Abstract
Background
There are contradictory effects regarding the effect of NAD + precursor on glucose metabolism and liver enzymes. In order to obtain a better viewpoint from them, this study aimed to comprehensively investigate the effects of NAD + precursor supplementation on glucose metabolism, C-reactive protein (CRP), and liver enzymes.
Methods
PubMed/MEDLINE, Web of Science, SCOPUS, and Embase databases were searched using standard keywords to identify all controlled trials investigating the glucose metabolism, CRP, and liver enzymes effects of NAD + precursor. Pooled weighted mean difference (WMD) and 95% confidence intervals (95% CI) were achieved by random-effects model analysis for the best estimation of outcomes.
Results
Forty-five articles with 9256 participants’ were included in this article. The pooled findings showed that NAD + precursor supplementation had a significant increase in glucose (WMD: 2.17 mg/dL, 95% CI: 0.68, 3.66, P = 0.004) and HbA1c (WMD: 0.11, 95% CI: 0.06, 0.16, P < 0.001) as well as a significant decrease in CRP (WMD: -0.93 mg/l, 95% CI -1.47 to -0.40, P < 0.001) compared with control group, and was not statistically significant with respect to insulin and homeostasis model assessment of insulin resistance (HOMA-IR). However, we found no systemic changes in aspartate transaminase (AST), alanine transaminase (ALT), or alkaline phosphatase (ALP) levels after NAD + precursor supplementation. The results of the subgroup analysis showed that the intake of NAD + precursor during the intervention of more than 12 weeks caused a greater increase in the glucose level. Furthermore, Nicotinic acid supplementation (NA) causes a greater increase in glucose and HbA1c levels than nicotinamide (NE) supplementation.
Conclusions
Overall, these findings suggest that NAD + precursor supplementation might have an increase effect on glucose metabolism as well as a decrease in CRP.
Similar content being viewed by others
Introduction
Diabetes is a chronic and progressive disease generally characterised by high fasting blood glucose concentrations [1, 2] or changes in levels of other factors such as glycated haemoglobin (HbA1c) [3, 4], and HOMA-IR [5]. Evidence suggests that diabetes impairs the function of different organs like the heart, kidneys, eyes, and especially the liver [6,7,8,9,10]. In actuality, diabetes causes liver function to be disrupted due to the increased lipid influx into the liver and de novo lipid syntheses [11, 12], which are shown by elevated liver enzymes (AST and ALT) [13]. In addition, diabetes causes disruption in mitochondrial function [14], metabolic dysregulation [15], oxidative damage [16], and NAD + redux abnormalities [17,18,19].
According to estimates, the number of individuals over 20 years old with diabetes will rise to more than 700 million by 2045 [20]. Therefore, conducting interventional studies in order to stop the complications of diabetes seems necessary.
The NAD + precursor and related compounds are of great interest due to their therapeutic effects, especially in the treatment of hyperlipidemia [21]; the findings indicate that the NAD + precursor, which is predominantly synthesised by the salvage pathways from the recovery of nicotinamide (NE) and nicotinic acid (NA) biogenesis, is an essential metabolic cofactor in cellular metabolism [22]. Thus, maintaining the cytosolic NAD+/NADH ratio within the normal range is critical. While this ratio decreases in diabetes and is referred to as pseudohypoxia, which leads to oxidative stress [19, 23]. Yoshinno et al. also reported the depletion of NAD + in mice liver due to the accumulation of fat caused by insulin resistance and impaired glucose metabolism [24]. In recent years, studies have shown that NAD + precursors can significantly cause hyperglycemia and reduce inflammation [18, 21] and have a moderate effect on liver enzymes [25, 26]. However, the NAD + supplementation effect on glucose metabolism measurement criteria and liver enzymes is still obscure. In order to assess the effects of different NAD + precursor supplements on fasting blood glucose, HbA1c, insulin, and HOMA-IR as well as CRP, ALT, AST, and ALP as liver enzymes, this systematic review and meta-regression analysis based on known clinical studies was conducted.
Methods
Search strategy
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) criteria were followed for conducting this study [27]. Without regard to language or time restrictions, a thorough search was carried out in the PubMed/MEDLINE, Web of Science, SCOPUS, and Embase databases from the beginning to April 2024. Additionally, similar papers and gray literature were considered in the search. Medical subject headings (MeSH) and Emtree (Embase subject headings) were selected to search the online databases, as follow: (“NAD” OR "NAD precursor" OR "Nicotinic Acids" OR "Niacin" OR "Niacinamide" OR "Nicotinamide Mononucleotide" OR Niaspan OR acipomax OR Niagen) AND (“Insulin Resistance” OR Insulin OR HOMA-IR OR Glucose OR “Glucose Intolerance” OR “Glycated Hemoglobin” OR “HbA1c” OR "C-Reactive Protein" OR "Inflammation" OR “Aspartate Transaminase” OR AST OR “Alanine Transaminase” OR ALT OR SGOT OR SGPT OR “Alkaline Phosphatase” OR ALP) AND ("Clinical Trials as Topic" OR "Cross-Over Studies" OR "Double-Blind Method" OR "Single-Blind Method" OR "Random Allocation" OR "Clinical Trial") (The specific search strategy is described in the Supplementary Appendix S1). The reference lists of the publications retrieved and linked review studies were manually searched to identify potentially overlooked qualifying trials. We also performed a “snowball search” to add other RCTs (not included in this study).
Eligibility criteria
Using titles, abstracts, or the complete texts of the research, two writers separately removed duplicate articles before finding and reviewing relevant publications. In the end, the papers were separated based on the following standards: 1) Randomized clinical trials studies; 2) NAD + precursor supplementation (nicotinic acid (NA) or nicotinamide (NE) supplementation) was given as an intervention in individual’s aged 18 and over; and 3) baseline and post in both group (intervention and control) glucose, insulin, HOMA-IR, HbA1c, CRP, ALP, AST, and ALT were recorded. The most recent or longest follow-up period was used when a research revealed results at more than one follow-up time. Studies with duplicated data, studies with ambiguous information, studies in which NAD + precursor was used as an intervention alongside other commonly prescribed medications, non-randomized trial designs, animal studies, studies without a control group, reviews, and meta-analysis studies were also excluded. The PICOS criteria for inclusion and exclusion of studies were as follows. Population: individual’s aged 18 and over; Intervention: NAD + precursor supplementation (nicotinic acid (NA) or nicotinamide (NE) supplementation); Comparator: other intervention or placebo; Outcomes: glucose, insulin, HOMA-IR, HbA1c, CRP, ALP, AST, and ALT; Study design: randomized clinical trials studies.
Data extraction
The qualifying studies were examined by two authors independently. The first author's name, the study's location, the year it was published, the sample size (for the intervention and control groups), the participant characteristics (such as the percentage of men, age, and health status), the type of outcomes, duration of the intervention, the dosage and type of the intervention, and the means and standard deviations (S.D.s) of the intended outcomes at baseline, post-intervention, and/or changes between baseline and post-intervention, were all extracted.
Quality assessment
Using the Cochrane risk-of-bias test for randomized trials (RoB 2), version 2, the quality of the included RCTs was methodologically evaluated [28]. Based on the following potential sources of bias: blinding of outcome assessment, allocation concealment, participant and staff blinding, random sequence generation, incomplete outcome data, selective reporting, and other bias, two authors independently rated each study as having a low, high, or unclear risk of bias. Any discrepancies were discussed with a third author in order to come to a consensus. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) grading method was also used to evaluate the quality of the current analytic research [29]. A reliable 10-point assessment system that assesses elements affecting study quality is the GRADE checklist. This scale has seven components: (1) risk of bias, (2) precision, (3) heterogeneity, (4) directness, (5) publishing bias, (6) funding bias, and (7) study design.
Data synthesis and statistical analysis
The data were examined using STATA version 12.0 software. Different data types were converted using a predetermined procedure to the mean and standard deviations (S.D.s) [30, 31]. For instance, in the absence of standard deviations, we calculated the change using the method below: The definition of standard deviation changes is square root [(S.D. baseline 2 + SD final 2)—(2R S.D. baseline 2 S.D. final)]. The following formula is used to convert the standard error of the mean (SEM) to standard deviation: S.D. is equal to SEM × √n, where n is the total number of participants in each group. The random-effects model was employed in the meta-analysis of research results. R codes used for analysis is described in the Supplementary Appendix S2. The weighting of the research followed the typical inverse variance technique. The data from the longest time point were used for the analysis, which allowed for the handling of many assessments within a single study group. Using Q Statistics and I-squared (I2), the degree of study heterogeneity was evaluated. Insignificant, low, moderate, and high heterogeneity were found with I2 values ranging from 0% to 25, 26% to 50%, 5% to 75%, and 76% to 100%, respectively [32]. To identify possible causes of heterogeneity, a pre-defined subgroup analysis based on the dosage, duration, and type of the intervention was conducted. A sensitivity analysis was done to determine the contribution of each research to the overall mean difference. In order to establish if there was publication bias, we utilized the official Egger's test [33].
Results
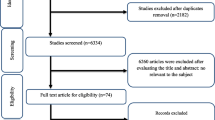
Figure 1 depicts a flowchart of the research selection process with exclusion criteria. This value indicates that the aforementioned electronic databases generated 2519 articles. After removing publications with duplicate research, there were 1422 total. Following an assessment of the research's titles and abstracts, 1345 papers were dropped since they didn't meet the inclusion requirements. 77 articles were found utilizing the full-text search during the secondary screening. For the reasons listed above, 32 of the investigations were dropped. Finally, 45 papers were included in the quantitative meta-analysis since they matched the qualifying requirements.
Study characteristics
The features of the pooled articles are shown in Table 1. Our surveys reveal that 19 studies have been carried out in USA, 14 articles in the European continent, 11 studies in the Asia and one article in the Egypt. Also, a multicenter study was conducted. All articles were published between 1998 and 2022 and follow up intervention ranged from 4 to 144 weeks. The mean age and percentage of male participants ranged from 26.3 to 71.1 years and 0–100%, respectively, at the baseline. Six studies were conducted with crossover design and the rest of the study was conducted in parallel. The doses prescribed in the studies were between 100 and 3000 mg per day, and in nine studies the supplement type was in the form of NE and the rest were in the form of NA. In addition, the population investigated in the studies included people with diabetes or glucose tolerance disorder, non-alcoholic fatty liver disease, metabolic syndrome, obese people, polycystic ovary syndrome, people with dyslipidemia or cardiovascular problems, as well as healthy people.
The findings of the evaluation of the eligible studies' quality are shown in Table 2. Additionally, a score of 7.6 (very good quality) was determined after the GRADE score system was used to assess the quality of the current meta-analysis. The Kappa result for the authors of our study for data screening and selection was about 0.92, which was interpreted as almost perfect agreement.
Meta-analysis results
The effect of NAD + precursor supplementation on glucose metabolism
With the use of random effects model, the pooled results indicated that NAD + precursor supplementation had a significant increased on glucose (WMD: 2.17 mg/dL, 95% CI: 0.68, 3.66, P = 0.004) and HbA1c (WMD: 0.11, 95% CI: 0.06, 0.16, P < 0.001) compared with control. However, compared to the control group, no significant effect on insulin (WMD: 0.68 μU/mL, 95% CI: -1.27, 2.64, P = 0.493) and HOMA-IR (WMD: 0.15, 95% CI: -0.27, 0.56, P = 0.488) was reported after receiving NAD + precursor. A high heterogeneity was shown in the trials for glucose (Cochran Q test, P < 0.001, I2 = 59.2–70.3%), insulin (Cochran Q test, P < 0.001, I2 = 67.1–73.9%) and HOMA-IR (Cochran Q test, P < 0.001, I2 = 69.6–77.8%). Although low heterogeneity was observed for HbA1c (Cochran Q test, P = 0.265, I2 = 11.4–17.1%) (Fig. 2).
Subgroups analysis
The results of the subgroup analysis showed that the intake of NAD + precursor during the intervention of more than 12 weeks caused a greater increase in the glucose level. Furthermore, Nicotinic acid supplementation (NA) causes a greater increase in glucose and HbA1c levels than nicotinamide (NE) supplementation (Supplementary Table).
The effect of NAD + precursor supplementation on liver enzymes and CRP
Pooled findings from the random-effects model indicated that ALT (WMD: -1.22 U/L, 95% CI: -2.67 to 0.22, P = 0.098), AST (WMD: -0.75 U/L, 95% CI: -3.66 to 0.16, P = 0.614), and ALP (WMD: -0.27 U/L, 95% CI: -3.05 to 2.50, P = 0.846) were not significantly changed after NAD + precursor supplementation compared to control group. Howeve, NAD + precursor supplementation significantly reduced CRP (WMD: -0.93 mg/l, 95% CI -1.47 to -0.40, P < 0.001) levels compared to the control group. Furthermore, a significant heterogeneity was found among the studies for CRP (Cochran Q test, P = 0.002, I2 = 96.5–99.2%), ALT (Cochran Q test, P = 0.025, I2 = 44.2–49.9%) and AST (Cochran Q test, P < 0.001, I2 = 92.6%), but a low heterogeneity was reported for ALP (Cochran Q test, P = 0.873, I2 = 0.5–0.9%; Fig. 3).
Subgroups analysis
The findings of the subgroup also show the greater effect of NAD + precursor supplementation on ALT increase in a duration of intervention ≤ 12 weeks. In addition, subgroup analysis showed that the increase in ALT was greater when receiving supplementation NE than NA supplementation (Supplementary Table). The reducing effect on CRP concentration was also greater in the dose of ≥ 2 g, the duration of the intervention was ≤ 12 weeks, and with the supplement of NA (Supplementary Table).
Meta-regression
Meta-regression between NAD + precursor and absolute mean differences in CRP, ALT, AST, glucose, insulin, HOMA-IR, and HbA1c based on dosage and duration of intervention was performed. Only, there was a significant relationship between duration of intervention with changes in ALT (coefficient (Coef) = 0.1589788, P = 0.004). However, meta-regression analysis not showed a significant linear relationship between dose and duration of intervention with changes in other variables (Supplementary Figs. 1–7).
Sensitivity analysis
We gradually removed each trial from the analysis in order to determine the impact of each article on the aggregated effect size for the levels of glucose, insulin, HOMA-IR, HbA1c, CRP, AST, ALT, and ALP. The robustness of the findings was demonstrated by the leave-one-out sensitivity analysis (Supplementary Figs. 8–9).
Publication bias
Based on the Egger's tests, no indication of publication bias was found for the following variables: glucose (P = 0.762), insulin (P = 0.788), HOMA-IR (P = 531), HbA1c (P = 436), CRP (P = 0.970), AST (P = 0.131), ALT (P = 0.622), and ALP (P = 0.327) (Supplementary Figs. 10–11).
Discussion
Our comprehensive review and meta-analysis revealed that supplementing with NAD + precursors increased blood glucose and HbA1c in humans much more than placebo or no therapy, but that it had no statistically significant effect on insulin or HOMA-IR. To the best of our knowledge, no meta-analysis has been done on the impact of NAD + precursors on healthy and other papulation people' glucose metabolism. The effects of nicotinamide adenine dinucleotide (NAD +) precursor supplementation on glucose and lipid metabolism in humans were examined in the meta-analysis carried out by Zhong et al. Only studies that allowed diabetes and works of English literature, however, were included [21]. Earlier meta-analysis investigated and described the effect of NAD + precursor supplementation on improving TG, TC, LDL, and HDL levels in humans, but resulted in hyperglycemia, compared with placebo or no treatment [21, 79]. Animal studies evaluating obese mice have shown an association between NAD + supplementation and improved indices of obesity as well as molecular regulation of adipocytes [80, 81].
To evaluate these results, it is worth noting that NAD + is an important molecule in energy and signal transduction, in addition to acting as a substrate for enzymes such as sirtuins, poly-ADP ribose polymerases (PARPs) and cyclic ADP ribose synthetases that regulate cellular processes key to energy metabolism, DNA damage repair, and calcium signaling [82]. And the relationship between NAD + precursor supplementation and increased blood glucose can be explained by the function of NAD + in pancreatic beta cells, responsible for insulin production [83]. Insulin is a hormone that regulates blood glucose levels, allowing cells to absorb glucose from the blood and use it as energy, a function that is increased by the use of intracellular NAD supplementation, as mentioned by Reimers et al. [84]. According to Yoshino et al. [85] NAD + supplementation can lead to increased insulin production by pancreatic beta cells resulting in increased glucose absorption by body cells.
Still, other important functions that could explain the significant increase in glucose and HbA1c in humans compared to placebo or no treatment, is that nicotinamide has the ability to scavenge free radicals, as well as provide protection against toxic stimuli and against depletion of intracellular NAD. However, when their levels are still high, as in the case of supplementation or even by endogenous pathways, they are able to inhibit NAD-dependent functions, causing an increase in glucose metabolism and preventing the process of aerobic glycolysis, consequently generating an increase in glucose [86, 87].
Thus, it can be evaluated that the insulin response and HOMA-IR tend not to present significant results, as occurred in this study, since this information regarding supplementation can generate conflicts, depending on the amount in the body of each individual, being a limiting factor of the response to supplementation.
NAD + supplementation may also affect glucose production in the liver. NAD + is required for the proper function of several hepatic enzymes involved in glucose metabolism, including gluconeogenesis, the process by which the liver produces glucose from non-glidic precursors. NAD + supplementation may increase the activity of these hepatic enzymes, resulting in an increase in glucose production by the liver [25]. However, we found no systemic changes in ALT, AST, or ALP levels after NAD + precursor supplementation when compared to the control group.
The biochemical regulation of nicotinamide in the blood takes place through hepatic regulation, involving its conversion to stored NAD through hydrolysis or the reverse cycle. This reaction helps maintain NAD levels within normal limits [88]. Therefore, in order to observe changes in liver enzymes, very high doses must be administered and controlled to avoid generating hepatotoxicity. However, the results of NAD + supplementation on liver enzymes did not change due to such factors. Additionally, the study duration was not sufficient to produce evident effects.
In this meta-analysis, we look at the relation between supplementation and HbA1c, or glycosylated hemoglobin, which is a test used to measure the average blood sugar level over the past 2–3 months [89]. It is formed when hemoglobin, a protein in red blood cells, binds to glucose in the bloodstream. The amount of HbA1c in the blood can be used as an indicator of how well a person's blood sugar has been controlled over time, which is important for managing diabetes. HbA1c and NAD + can be important for maintaining overall health and wellness. Proper blood sugar control is crucial for managing diabetes and reducing the risk of complications, while NAD + plays a vital role in cellular energy production and DNA repair. Given the evidence of supplementation and increased glucose and HbA1c, it is important to emphasize that NAD + supplementation should not be seen carried out in pathological settings because an increase in blood glucose is undesirable in some conditions, such as diabetes, and, as a result, NAD + supplementation should be carried out under appropriate supervision [90].
With respect to the significant reduction in CRP concentration in treated individuals, our findings showed a potential anti-inflammatory effect with NAD + precursors supplementation. Although the exact mechanism to explain this relationship has not yet been established, it is suggested that the reduction in CRP concentration in individuals supplemented with NAD + precursors, such as NA, may also be related to its effects as lipid lowering agents [91].
Our results showed that the magnitude of the reduction in CRP concentration was also greater with a dosage equal to or greater than 2 g of NAD + precursors. However, contrary to what was observed for BP, the effect was more expressive with a treatment time equal to or less than three months. Such findings suggest that for acute biochemical parameters of cardiovascular importance, such as CRP, a treatment with high doses but with a shorter duration is more effective.
Our study had some limitations that jeopardized the extraction of robust conclusions. Clinically and statistically significant heterogeneities was found for adiponectin. These may be explained by the differences in the intervention-specific factors (e.g., type, dose, administration route, and duration of drugs) and blood pressure/inflammation-specific factors (e.g., age, sex, physiology, genetics, familial history, race/ethnicity, physical activity, socioeconomic status, dietary intakes, and drug, tobacco, or alcohol consumption) [92]. Nonetheless, we attempted to identify some possible sources of heterogeneity in data by performing a subgroup analysis. As a limitation of this systematic review we only include studies with an intervention duration of more than 4 weeks. We have included this limitation to ensure the validity of the results as well as the quality design of the studies in this meta-analysis study. In addition, lack of registration of the current study in PROSPERO due to time limit was another limitation of this study. Despite its limitations, the current study has several positive features: a rigorous methodology was used based on the PRISMA guidelines [93]; A thorough literature search using multiple independent databases; two researchers independently and in duplicate searched, selected, and extracted data from the selected studies; To resolve disputes, a third party was consulted [94, 95]. Furthermore, the present study likely included the largest effect size for each outcome assessed at glucose metabolism and liver enzymes.
Overall, these findings suggest that NAD + precursor supplementation might have a significant effect on glucose metabolism and CRP but does not appear to have a significant effect on liver enzymes. The results highlight the importance of considering the duration and the type of NAD + precursor supplementation when evaluating its effects on glucose metabolism. Further interventional studies with a major period (> 4 weeks) are needed to clarify the mechanisms of action and potential long-term effects of NAD + precursor supplementation on glucose metabolism and liver enzymes.
Availability of data and materials
Data will not be made available in a public repository as we have not obtained ethical clearance to share data publicly. However, on request from corresponding author data could be provided while maintaining anonymity.
References
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62.
Fonseca VA. Defining and Characterizing the Progression of Type 2 Diabetes. Diabetes Care. 2009;32(suppl_2):S151–6.
Gillett MJ. International expert committee report on the role of the A1c assay in the diagnosis of diabetes: diabetes care 2009; 32 (7): 1327–1334. Clin Biochem Rev. 2009;30(4):197.
Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–73.
Hirata T, Higashiyama A, Kubota Y, Nishimura K, Sugiyama D, Kadota A, et al. HOMA-IR values are associated with glycemic control in Japanese subjects without diabetes or obesity: the KOBE study. J Epidemiol. 2015;25(6):407–14.
Ho KL, Karwi QG, Connolly D, Pherwani S, Ketema EB, Ussher JR, et al. Metabolic, structural and biochemical changes in diabetes and the development of heart failure. Diabetologia. 2022;65(3):411–23.
Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–32.
Wu S, Mo X. Optic Nerve Regeneration in Diabetic Retinopathy: Potentials and Challenges Ahead. Int J Mol Sci. 2023;24(2):1447.
El–Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology. 2002;122(7):1822–8.
El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8.
Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967(1):363–78.
Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Investig. 2005;115(5):1139–42.
Mainous AG, Diaz VA, King DE, Everett CJ, Player MS. The relationship of hepatitis antibodies and elevated liver enzymes with impaired fasting glucose and undiagnosed diabetes. J Am Board Fam Med. 2008;21(6):497–503.
Wada J, Nakatsuka A. Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med Okayama. 2016;70(3):151–8.
Palomer X, Salvadó L, Barroso E, Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. 2013;168(4):3160–72.
Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41(9):1144–9.
Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal. 2007;9(7):931–42.
Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, et al. Hyperglycemic Pseudohypoxia and Diabetic Complications. Diabetes. 1993;42(6):801–13.
Fan L, Cacicedo JM, Ido Y. Impaired nicotinamide adenine dinucleotide (NAD+) metabolism in diabetes and diabetic tissues: Implications for nicotinamide-related compound treatment. J Diabetes Investig. 2020;11(6):1403–19.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Zhong O, Wang J, Tan Y, Lei X, Tang Z. Effects of NAD+ precursor supplementation on glucose and lipid metabolism in humans: a meta-analysis. Nutr Metab. 2022;19(1):20.
Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 2018;27(3):529–47.
Gumaa K, McLean P, Greenbaum A. Compartmentation in relation to metabolic control in liver. Essays Biochem. 1971;7:39–86.
Yoshino J, Mills KF, Yoon MJ, Imai SI. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–36.
Dall M, Hassing AS, Treebak JT. NAD+ and NAFLD–caution, causality and careful optimism. J Physiol. 2022;600(5):1135–54.
Han X, Bao X, Lou Q, Xie X, Zhang M, Zhou S, et al. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ. 2019;7:e7568.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions. 2019:205–28.
Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, et al. Perspective: NutriGrade: A Scoring System to Assess and Judge the Meta-Evidence of Randomized Controlled Trials and Cohort Studies in Nutrition Research. Adv Nutr. 2016;7(6):994–1004 PubMed PMID: 28140319. eng.
Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. www cochrane-handbook org. 2011.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Dollerup OL, Trammell SA, Hartmann B, Holst JJ, Christensen B, Møller N, et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab. 2019;104(11):5703–14.
Canner PL, Furberg CD, McGovern ME. Benefits of niacin in patients with versus without the metabolic syndrome and healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol. 2006;97(4):477–9.
Linke A, Sonnabend M, Fasshauer M, Höllriegel R, Schuler G, Niebauer J, et al. Effects of extended-release niacin on lipid profile and adipocyte biology in patients with impaired glucose tolerance. Atherosclerosis. 2009;205(1):207–13.
Villines TC, Stanek EJ, Devine PJ, Turco M, Miller M, Weissman NJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6–HDL and LDL Treatment Strategies in Atherosclerosis) Final Results and the Impact of Medication Adherence, Dose, and Treatment Duration. J Am Coll Cardiol. 2010;55(24):2721–6.
Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51(16):1564–72.
Philpott AC, Hubacek J, Sun YC, Hillard D, Anderson TJ. Niacin improves lipid profile but not endothelial function in patients with coronary artery disease on high dose statin therapy. Atherosclerosis. 2013;226(2):453–8.
Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, et al. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95(6):2727–35.
Abdi A, Khosravi A, Sarrafzadegan N, Ansari R, Mehr GK, Roghani F, et al. The effect of low-dose niacin added to simvastatin on lipoprotein profile. arya Atherosclerosis.3(1):8.
Aye M, Kilpatrick E, Afolabi P, Wootton S, Rigby A, Coady A, et al. Postprandial effects of long-term niacin/laropiprant use on glucose and lipid metabolism and on cardiovascular risk in patients with polycystic ovary syndrome. Diabetes Obes Metab. 2014;16(6):545–52.
Huang H. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Frontiers in aging. 2022;3:851698.
Bregar U, Jug B, Keber I, Cevc M, Sebestjen M. Extended-release niacin/laropiprant improves endothelial function in patients after myocardial infarction. Heart Vessels. 2014;29:313–9.
Hamilton SJ, Chew GT, Davis TM, Watts GF. Niacin improves small artery vasodilatory function and compliance in statin-treated type 2 diabetic patients. Diab Vasc Dis Res. 2010;7(4):296–9.
Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343–53.
Westphal S, Borucki K, Taneva E, Makarova R, Luley C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis. 2007;193(2):361–5.
Vittone F, Chait A, Morse JS, Fish B, Brown BG, Zhao X-Q. Niacin plus simvastatin reduces coronary stenosis progression among patients with metabolic syndrome despite a modest increase in insulin resistance: A subgroup analysis of the HDL-Atherosclerosis treatment study. J Clin Lipidol. 2007;1(3):203–10.
Thoenes M, Oguchi A, Nagamia S, Vaccari CS, Hammoud R, Umpierrez G, et al. The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int J Clin Pract. 2007;61(11):1942–8.
Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22(11):2243–50.
Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121(1):110–22.
Savinova OV, Fillaus K, Harris WS, Shearer GC. Effects of niacin and omega-3 fatty acids on the apolipoproteins in overweight patients with elevated triglycerides and reduced HDL cholesterol. Atherosclerosis. 2015;240(2):520–5.
Chow DC, Stein JH, Seto TB, Mitchell C, Sriratanaviriyakul N, Grandinetti A, et al. Short-term effects of extended-release niacin on endothelial function in HIV-infected patients on stable antiretroviral therapy. AIDS. 2010;24(7):1019–23.
Goldberg RB, Bittner VA, Dunbar RL, Fleg JL, Grunberger G, Guyton JR, et al. Effects of extended-release niacin added to simvastatin/ezetimibe on glucose and insulin values in AIM-HIGH. Am J Med. 2016;129(7):753. e13-. e22.
Nash MS, Lewis JE, Dyson-Hudson TA, Szlachcic Y, Yee F, Mendez AJ, et al. Safety, tolerance, and efficacy of extended-release niacin monotherapy for treating dyslipidemia risks in persons with chronic tetraplegia: a randomized multicenter controlled trial. Arch Phys Med Rehabil. 2011;92(3):399–410.
Warnholtz A, Wild P, Ostad MA, Elsner V, Stieber F, Schinzel R, et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis. 2009;204(1):216–21.
Osar Z, Samanci T, Demirel GY, Damci T, Ilkova H. Nicotinamide effects oxidative burst activity of neutrophils in patients with poorly controlled type 2 diabetes mellitus. J Diabetes Res. 2004;5:155–62.
Kei A, Liberopoulos E, Mikhailidis D, Elisaf M. Comparison of switch to the highest dose of rosuvastatin vs. add-on nicotinic acid vs. add-on fenofibrate for mixed dyslipidaemia. Int J Clin Pract. 2013;67(5):412–9.
Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. JAMA. 2000;284(10):1263–70.
Igarashi M, Nakagawa-Nagahama Y, Miura M, Kashiwabara K, Yaku K, Sawada M, et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging. 2022;8(1):5.
Otto C, Parhofer KG, Ritter MM, Richter WO, Schwandt P. Effects of acipimox on haemorheology and plasma lipoproteins in patients with mixed hyperlipoproteinaemia. Br J Clin Pharmacol. 1998;46(5):473–8.
Ko GT, Mak TW, Yeung VT, Chan DC, Lam CW, Tsang LW, et al. Short-term Efficacy and Tolerability of Combination Therapy with Lovastatin and Acipimox in Chinese Patients with Type 2 Diabetes Mellitus and Mixed Dyslipidemia. J Clin Pharmacol. 1998;38(10):912–7.
Song S, Lee CJ, Oh J, Park S, Kang S-M, Lee S-H. Effect of niacin on carotid atherosclerosis in patients at low-density lipoprotein-cholesterol goal but high lipoprotein (a) level: A 2-year follow-up study. J Lipid Atheroscler. 2019;8(1):58.
Kang HJ, Kim DK, Lee SM, Kim KH, Han SH, Kim KH, et al. Effects of low-dose niacin on dyslipidemia and serum phosphorus in patients with chronic kidney disease. Kidney Res Clin Pract. 2013;32(1):21–6.
Lee JM, Robson MD, Yu L-M, Shirodaria CC, Cunnington C, Kylintireas I, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54(19):1787–94.
Bays HE, Shah A, Lin J, Sisk CM, Paolini JF, Maccubbin D. Efficacy and tolerability of extended-release niacin/laropiprant in dyslipidemic patients with metabolic syndrome. J Clin Lipidol. 2010;4(6):515–21.
Owada A, Suda S, Hata T. Antiproteinuric effect of niceritrol, a nicotinic acid derivative, in chronic renal disease with hyperlipidemia: a randomized trial. Am J Med. 2003;114(5):347–53.
Okabe K, Yaku K, Uchida Y, Fukamizu Y, Sato T, Sakurai T, et al. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front Nutr. 2022;9:868640.
El-Kady RR, Ali AK, El Wakeel LM, Sabri NA, Shawki MA. Nicotinamide supplementation in diabetic nonalcoholic fatty liver disease patients: randomized controlled trial. Therapeutic advances in chronic disease. 2022;13:20406223221077960.
Goldberg A, Alagona P Jr, Capuzzi DM, Guyton J, Morgan JM, Rodgers J, et al. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am J Cardiol. 2000;85(9):1100–5.
Moore A, Phan BAP, Challender C, Williamson J, Marcovina S, Zhao X-Q. Effects of adding extended-release niacin and colesevelam to statin therapy on lipid levels in subjects with atherosclerotic disease. J Clin Lipidol. 2007;1(6):620–5.
Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep. 2019;9(1):9772.
Vidal J, Fernandez-Balsells M, Sesmilo G, Aguilera E, Casamitjana R, Gomis R, et al. Effects of nicotinamide and intravenous insulin therapy in newly diagnosed type 1 diabetes. Diabetes Care. 2000;23(3):360–4.
Airan-Javia SL, Wolf RL, Wolfe ML, Tadesse M, Mohler E, Reilly MP. Atheroprotective lipoprotein effects of a niacin-simvastatin combination compared to low-and high-dose simvastatin monotherapy. Am Heart J. 2009;157(4):687.e1-. e8.
Shah S, Ceska R, Gil-Extremera B, Paolini J, Giezek H, Vandormael K, et al. Efficacy and safety of extended-release niacin/laropiprant plus statin vs. doubling the dose of statin in patients with primary hypercholesterolaemia or mixed dyslipidaemia. Int J Clin Pract. 2010;64(6):727–38.
Karacaglar E, Atar I, Altin C, Yetis B, Cakmak A, Bayraktar N, et al. The effects of niacin on inflammation in patients with non-ST elevated acute coronary syndrome. Acta Cardiologica Sinica. 2015;31(2):120.
Fazio S, Guyton J, Lin J, Tomassini J, Shah A, Tershakovec A. Long-term efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in hyperlipidaemic patients with diabetes or metabolic syndrome. Diabetes Obes Metab. 2010;12(11):983–93.
Lee K, Ahn TH, Kang WC, Han SH, Choi IS, Shin EK. The effects of statin and niacin on plaque stability, plaque regression, inflammation and oxidative stress in patients with mild to moderate coronary artery stenosis. Korean Circ J. 2011;41(11):641–8 PubMed PMID: 22194758. Pubmed Central PMCID: PMC3242018. Epub 2011/12/24. eng.
Sahebkar A, Reiner Ž, Simental-Mendia LE, Ferretti G, Cicero AF. Effect of extended-release niacin on plasma lipoprotein (a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. 2016;65(11):1664–78.
Roh E, Myoung Kang G, Young Gil S, Hee Lee C, Kim S, Hong D, et al. Effects of chronic NAD supplementation on energy metabolism and diurnal rhythm in obese mice. Obesity. 2018;26(9):1448–56.
Zhang Y, Zhu W, Wang M, Xi P, Wang H, Tian D. Nicotinamide mononucleotide alters body composition and ameliorates metabolic disorders induced by a high-fat diet. IUBMB Life. 2023;75(6):548–62. https://doi.org/10.1002/iub.2707. Epub 2023 Feb 13.
Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513–28.
Yamaguchi S, Yoshino J. Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. BioEssays. 2017;39(5):1600227.
Reimers J, Andersen H, Pociot F. Nikotinamid og forebyggelse af insulinkraevende diabetes mellitus. Rationale, virkningsmekanisme, toksikologi og kliniske erfaringer. ENDIT Gruppe. Ugeskr Laeger. 1994;156(4):461–5.
Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372(6547):1224–9.
Gale E. Molecular Mechanisms of Beta-Cell Destruction in IDDM The Role of Nicotinamide. Hormone Research in Paediatrics. 1996;45(Suppl. 1):40–3.
Braidy N, Berg J, Clement J, Khorshidi F, Poljak A, Jayasena T, et al. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid Redox Signal. 2019;30(2):251–94.
Roberti A, Fernández AF, Fraga MF. Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation. Mol Metab. 2021;45:101165. https://doi.org/10.1016/j.molmet.2021.101165. Epub 2021 Jan 14.
Yazdanpanah S, Rabiee M, Tahriri M, Abdolrahim M, Rajab A, Jazayeri HE, et al. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: A comprehensive review. Crit Rev Clin Lab Sci. 2017;54(4):219–32.
Blanco-Vaca F, Rotllan N, Canyelles M, Mauricio D, Escolà-Gil JC, Julve J. NAD+-Increasing Strategies to Improve Cardiometabolic Health? Front Endocrinol. 2022;12:1820.
Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24(1):33–50.
Jéquier E. Tappy LJPr. Regulation of body weight in humans. 1999;79(2):451–80.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10.
Guimarães NS, Ferreira AJ, Silva RdCR, de Paula AA, Lisboa CS, Magno L, et al. Deduplicating records in systematic reviews: There are free, accurate automated ways to do so. J Clin Epidemiol. 2022;152:110–5.
Acknowledgements
This study is part of the project No. 1402/63015 of the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We thank the Student Research Committee and the Research & Technology Chancellor of Shahid Beheshti University of Medical Sciences for their financial support of this study.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Mh.S and A.H contributed in conception, design, and statistical analysis. Mh.S, S.T, M.GR, and N.SG contributed in data collection and manuscript drafting. Mh.S and A.H supervised the study. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the research council and ethics committee Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sohouli, M.H., Tavakoli, S., Reis, M.G. et al. Changes in glucose metabolism, C-reactive protein, and liver enzymes following intake of NAD + precursor supplementation: a systematic review and meta‐regression analysis. Nutr Metab (Lond) 21, 35 (2024). https://doi.org/10.1186/s12986-024-00812-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-024-00812-0