Abstract
Background
Kawasaki Disease (KD) involves arterial inflammation, primarily affecting the coronary arteries and leading to coronary artery lesions. Recent advancements in understanding the immunomodulatory roles of vitamin D have prompted investigations into the potential correlation between serum vitamin D levels and the risk of coronary artery lesions (CAL) in KD. This review aims to explore this association.
Methods
A systematic search utilizing relevant keywords related to Kawasaki disease and coronary artery lesions was conducted across four databases (PubMed, Embase, Scopus, and Web of Science). The quality of the incorporated studies was assessed utilizing the Newcastle-Ottawa Scale. The study protocol is registered in PROSPERO under the registry code CRD42024493204.
Results
In a review of five studies involving 442 KD patients and 594 healthy controls, KD patients generally had lower serum vitamin D levels compared to controls, with mixed findings on the association with coronary artery lesions and IVIG resistance. While three studies supported lower vitamin D in KD, one showed no significant difference. Regarding CAL, one study found lower vitamin D, another found higher levels associated with CAL, and two found no significant difference.
Conclusions
Overall, the evidence is inconclusive, but there’s a trend suggesting potential benefits of sufficient vitamin D levels in Kawasaki disease rather than evidence refuting any association with clinical outcomes.
Similar content being viewed by others
Background
Kawasaki Disease (KD) is a systemic vasculitis characterized by inflammation of small to medium-sized arteries, with coronary artery involvement representing a critical complication [1]. The spectrum of coronary lesions associated with KD includes coronary stenosis, ectasia, aneurysms, and myocardial infarction. Timely diagnosis and treatment with IVIG are pivotal in preventing coronary artery lesions (CAL) in KD, as delayed intervention escalates the risk of these serious complications [1]. However, up to 20% of patients do not respond to intravenous immunoglobulin (IVIG) and require adjunctive therapies such as monoclonal antibodies [2]. The intricate pathogenesis involves systemic inflammatory processes and immune activation during the acute phase of KD. This phase is characterized by heightened proliferation and activation of immune cells, including T cells, neutrophils, and macrophages. These immune cells exhibit activation of nuclear transcription factor-B (NF-κB) and an increased release of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-1β, IL-6, and monocyte chemoattractant protein-1 [3,4,5,6]. Additionally, endothelial cells demonstrate increased expression of adhesion molecules like intercellular adhesion molecule-1 and E-selectin, coupled with the activation of NF-κB, leading to heightened leucocyte infiltration and cytokine release [3, 7].
In recent years, several studies demonstrated that 1,25dihydroxyvitamin D3 (1,25-(OH)2D3), the active form of vitamin D, not only fulfills its conventional role in calcium and phosphorus homeostasis but also exhibits significant anti-inflammatory and immunomodulatory effects [6, 8]. It plays a pivotal role in suppressing immune responses by inhibiting dendritic cells and macrophages’ activation and restraining T and B cell proliferation through adjusting various signaling pathways like ERK1/2 [9, 10]. 1,25-(OH)2D3 can also upregulate regulatory T cells and induce immune tolerance [9]. Furthermore, 1,25-(OH)2D3 hinders the synthesis of immunoglobulins and downregulates the transcription of various cytokines, including IL-1, IL-2, IL-6, IL-12, TNF-α, and INF-γ [11,12,13]. NF-κB activation in macrophages and TNF-α-induced expression of adhesion molecules in endothelial cells are also modulated by 1,25-(OH)2D3 [8, 14]. Given the substantial role of 1,25-(OH)2D3 in the inflammatory process of KD, prior studies propose that 1,25-(OH)2D3, as a potential immunomodulatory agent, could be advantageous in Kawasaki patients, particularly in those resistant to IVIG and in need of adjunctive therapy.
In this systematic review, we aim to comprehensively evaluate the association between serum vitamin D levels and the risk of coronary artery involvement in Kawasaki Disease, shedding light on the potential therapeutic role of vitamin D in managing this vasculitis.
Methods
This systematic review was performed adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [15]. The study protocol also was logged in PROSPERO under the registration code CRD42024493204.
Eligibility criteria
This study included all peer-reviewed observational and interventional human studies investigating the correlation between serum 25(OH)-vitamin D levels and developing coronary artery lesions in patients with KD. We excluded studies that investigated animal studies, review studies, and conference abstracts.
Search strategy
A systematic search was conducted using all pertinent keywords related to Kawasaki disease, coronary artery lesion, and coronary aneurysm across the PubMed, Embase, Scopus, and Web of Science databases until December 10th, 2023. There were no restrictions regarding language and publication dates. Detailed search strategies can be found in Supplementary Table 1.
Selection process
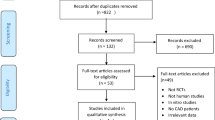
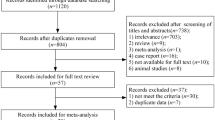
Two independent reviewers (Z.A. and E.K.) initially screened the records by title and abstract utilizing the Rayyan web app. The publications were labeled as either “Excluded” or “Maybe” during the screening based on the pre-specified eligibility criteria. Records marked as “Maybe” went through a full-text evaluation for probable addition in the systematic review. In a disagreement between the two authors, a third author (M.G.) expertly intervened to resolve discrepancies and made the final decision. Figure 1 shows an overview of the entire selection process.
Data extraction
Two reviewers (Z.A. and E.K.) independently extracted data from all studies meeting the eligibility criteria and resolved disagreements through consensus. The extracted data encompassed the first author, publication year, country, study subjects, study design, sample size, sample characteristics, serum vitamin D levels, vitamin D insufficiency definitions, and incidence rates of CALs and IVIG resistance. Both significant and non-significant findings were comprehensively extracted, with a p-value of < 0.05 considered as the significance level.
Risk of bias and study quality assessment
The quality of the animal studies was assessed using the Newcastle-Ottawa Scale (NOS) checklist [16]. Two independent reviewers (Z.A. and E.K.) comprehensively evaluated the quality of each included study. A third reviewer (M.G.) resolved disagreements.
Results
Literature search and baseline characteristic of the included studies
The initial search yielded 259 results, comprising 32 from PubMed, 70 from Embase, 93 from the Web of Science, and 64 from Scopus. Subsequent removal of duplicates (N = 81) led to 173 unique studies, which underwent screening based on titles and abstracts. Following this initial screening, 24 studies remained for comprehensive full-text evaluation. After applying exclusion criteria for various reasons, five peer-reviewed studies were ultimately included in this review [17,18,19,20,21]. The selection process and rationale for exclusions are elucidated in Fig. 1.
The included studies, conducted between 2014 and 2022 in China [17, 19], Italy [20], Korea [18], and Japan [19], each contributed one study. In total, the investigation involved 442 patients with Kawasaki Disease (KD), comprising 85 with coronary artery lesions (CAL) and 357 without CAL, alongside 594 healthy control cases. All the included studies were observational, with no experimental studies on the administration of vitamin D in KD found.
Regarding serum sampling for 25(OH)-vitamin D level assessment, three studies collected serum samples before IVIG treatment, one collected samples both before and after IVIG treatment, and one study did not provide information in this area. Three of the included studies enrolled both complete and incomplete KD patients, one enrolled only complete KD patients, and one study did not specify whether the patients were complete or incomplete. Notably, none of the included studies performed an analysis to compare vitamin D status between complete and incomplete patients. Regarding the risk assessment of the studies, the Newcastle-Ottawa Scale (NOS) scores ranged from 4 to 7, with an average score of 5.8. Table 1 provides a summary of the included studies.
Serum vitamin D levels in KD patients compared to healthy controls
Four studies compared serum vitamin D levels in KD patients with those in healthy subjects [17, 19,20,21]. In the study by Stagi et al., it was observed that before receiving treatment for KD, 98.7% of KD patients exhibited insufficient or deficient levels of serum 25(OH)-vitamin D (< 30 ng/mL). This prevalence was significantly higher than the 78.6% found in healthy control groups. Additionally, the serum 25(OH)-vitamin D levels in the KD group were significantly lower than those in the healthy controls, measuring 9.17 ± 4.94 ng/mL and 23.3 ± 10.6 ng/mL, respectively [20].
Similarly, Zhang et al. observed that the CAL and NCAL subgroups of KD patients had significantly lower serum 25(OH)-vitamin D levels compared to healthy controls (40 ± 10 ng/mL for healthy controls vs. 22 ± 5 ng/mL for NCAL patients and 15 ± 4 ng/mL for CAL patients) [21].
In a recent study, Okazaki et al. consistently corroborated that 25(OH)-vitamin D serum levels in KD patients were notably lower than controls (median: 17 ng/mL vs. 25 ng/mL). The number of patients with sub-normal 25(OH)-vitamin D serum levels (< 20 ng/ mL) (64% vs. 27% for controls) and deficient status (< 12 ng/ mL) (37% vs. 7% for controls) was also significantly higher in KD patients [19].
Conversely, Chen et al. reported that serum 25-(OH)-vitamin D levels in both CAL and NCAL groups were higher than healthy controls. However, the difference between NCAL and healthy controls did not reach statistical significance, with measurements of 83.9 ± 26.3 ng/ml, 49.2 ± 23.8 ng/ml and 44.1 ± 30.2 ng/ml for CAL, NCAL, and healthy controls, respectively [17].
Chen et al. did not mention the overall 25-(OH)-vitamin D serum level for KD patients. However, with respect to measured indices for CAL and NCAL subgroups of KD patients, the approximate serum 25-(OH)-vitamin D level for all KD patients could be calculated as 58 ± 24 ng/ml using a formula for pooling the mean and standard deviation. This estimated value is numerically higher than the serum 25-(OH)-vitamin D levels for healthy controls (58 ± 24 ng/mL vs. 44.1 ± 30.2 ng/ml), which is quite unexpected with regard to the previously mentioned studies [17].
Association between serum 25-(OH)-vitamin D levels and coronary artery involvement in KD patients
The reports on the association between 25-(OH)-vitamin D serum levels and developing CAL in KD patients were highly heterogeneous, both in terms of outcome measures and results.
Stagi et al. observed that the development of coronary artery aneurysm (CAA) was associated with significantly lower serum levels of 25-(OH)-vitamin D (4.92 ± 1.36 ng/mL) compared to patients without CAA (9.41 ± 4.95 ng/mL) [20]. Similarly Zhang et al., demonstrated that KD patients with CAL had lower serum levels of 25-(OH)-vitamin D both before or after IVIG treatment compared to those without CAL [21].
In contrast, Okazaki et al. found no association between 25(OH)-vitamin D levels at the time of KD diagnosis and CAL [19]. Similarly, Jun et al. reported no significant difference in developing CAL in KD patients with vitamin D deficiency (< 20 ng/mL) compared to those without deficiency (> 20 ng/mL) (17.9% vs. 7.7%) [18].
Interestingly, Chen et al. observed that serum 25-(OH)-vitamin D levels were markedly elevated in children with CALs compared to ones without (83.9 ± 26.3 ng/ml vs. 49.2 ± 23.8 ng/ml). They introduced a cutoff point of 65 ng/ml to predict subsequent CALs, possessing a 0.73 specificity, 0.78 sensitivity, and 0.74 diagnostic accuracy [17].
Association between serum vitamin D levels and IVIG resistance in KD patients
Three studies reported the association between serum 25-(OH)-vitamin D levels and IVIG resistance in KD patients. Jun et al. revealed an increase in IVIG resistance incidence in KD patients with vitamin D deficiency (< 20 ng/mL) compared to those without deficiency (> 20 ng/ml) (30.8% vs. 11.5%) [18]. Zhang et al. similarly reported lower serum levels for IVIG resistance KD patients compared to responders (14 ± 4 ng/ml vs. 21 ± 6 ng/ml) [21]. However, the other study by Okazaki et al. did not find any association between 25-(OH)-vitamin D serum levels at the time of diagnosis of KD and IVIG resistance [19].
Discussion
This systematic review identified evidence from observational studies of an association between serum vitamin D status and susceptibility to coronary artery lesions in KD patients. Additionally, comparisons were made between serum vitamin D levels in KD patients and healthy controls, as well as associations between serum vitamin D and IVIG resistance. No evidence from intervention studies was found.
The primary finding indicates that KD patients generally have lower serum vitamin D compared to healthy controls. Three out of four included studies support this finding, although one study revealed conflicting results with higher serum vitamin D in KD patients versus controls [17, 19,20,21]. Regarding the association between serum vitamin D and developing CAL in KD, this review failed to reach a consensus due to heterogeneous study results. Two studies reported lower vitamin D levels in KD patients with CAL compared to those without [20, 21]. However, two other studies found no significant association between vitamin D status and CAL, and notably one study demonstrated an inverse correlation, with higher serum vitamin D levels associated with increased risk of CAL [17,18,19]. For IVIG resistance, trends leaned towards lower serum vitamin D being associated with higher resistance [18, 21].
Several factors may explain the inconsistent study results, including differences in sample size, variations in vitamin D status classification and CAL determination, population heterogeneity, and timing of outcome assessments.
In vitro studies support the immunomodulatory functions of vitamin D in KD vasculitis [22, 23]. Kudo et al. found vitamin D significantly inhibited TNF-α-induced vascular cellular adhesion molecule-1 expression and interleukin-8 production [8]. Furthermore, Suzuki et al. showed the anti-inflammatory effects of 1α,25-dihydroxyvitamin D3 in human coronary arterial endothelial cells, suggesting vitamin D could modulate KD inflammation via NF-kappaB activation [14]. Beyond KD, previous studies demonstrated vitamin D deficiency accelerates coronary artery disease progression by increasing chronic inflammation, while supplementation benefits disease progression by dampening vascular inflammation and atherosclerosis [6, 24, 25].
Given the anti-inflammatory functions of vitamin D, it remains biologically plausible that insufficient 25(OH)-vitamin D levels can negatively impact coronary artery complications in KD patients. However, due to the limited number of studies whether vitamin D deficiency increases CAL risk cannot be definitively concluded.
Strengths and limitations
This systematic review has several strengths. It is the most comprehensive review assessing serum vitamin D and the development of CAL in Kawasaki disease. The systematic approach to identifying studies makes it unlikely that relevant studies were missed. No language or time limitations were imposed, and the review assessed the risk of bias of evidence. However, an inability to quantitatively analyze data due to study heterogeneity and insufficient information represented limitations. The small number of included studies from only four countries reduced generalizability and challenged result interpretation. Finally, as studies only evaluated associations between vitamin D and CAL incidence, the therapeutic value of vitamin D in KD remains unknown.
Conclusions
Although the current evidence is inconclusive overall, the results show more support for a potential benefit of sufficient vitamin D levels in Kawasaki disease rather than evidence that refutes any association with clinical outcomes.
Data availability
The datasets used (extracted from included studies) in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CAA:
-
Coronary artery aneurysms
- CAL:
-
Coronary artery lesions
- HC:
-
Healthy controls
- IVIG:
-
Intravenous immunoglobulin
- KD:
-
Kawasaki disease
- NACVL:
-
Non-aneurysmatic cardiovascular lesions
- NCAL:
-
No coronary artery lesions
References
Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67(14):1738–49.
Portman MA, et al. Etanercept as adjunctive treatment for acute Kawasaki disease: study design and rationale. Am Heart J. 2011;161(3):494–9.
Takahashi K, Oharaseki T, Yokouchi Y. Pathogenesis of Kawasaki disease. Clin Experimental Immunol. 2011;164(Supplement1):20–2.
Hara T, Yamamura K, Sakai Y. The up-to-date pathophysiology of Kawasaki disease. Clin Transl Immunol. 2021;10(5):e1284.
Ichiyama T, et al. NF-kappaB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin Immunol. 2001;99(3):373–7.
Amirsardari Z, et al. Bridging the gap: navigating the impact of dietary supplements on abdominal aortic aneurysm progression-A systematic review. PLoS ONE. 2024;19(6):e0305265.
Kim DS, Lee KY. Serum soluble E-selectin levels in Kawasaki disease. Scand J Rheumatol. 1994;23(5):283–6.
Kudo K, et al. 1alpha,25-Dihydroxyvitamin D(3) inhibits vascular cellular adhesion molecule-1 expression and interleukin-8 production in human coronary arterial endothelial cells. J Steroid Biochem Mol Biol. 2012;132(3–5):290–4.
Qi XL, et al. 1,25-Dihydroxyvitamin D3 regulates T lymphocyte proliferation through activation of P53 and inhibition of ERK1/2 signaling pathway in children with Kawasaki disease. Eur Rev Med Pharmacol Sci. 2017;21(16):3714–22.
Penna G, Adorini L. 1α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–11.
Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4 + T cells. J Immunol. 2002;168(3):1181–9.
Muller K, Odum N, Bendtzen K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett. 1993;35(2):177–82.
D’Ambrosio D, et al. Inhibition of IL-12 production by 1, 25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Investig. 1998;101(1):252–62.
Suzuki Y, et al. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol. 2009;113(1–2):134–8.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
Wells GA et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
Chen YL, Wang JL, Li WQ. Prediction of the risk of coronary arterial lesions in Kawasaki disease by serum 25-hydroxyvitamin D3. Eur J Pediatr. 2014;173(11):1467–71.
Jun JS, Jung YK, Lee DW. Relationship between vitamin D levels and intravenous immunoglobulin resistance in Kawasaki disease. Korean J Pediatr. 2017;60(7):216–20.
Okazaki N, et al. The impact of vitamin D on the onset and progress of Kawasaki disease. Pediatr Int. 2022;64(1):e15191.
Stagi S, et al. Severe vitamin D deficiency in patients with Kawasaki disease: a potential role in the risk to develop heart vascular abnormalities? Clin Rheumatol. 2016;35(7):1865–72.
Zhang YD, et al. [Changes in 25-hydroxyvitamin D3 level and its significance in children with Kawasaki disease]. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18(3):211–4.
Konijeti GG, Vitamin D, Supplementation Modulates T, et al. Cell-mediated immunity in humans: results from a Randomized Control Trial. J Clin Endocrinol Metab. 2016;101(2):533–8.
Ao T, Kikuta J, Ishii M. The effects of vitamin D on Immune System and Inflammatory diseases. Biomolecules, 2021. 11(11).
Legarth C et al. Potential Beneficial effects of vitamin D in coronary artery disease. Nutrients, 2019. 12(1).
Chen S, et al. Vitamin D Deficiency accelerates coronary artery Disease Progression in Swine. Arterioscler Thromb Vasc Biol. 2016;36(8):1651–9.
Acknowledgements
Not applicable.
Funding
This systematic review was carried out without external financial support or research grants.
Author information
Authors and Affiliations
Contributions
Conceptualization: ZA and MG. Design, Literature Search, Study Selection, Screening, and Data Extraction: ZA and EK. Writing-Original Draft Preparation: ZA and EK. Writing-Review and Editing: MG and VZ. Supervision: MG and VZ. All authors have reviewed and approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used Claude 2.1 to ensure English language fluency and native-quality writing. Claude was consulted regarding grammar, word choice, sentence structure, and overall clarity of expression. After using this service, the authors reviewed and edited the content as needed and take full responsibility for the publication’s content.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agree with the publication of this systematic review.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Amirsardari, Z., Amirsardari, F., Kohansal, E. et al. Exploring the association between serum Vitamin D levels and the development of coronary artery lesions in Kawasaki disease - a systematic review. Pediatr Rheumatol 22, 71 (2024). https://doi.org/10.1186/s12969-024-01010-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-024-01010-1