Abstract
Background
A new, green and environmentally friendly protocol has been developed for the synthesis of tetrahydrodipyrazolopyridine derivatives. The structures of these products were determined in terms of melting point, FTIR, NMR and Mass spectroscopy.
Results
The tetrahydrodipyrazolopyridine derivatives were synthesized in water through a catalyst-free pseudo-six-component reaction of hydrazine hydrate, ethyl acetoacetate, ammonium acetate and aldehyde at room temperature.
Conclusions
This novel procedure has some advantages such as aqueous media, high yield and simple work-up.
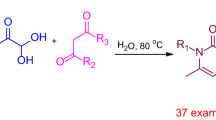
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Multicomponent reactions (MCRs) are selective, simple and effective as compared to the conventional multistep synthesis [1,2,3,4]. They have been utilized to reduce environmental pollution. Water, as a green solvent, is presumed to speed up some organic reactions through hydrophobic effects [5]. Therefore, catalyst-free organic reactions in water that yield resolvable products are attractive for many organic chemists [6, 7]. THDPPs fuse the heterocyclic moieties of pyrazolopyridine with pharmaceutical activities, which exist in Etazolate, Cartazolate and Tracazolate drugs [8] (Fig. 1).
Meanwhile, Due to the existence of two biologically active moieties, namely pyrazole and 1,4-dihydropyridine pyrazole in the structure of THDPPs, these compounds have various pharmaceutical applications such as antiallergic, anti-herpetic and anxiolytic effects [9, 10], anti-Leishmania activities [11] and HIF 1α-prolyl hydroxylase inhibition [12].
A common protocol for the synthesis of THDPP is the reaction of aldehydes, hydrazine hydrate, ethyl acetoacetate, and ammonium acetate in a multi-component manner with the presence of a catalyst. Previously, pseudopolymeric magnetic nanoparticles [13], KCC-1-NH2-DPA [14], CuFe2O4@HNTs [15], acetic acid [16], and carbonaceous material (CSO3H) [17], Fe3O4/KCC1/IL/HPWMNPs [24], Nano-CdZr4(PO4)6 [25], Nano-Fe3O4@SiO2–SO3H [23], FeNi3-ILs MNPs [21], Nano-CuCr2O4 [26], Nano-ovalbumin [20], M(II)/Schiff base@MWCNT-Fe3O4/SiO2 [27]. Meanwhile, a catalyst free protocol using ammonium carbonate instead of ammonium acetate has been reported [16], Ammonium carbonate is a basic salt and can catalysis the synthesis of THDPP’s. In here, we have used ammonium acetate as neutral salt for production of ammonia in water and absence of any catalyst.
In this study, THDPPs are synthesized with a green catalyst-free protocol implemented in a water medium at room temperature (Scheme 1).
Experimental
Materials and methods
General
A Bruker, Equinox 55 spectrometer was used to record the Fourier transforms infrared (FT-IR) spectra. A Bruker (DRX-400 Avance) nuclear magnetic resonance (NMR) instrument was also used to record the NMR spectra. In addition, a Buchi B-540 B.V.CHI apparatus served to determine the melting points of the compounds. Mass spectrometry spectra were recorded with a Agilent Technology (HP), Model: 5793, Ion source: Electron Impact (EI), 20-EV, 230 °C, and Quadrupole analyzer.
Chemistry
General procedure for the synthesis of THDPP’s
Firstly, a solution of 2.0 mmol of hydrazine hydrate, 2.0 mmol of ethyl acetoacetate and 3 mL of water was stirred in a 25-mL round-bottom flask at room temperature. Secondly, 1.0 mmol of aldehyde and 4.0 mmol of ammonium acetate were added to it and stirred at room temperature. The reaction was monitored by thin-layer chromatography (TLC; n-hexane:EtOAc, 70:30). After the completion of the reaction, the solution was diluted with cold water, and the product was appeared as water insoluble solid which isolated by simple filtration.
Results and discussion
Although, catalytic synthesis of THDPPs protocols have many advantages such as high yields of products and short reaction time. But the hard work-up and expensive catalysts are some of drawbacks of them. Therefore, we have decided to design a catalyst-free protocol for synthesis of THDPPs.
In order to optimize the reaction conditions for the preparation of THDPPs in the absence of a catalyst, some reactions were performed between ethylacetoacetate, ammonium acetate, 4-chlorobenzaldehyde and hydrazine hydrate in the presence of different solvents (Table 1). As the results indicated, tetrahydrodipyrazolopyridines could be synthesized in good-to-high yields and short reaction times.

Hydrazine and ammonium acetate are soluble in water, ethyl acetoacetate is partially soluble and aldehyde is insoluble in water. In the first step, hydrazine hydrate and ethyl acetoacetate react in water to produce a insoluble intermediate which react with aldehyde in water cage.
Regarding the conditions for the synthesis of THDPPs, the optimization process was implemented with different aldehydes, hydrazine hydrate, ethyl acetoacetate and ammonium acetate (Table 2).

Meanwhile, we have synthesis THDPP using citral as aliphatic aldehyde (Scheme 2).
A mechanism proposed for the catalyst-free synthesis of THDPPs is shown in Scheme 3. Ammonium acetate was used as a source of nitrogen for the formation of 1,4-dihydropyridine ring in the THDPPs. Hydrazine is soluble and ethylacetoacetate is partially soluble (2.86 g/100 mL) in water. Initially, the reaction was begun with the versatile condensation of ethyl acetoacetate with hydrazine and then the elimination of ethanol to form pyrazolone A (B, tautomer of A) as a water insoluble solid. The A (B) and aldehyde are hydrophobia materials and react in a water cage to form the intermediate C through the Knoevenagel condensation. Then, the second molecule of B was condensed with C via Michael reaction to produce bipyrazolone D. Ammonia, which was produced from ammonium acetate, condensed with D to form imine E which produced product F through intramolecular cyclization, tautomerization and water removal.
In order to show the superiority of the THDPPs synthesis process in the absence of catalysts, this process was compared to some others in terms of conditions, reaction time and yield. The results are listed in Table 3.

Conclusions
In this study, an environmentally friendly protocol is introduced for the synthesis of THDPPs in a neutral aqueous medium without using any catalyst or organic solvent. In this green versatile protocol hydrophobia intermediate and aldehyde had been reacted under high pressure condition in water cage. In solubility of products in water caused having a simple work-up and purification of them. The attractive advantages of the protocol are excellent yields, mild reaction conditions, less pollution, short time reaction, simple workup and high-purity products.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- THDPP’s:
-
Tetrahydrodipyrazolopyridines
- MCRs:
-
Multi-component reactions
- FT-IR:
-
Fourier transform infrared
- NMR:
-
Nuclear magnetic resonance
- TLC:
-
Thin layer chromatography
References
Ahmadi T, Ziarani GM, Gholamzadeh P, Mollabagher H. Recent advances in asymmetric multicomponent reactions (AMCRs). Tetrahedron Asymmetry. 2017;28(5):708–24.
Hulme C, Chappeta S, Griffith C, Lee Y-S, Dietrich J. An efficient solution phase synthesis of triazadibenzoazulenones:‘designer isonitrile free’methodology enabled by microwaves. Tetrahedron Lett. 2009;50(17):1939–42.
Domling A, Wang W, Wang K. Chemistry and biology of multicomponent reactions. Chem Rev. 2012;112(6):3083–135.
Gu Y. Multicomponent reactions in unconventional solvents: state of the art. Green Chem. 2012;14(8):2091–128.
Lubineau A, Augé J. Water as solvent in organic synthesis. In: Knochel P, editor. Modern solvents in organic synthesis. Topics in current chemistry, vol. 206. Berlin: Springer; 1999. p. 1–39.
Katada M, Kitahara K, Iwasa S, Shibatomi K. Catalyst-free decarboxylative fluorination of tertiary β-keto carboxylic acids. Synlett. 2018;29(18):2408–11.
Tian Y, Liu Q, Liu Y, Zhao R, Li G, Xu F. Catalyst-free Mannich-type reactions in water: expedient synthesis of naphthol-substituted isoindolinones. Tetrahedron Lett. 2018;59(15):1454–7.
Manjunatha UH, Vinayak S, Zambriski JA, Chao AT, Sy T, Noble CG, Bonamy GM, Kondreddi RR, Zou B, Gedeck P. A cryptosporidium PI (4) K inhibitor is a drug candidate for cryptosporidiosis. Nature. 2017;546(7658):376–80.
Yu G, Mason H, Wu X, Wang J, Chong S, Beyer B, Henwood A, Pongrac R, Seliger L, He B. Substituted pyrazolopyridopyridazines as orally bioavailable potent and selective PDE5 inhibitors: potential agents for treatment of erectile dysfunction. J Med Chem. 2003;46(4):457–60.
Liu C, Li Z, Zhao L, Shen L. One-step, facile synthesis of pyrazolopyridines and tetrahydropyrazolopyridines through disproportionation of initially formed pyrazolo Hantzsch dihydropyridine. ARKIVOC. 2009;2:258–68.
de Mello H, Echevarria A, Bernardino AM, Canto-Cavalheiro M, Leon LL. Antileishmanial pyrazolopyridine derivatives: synthesis and structure-activity relationship analysis. J Med Chem. 2004;47(22):5427–32.
Warshakoon NC, Wu S, Boyer A, Kawamoto R, Renock S, Xu K, Pokross M, Evdokimov AG, Zhou S, Winter C. Design and synthesis of a series of novel pyrazolopyridines as HIF 1-α prolyl hydroxylase inhibitors. Bioorg Med Chem Lett. 2006;16(21):5687–90.
Dashteh M, Yarie M, Zolfigol MA, Khazaei A, Makhdoomi S. Novel pseudopolymeric magnetic nanoparticles as a hydrogen bond catalyst for the synthesis of tetrahydrodipyrazolopyridine derivatives under mild reaction conditions. Appl Organomet Chem. 2021;35(6):e6222.
Azizi S, Shadjou N, Hasanzadeh M. KCC-1-NH2-DPA: an efficient heterogeneous recyclable nanocomposite for the catalytic synthesis of tetrahydrodipyrazolopyridines as a well-known organic scaffold in various bioactive derivatives. Nanocomposites. 2019;5(4):124–32.
Maleki A, Hajizadeh Z, Salehi P. Mesoporous halloysite nanotubes modified by CuFe2O4 spinel ferrite nanoparticles and study of its application as a novel and efficient heterogeneous catalyst in the synthesis of pyrazolopyridine derivatives. Sci Rep. 2019;9(1):1–8.
Ghaedi A, Bardajee G, Mirshokrayi A, Mahdavi M, Shafiee A, Akbarzadeh T. Facile, novel and efficient synthesis of new pyrazolo [3,4-b] pyridine products from condensation of pyrazole-5-amine derivatives and activated carbonyl groups. RSC Adv. 2015;5(109):89652–8.
Chen Z, Shi Y, Shen Q, Xu H, Zhang F. Facile and efficient synthesis of pyrazoloisoquinoline and pyrazolopyridine derivatives using recoverable carbonaceous material as solid acid catalyst. Tetrahedron lett. 2015;56(33):4749–52.
Zhao K, Lei M, Ma L, Hu L. A facile protocol for the synthesis of 4-aryl-1, 4, 7, 8-tetrahydro-3, 5-dimethyldipyrazolo [3, 4-b: 4′, 3′-e] pyridine derivatives by a Hantzsch-type reaction. Monatsh Chem. 2011;142(11):1169–73.
Mirjalili BF, Jalili Bahabadi N, Bamoniri A. Triethanolamine–sodium acetate as a novel deep eutectic solvent for promotion of tetrahydrodipyrazolopyridines synthesis under microwave irradiation. J Iran Chem Soc. 2021;18:1–7.
Salehi N, Mirjalili BF. Nano-ovalbumin: a green biocatalyst for biomimetic synthesis of tetrahydrodipyrazolo pyridines in water. Res Chem Intermed. 2018;44(11):7065–77.
Safaei-Ghomi J, Sadeghzadeh R, Shahbazi-Alavi H. A pseudo six-component process for the synthesis of tetrahydrodipyrazolo pyridines using an ionic liquid ilized on a FeNi3 nanocatalyst. RSC Adv. 2016;6(40):33676–85.
Shabalala NG, Pagadala R, Jonnalagadda SB. Ultrasonic-accelerated rapid protocol for the improved synthesis of pyrazoles. Ultrason Sonochem. 2015;27:423–9.
Safaei-Ghomi J, Shahbazi-Alavi H. A flexible one-pot synthesis of pyrazolopyridines catalyzed by Fe3O4@SiO2–SO3H nanocatalyst under microwave irradiation. Sci Iran. 2017;24(3):1209–19.
Sadeghzadeh SM. A heteropolyacid-based ionic liquid immobilized onto magnetic fibrous nano-silica as robust and recyclable heterogeneous catalysts for the synthesis of tetrahydrodipyrazolopyridines in water. RSC Adv. 2016;6(79):75973–80.
Chioua M, Samadi A, Soriano E, Lozach O, Meijer L, Marco-Contelles J. Synthesis and biological evaluation of 3, 6-diamino-1H-pyrazolo [3, 4-b] pyridine derivatives as protein kinase inhibitors. Bioorg Med Chem Lett. 2009;19(16):4566–9.
Shahbazi-Alavi H, Safaei-Ghomi J, Eshteghal F, Zahedi S, Nazemzadeh SH, Alemi-Tameh F, Tavazo M, Basharnavaz H, Lashkari MR. Nano-CuCr2O4: an efficient catalyst for a one-pot synthesis of tetrahydrodipyrazolopyridine. J Chem Res. 2016;40(6):361–3.
Lashanizadegan M, Nikoofar K, Aghaei A, Mehrikaram F, Mirzazadeh H. Immobilized Cu(II) and Co(II) Schiff base complexes on the surface of functionalized magnetized multiwalled carbon nanotubes: Novel catalysts for oxidation and solvent-free pseudo six-component condensation reaction. Solid State Sci. 2019;95:105937.
Acknowledgements
The Research Council of Yazd University gratefully acknowledged for the financial support for this work.
Funding
This study was financially supported by Yazd University. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
M.K. wrote the main manuscript and prepared figures. B.F.M edited the manuscript and submit it as corresponding author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Spectroscopic data for the synthesized tetrahydrodipyrazolo[3,4-b:4′,3′-e] pyridine derivatives.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Keihanfar, M., Mirjalili, B.B.F. Catalyst-free synthesis of tetrahydrodipyrazolopyridines via an one-pot tandem and green pseudo-six-component reaction in water. BMC Chemistry 16, 9 (2022). https://doi.org/10.1186/s13065-022-00802-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-022-00802-4