Abstract
Background
Fulminant myocarditis (FM) is a form of severe inflammatory carditis with rapidly developing acute heart failure.
Case presentation
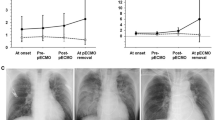
We report three cases of successful intensive treatment by Impella of FM without any complications. In all cases, impairment of microcirculation as measured by blood lactate level and the hemodynamic value as indicated by cardiac index were improved within 24–48 h and 7 days after Impella implantation, respectively. Interestingly, our data also suggested that treatment by Impella CP or 5.0 may lead to faster recovery of microcirculation and cardiac function than treatment by Impella 2.5.
Conclusion
Our findings demonstrate that the appropriate selection of Impella devices guided by body surface area measurements may help to improve clinical outcomes of severe heart failure including FM.
Similar content being viewed by others
Background
Percutaneous left ventricular assist device “Impella” (Abiomed, Danvers, MA) is a microaxial pump system [1], which can be inserted without thoracotomy and was first covered by health insurance in September 2017 in Japan. Impella has since been considered a highly regarded treatment strategy due to its utility of circulatory support [2, 3]. We have previously demonstrated findings from the first case using Impella in Japan to a patient who suffered from ventricular septal perforation after acute myocardial infarction [4]. In our case series, we report three cases of Impella to patients with fulminant myocarditis (FM), severe inflammatory carditis with rapidly developing acute heart failure, cardiogenic shock, and fatal arrhythmia.
Case presentation
Case 1
A 22-year-old man presented fever that had begun 5 days before and had taken antibiotics (Table 1). The patient had no past medical history. On admission, electrocardiogram showed severe tachycardia (Fig. 1A), and a blood test showed high level of lactate (Table 2). Transthoracic echocardiography (TTE) revealed impaired left ventricular function (left ventricular ejection fraction (LVEF): 15%) (Table 3). In addition, Coxsackievirus A was detected by blood culture. The patient was transferred to our hospital for detailed analysis and treatment of fulminant myocarditis. Due to unstable vital signs under high doses of inotropic agents, Impella CP (assist flow: 2.439 L/min/m2) was implanted to the right femoral artery (Table 1). All the hemodynamic values improved within the first 48 h of Impella support. After Impella implantation, extracorporeal membrane oxygenation (ECMO) was inserted because of impaired right ventricular function. Immediately after treatment of Impella and ECMO, the level of lactate significantly improved. Inotropic agents were reduced gradually, and ECMO was removed on the 5th day after insertion, and Impella was removed after another 5 days (Fig. 2A). LVEF improved to 52% after Impella removal (Table 3). The recovery of symptoms and postoperative blood tests progressed satisfactorily. The patient was discharged on day 19 without serious complications.
Case 2
A 16-year-old man with no past medical history presented sudden fever, chest pain, and loss of consciousness, resulting in cardiopulmonary arrest (Table 1). The patient was transferred to another hospital in a state of cardiopulmonary arrest. ECMO and intra-aortic balloon pump were inserted and while return of spontaneous circulation was confirmed, ventricular tachycardia (VT) was not controlled (Fig. 1B). The patient was transferred to our hospital for a more detailed analysis. A blood test on admission showed high level of creatinine kinase and lactate (Table 2). TTE revealed impaired left ventricular function (LVEF: 14%) (Table 3). Despite high doses of inotropic agents, VT storm was difficult to control. In addition, Coxsackievirus A4 was detected by blood culture, and the patient was diagnosed with fulminant myocarditis. Due to unstable vital signs and drug-resistance arrhythmia, Impella 5.0 (assist flow: 2.793 L/min/m2) was implanted via prosthetic graft to the right axillary artery after removal of intra-aortic balloon pumping and insert of ECMO (Tables 1, 2, 3). All the hemodynamic values and lactate levels improved within the first 48 h of Impella support. Impella was removed 5 days after implantation (Fig. 2B). LVEF improved to 59% after Impella removal (Table 3). The patient started cardiac rehabilitation and was discharged on day 23 without serious complications.
Case 3
A 68-year-old man with no medical history presented cold-like symptoms and mild breathlessness that had begun a couple of days before; however, the symptoms did not improve. Initially suspected of asthma and hepatic dysfunction, the patient was admitted to our hospital (Table 2). TTE on admission revealed significantly impaired ventricular function (LVEF: 27%) (Table 3). Unfortunately, any causative microorganisms were not detected by blood culture, and we diagnosed fulminant myocarditis based on the clinical course and examination findings. Because the patient was in cardiogenic shock on the first day of admission, Impella 2.5 (assist flow: 1.722 L/min/m2) was implanted to the right femoral artery (Table 1). ECMO was used because the hemodynamics supported by Impella and recovery of peripheral circulation were insufficient (Fig. 2C). As respiratory condition gradually improved, ECMO and Impella were removed on day 3 and 7, respectively. LVEF improved to 46% after Impella removal (Table 3). The patient started cardiac rehabilitation and was discharged on day 59 without serious complications.
Discussion
FM is a well-known severe inflammatory myocardial disease with rapidly developing heart failure, cardiac shock, and life-threatening arrhythmia. Generally, FM requires intensive treatments including mechanical circulatory support, and heart transplantation in some cases [5]. We describe three cases of successful intensive treatment by Impella of fulminant myocarditis without any complications. At the time of cardiogenic shock on admission, right ventricular function in all cases were relatively normal; however, indication for ECMO was required because of the presentation of right ventricular failure or deterioration of respiratory condition in the clinical course of all cases. Although there is a slight time gap, impairment of microcirculation with blood lactate level as an index was improved within 24–48 h. Additionally, the hemodynamic value with cardiac index (CI) as an index was improved within 7 days after Impella implantation. Although a direct comparison cannot be made, our data suggested that a case treated by Impella 2.5 may have slower recovery of microcirculation than other cases treated by Impella CP or 5.0. Additionally, the case treated by Impella 2.5 had the longest hospital stay (59 days). As one of the possibilities, the poor outcomes by Impella 2.5 may be a result of body surface area (BSA) mismatch and consequently insufficient unload. To maintain 2.2 L/min/m2 of CI needed to improve heart failure, a proper Impella device based on BSA should be selected. For example, in cases with < 1.14 of BSA, 1.15–1.6, and > 1.6, Impella 2.5, CP, and 5.0 need to be used for securing 2.2 L/min/m2 of CI, respectively. Our data demonstrated that Case 1 with Impella CP (BSA 1.44) and Case 2 with Impella 5.0 (BSA 1.80) had satisfactory improvement of cardiac function. However, Case 3 (BSA 1.45) treated by Impella 2.5 had slower recovery of cardiac function (Fig. 2C). Taking into account the degree of unloading in this case, Impella 5.0 may have been the more advisable choice. Nevertheless, the fact that Impella 5.0 takes longer for implantation is also worth consideration in these cases. Taken together, this case series may be a good implication when we wonder which device to indicate in patients with a small physique.
Conclusion
Our findings regarding three cases of Impella treatment to patients with FM indicate that the proper selection of Impella devices according to BSA measurements may help to improve clinical outcomes of severe heart failure including FM.
Availability of data and materials
The datasets used are available from the corresponding author on reasonable request.
Abbreviations
- ECMO:
-
Extracorporeal membrane oxygenation
- LVEF:
-
Left ventricular ejection fraction
- FM:
-
Fulminant myocarditis
- TTE:
-
Transthoracic echocardiography
- VT:
-
Ventricular tachycardia
References
Garatti A, Colombo T, Russo C, Lanfranconi M, Milazzo F, Catena E, et al. Different applications for left ventricular mechanical support with the Impella Recover 100 microaxial blood pump. J Heart Lung Transplant. 2005;24:481–5.
Strecker T, Fischlein T, Pfeiffer S. Impella Recover 100: successful perioperative support for off pump coronary artery bypass grafting surgery in a patient with end-stage ischemic cardiomyopathy. J Cardiovasc Surg. 2004;45:381–4.
Iida M, Shimokawa T. Impella®, percutaneous left ventricular assist device for cardiogenic shock: our experiences. J Coron Artery Dis. 2021;27:1–6.
Iida M, Uchiyama M, Shimokawa T. A successful case of percutaneous left ventricular assist device “Impella” to postmyocardial infarction ventricular septal perforation in Japan. Artif Organs. 2019;43:806–7.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48.
Acknowledgements
We are grateful to Mr. Kento Kawai, D. Phil, for editorial assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
T.H., M.I., M.U., and T.S. designed the study, analyzed the data and wrote the manuscript. M.I. and M.U. helped in gathering patient information and performed data analysis. M.I. and M.U. performed graphic and tables. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent for this study has been obtained from participants.
Consent for publication
All subjects enrolled in this research have given their informed consent which alongside the described protocol.
Competing interests
All authors have no conflict of interest to disclosure as described by Journal of Cardiothoracic Surgery.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hori, T., Iida, M., Uchiyama, M. et al. Successful cases of percutaneous left ventricular assist device “Impella” to fulminant myocarditis. J Cardiothorac Surg 17, 72 (2022). https://doi.org/10.1186/s13019-022-01821-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-022-01821-x