DMSO (1%) or compound 11b for 48 h. The cells were then collected, washed with PBS, and stained with FITC-Annexin-V and PI (Promega Corporation, USA). Finally, the apoptosis of A549 cells was detected by flow cytometry (Becton Dickinson and Company, USA; FACS Calibur).
Western blotting
The cells were plated in 6-well plates and incubated with different concentrations of compound 11b for a specified time. The cells were then collected, and tested using a standard western blot as described before. [26, 31, 32]
Prediction of ADMET properties
The properties of compound 11b were predicted and analyzed using the admetSAR and SwissADME prediction website (http://lmmd.ecust.edu.cn/admetsar2/, and http://www.swissadme.ch/) [36].
Statistical analysis
All results were presented as the means ± SD. The statistical significance of differences was determined using Student’s t-test, and one-way analysis of variance (ANOVA) was used. And, Tukey’s in post hoc analysis was applied. Data were analyzed with Prism 9.0 (Graph Pad Software, San Diego, CA, USA). P < 0.05 were considered statistically significant.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hunia J, Gawalski K, Szredzka A, Suskiewicz MJ, Nowis D. The potential of PARP inhibitors in targeted cancer therapy and immunotherapy, Front Mol Biosci 9 (2022).
Wu K, Chen M, Peng X, Li Y, Tang G, Peng J, Cao X. Recent progress in the Research on Benzimidazole PARP-1 inhibitors. Mini Rev Med Chem. 2022;22:2438–62.
Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond, Nat. Rev Cancer. 2010;10:293–301.
Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13:411–24.
Herlina T, Gaffar S, Widowati W. Cytotoxic activity of erypogein d from erythrina poeppigiana (leguminosae) against Cervical cancer (HeLa), Breast cancer (MCF-7) and Ovarian cancer (SKOV-3) cells, in: J Phys Conf Ser 1013 (2018).
Samaga KKL, Rao GV, Chandrashekara Reddy G, Kush AK, Diwakar L. Synthetic racemates of abyssinone i and II induces apoptosis through mitochondrial pathway in human cervix carcinoma cells. Bioorg Chem. 2014;56:54–61.
Shanta A, Bernard FJ, Japheth OO, Runner RTM, Ephraim TG. Effect of erythrinaline alkaloids from Erythrina lysistemon on human recombinant caspase-3. Afr J Pharm Pharmacol. 2018;12:183–7.
Kumar S, Pathania AS, Saxena AK, Vishwakarma RA, Ali A, Bhushan S. The anticancer potential of flavonoids isolated from the stem bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signaling pathway in human Leukemia HL-60 cells. Chem Biol Interact. 2013;205:128–37.
Iranshahi M, Vu H, Pham N, Zencak D, Forster P, Quinn RJ. Cytotoxic evaluation of alkaloids and isoflavonoids from the Australian tree erythrina vespertilio. Planta Med. 2012;78:730–6.
Qing Z-X, Huang J-L, Yang X-Y, Liu J-H, Cao H-L, Xiang F, Cheng P. Zeng, Anticancer and reversing Multidrug Resistance activities of Natural Isoquinoline alkaloids and their structure-activity relationship. Curr Med Chem. 2017;25:5088–114.
Yu L, wei Yan Z, de Wang Y, Miao H, Zhao Jyi, Pang C, Li S. Recent advances in structural types and medicinal chemistry of PARP-1 inhibitors. Med Chem Res. 2022;31:1265–76.
Anscher MS, Chang E, Gao X, Gong Y, Weinstock C, Bloomquist E, Adeniyi O, Charlab R, Zimmerman S, Serlemitsos-Day M, Ning YM, Mayrosh R, Fuller B, Trentacosti AM, Gallagher P, Bijwaard K, Philip R, Ghosh S, Fahnbulleh F, Diggs F, Arora S, Goldberg KB, Tang S, Amiri-Kordestani L, Pazdur R, Ibrahim A, Beaver JA. FDA approval Summary: Rucaparib for the treatment of patients with deleterious BRCA-Mutated metastatic castrate-resistant Prostate Cancer, oncologist. 26 (2021) 139–46.
He X, Li XY, Liang JW, Cao C, Li S, Zhang TJ, Meng FH. Design, synthesis and anticancer activities evaluation of novel 5H-dibenzo[b,e]azepine-6,11-dione derivatives containing 1,3,4-oxadiazole units, Bioorganic Med. Chem Lett. 2018;28:847–52.
Shi D, Pang Q, Qin Q, Yao X, Yao X, Yu Y. Discovery of novel anti-tumor compounds targeting PARP-1 with induction of autophagy through in silico and in vitro screening, Front Pharmacol 13 (2022).
Wei L, Wang M, Wang Q, Han Z. Dual targeting, a new strategy for novel PARP inhibitor discovery. Drug Discov Ther. 2021;15:300–9.
Ge J, Yin Y, Li Y, Deng Y, Fu H. Dual-target inhibitors based on PARP1: new trend in the development of anticancer research. Future Med Chem. 2022;14:511–25.
Hinchcliff E, Chelariu-Raicu A, Westin SN. Current and future landscape of poly (ADP-ribose) polymerase inhibition resistance. Curr Opin Obstet Gynecol. 2021;33:19–25.
Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov. 2020;19:711–36.
Zhao Y, Zhang LX, Jiang T, Long J, Ma ZY, Lu AP, Cheng Y, Cao DS. The ups and downs of poly(ADP-ribose) Polymerase-1 inhibitors in cancer therapy–current progress and future direction. Eur J Med Chem. 2020;203:112570.
Leal TA, Sharifi MN, Chan N, Wesolowski R, Turk AA, Bruce JY, O’Regan RM, Eickhoff J, Barroilhet LM, Malhotra J, Mehnert J, Girda E, Wiley E, Schmitz N, Andrews S, Liu G, Wisinski KB. A phase I study of talazoparib (BMN 673) combined with carboplatin and paclitaxel in patients with advanced solid tumors (NCI9782). Cancer Med. 2022;11:3969–81.
Wang L, Yang C, Xie C, Jiang J, Gao M, Fu L, Li Y, Bao X, Fu H, Lou L. Pharmacologic characterization of fluzoparib, a novel poly(ADP-ribose) polymerase inhibitor undergoing clinical trials. Cancer Sci. 2019;110:1064–75.
Lee A. Fuzuloparib: First Approval Drugs. 2021;81:1221–6.
Matin MM, Matin P, Rahman MR, Ben Hadda T, Almalki FA, Mahmud S, Ghoneim MM, Alruwaily M, Alshehri S. Triazoles and their derivatives: Chemistry, Synthesis, and therapeutic applications, Front Mol Biosci 9 (2022).
Fang Z, Kang D, Zhang L, Huang B, Liu H, Pannecouque C, De Clercq E, Zhan P, Liu X. Synthesis and biological evaluation of a series of 2-((1-substituted-1H-1,2,3-triazol-4-yl)methylthio)-6-(naphthalen-1-ylme thyl)pyrimidin-4(3H)-one as potential HIV-1 inhibitors. Chem Biol Drug Des. 2015;86:614–8.
Sandip G, Agalave D, Suleman R, Maujan. Dr. Vandana S. Pore., click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem Asian J. 2011;6:2696–718.
Li S, yang Li X, jian Zhang T, Kamara MO, Liang Jwei, Zhu J, Meng F. Design, synthesis and biological evaluation of homoerythrina alkaloid derivatives bearing a triazole moiety as PARP-1 inhibitors and as potential antitumor drugs, Bioorg. Chem. 94 (2020) 103385.
Chekir S, Debbabi M, Regazzetti A, Dargère D, Laprévote O, Ben Jannet H, Gharbi R. Design, synthesis and biological evaluation of novel 1,2,3-triazole linked coumarinopyrazole conjugates as potent anticholinesterase, anti-5-lipoxygenase, anti-tyrosinase and anti-cancer agents. Bioorg Chem. 2018;80:189–94.
Xu Z, Zhao S-J, Liu Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: current developments, action mechanisms and structure-activity relationships. Eur J Med Chem. 2019;183:111700.
Jain PG, Patel BD. Medicinal chemistry approaches of poly ADP-Ribose polymerase 1 (PARP1) inhibitors as anticancer agents - a recent update. Eur J Med Chem. 2019;165:198–215.
Lu G, Nie W, Xin M, Meng Y, Gu J, Miao H, Cheng X, Chan ASC, Zou Y. Design, synthesis, biological evaluation and molecular docking study of novel urea-based benzamide derivatives as potent poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors, Eur. J Med Chem. 2022;243:114790.
Li S, yang Li X, jian Zhang T, Zhu J, han Xue W, Qian X, Meng F. Design, synthesis and biological evaluation of erythrina derivatives bearing a 1,2,3-triazole moiety as PARP-1 inhibitors, Bioorg. Chem. 96 (2020) 103575.
Li S, yang Li X, jian Zhang T, Zhu J, li Liu K, pu Wang D, Meng F. Novel 4,5-dihydrospiro[benzo[c]azepine-1,1′-cyclohexan]-3(2H)-one derivatives as PARP-1 inhibitors: Design, synthesis and biological evaluation, Bioorg. Chem. 111 (2021) 104840.
Pantelidou C, Sonzogni O, Taveira MDO, Mehta AK, Kothari A, Wang D, Visal T, Li MK, Pinto J, Castrillon JA, Cheney EM, Bouwman P, Jonkers J, Rottenberg S, Guerriero JL, Wulf GM, Shapiro GI. Parp inhibitor efficacy depends on CD8 + T-cell recruitment via intratumoral sting pathway activation in brca-deficient models of triple-negative Breast cancer. Cancer Discov. 2019;9:722–37.
Ning JF, Stanciu M, Humphrey MR, Gorham J, Wakimoto H, Nishihara R, Lees J, Zou L, Martuza RL, Wakimoto H, Rabkin SD. Myc targeted CDK18 promotes ATR and homologous recombination to mediate PARP inhibitor resistance in glioblastoma. Nat Commun. 2019;10:2910.
Fang Y, McGrail DJ, Sun C, Labrie M, Chen X, Zhang D, Ju Z, Vellano CP, Lu Y, Li Y, Jeong KJ, Ding Z, Liang J, Wang SW, Dai H, Lee S, Sahni N, Mercado-Uribe I, Kim Tbeom, Chen K, Lin SY, Peng G, Westin SN, Liu J, O’Connor MJ, Yap TA, Mills GB. Sequential therapy with PARP and WEE1 inhibitors minimizes toxicity while maintaining efficacy. Cancer Cell. 2019;35:851–67.
li Liu K, yang Li X, Wang D, han Xue W, hua Qian X, heng Li Y, qi Lin Q, Li S. F. hao Meng, Novel Allosteric Inhibitors of Deoxyhypusine Synthase against Malignant Melanoma: Design, Synthesis, and Biological Evaluation, J Med Chem. (64) 2021 13356–13372.
Acknowledgements
The authors would like to thank Chengde Medical University and China Medical University for providing all equipment and resources during this work.
Funding
This work was supported by the National Natural Science Foundation of China (No.82204210), the Biological Medicine Joint Fund of Natural Science Foundation of Hebei Province (NO. H2022406062), the Funded by Science and Technology Project of Hebei Education Department (NO. QN2022161), the 2022 Research Start-up Fund for High‐level Talents of Chengde Medical University (NO. 202207), and the Chengde Medical University basic research funds special project (NO. KY202315).
Author information
Authors and Affiliations
Contributions
Conceptualization: L.Z., and S.L.; Methodology: L.Y., and J.L.; Software: Y.W., and Z.Y. Validation: L.Y., and S.L.; Data curation: L.Y.; Writing—original draft preparation: L.Y.; Project administration: J.Z.; Funding acquisition: S.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, L., Li, Jh., Zhu, J. et al. Discovery of novel 2,3,4,5-tetrahydrospiro[benzo[c]azepine-1,1’-cyclohexan]-5-ol derivatives as PARP-1 inhibitors. BMC Chemistry 17, 147 (2023). https://doi.org/10.1186/s13065-023-01060-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-01060-8