Abstract
Prostate cancer (PC) is an age-related disease and represents, after lung cancer, the second cause of cancer death in males worldwide. Mortality is due to the metastatic disease, which mainly involves the bones, lungs, and liver. In the last 20 years, the incidence of metastatic PC has increased in Western Countries, and a further increase is expected in the near future, due to the population ageing. Current treatment options, including state of the art cancer immunotherapy, need to be more effective to achieve long-term disease control. The most significant anatomical barrier to overcome to improve the effectiveness of current and newly designed drug strategies consists of the prostatic stroma, in particular the fibroblasts and the extracellular matrix, which are the most abundant components of both the normal and tumor prostatic microenvironment. By weaving a complex communication network with the glandular epithelium, the immune cells, the microbiota, the endothelium, and the nerves, in the healthy prostatic microenvironment, the fibroblasts and the extracellular matrix support organ development and homeostasis. However, during inflammation, ageing and prostate tumorigenesis, they undergo dramatic phenotypic and genotypic changes, which impact on tumor growth and progression and on the development of therapy resistance. Here, we focus on the characteristics and functions of the prostate associated fibroblasts and of the extracellular matrix in health and cancer. We emphasize their roles in shaping tumor behavior and the feasibility of manipulating and/or targeting these stromal components to overcome the limitations of current treatments and to improve precision medicine’s chances of success.
Similar content being viewed by others
Introduction
The global burden of prostate cancer (PC) is substantial, ranking among the top five cancers for both incidence and mortality [1]. It is particularly common in developed Countries since, in addition to individual biological and genetic factors, environment and lifestyle impact the risk of developing the disease and surviving [2]. In the last 20 years the incidence of metastatic PC, which still lacks effective treatment, has increased in the U.S. from 11.58 cases per 100.000 men (4% of the total PC incidence) to 17.30 cases per 100.000 men (6% of the total PC incidence) [3], and an increase is expected due to the worldwide population aging. Improving the effectiveness of the therapeutic landscape, including the newly approved immunotherapies, for long-lasting control of advanced PC is a major challenge in urological oncology.
The histopathological architecture of the prostate, in which the stroma is widely represented, and plays an active role in tumorigenesis, raises the question whether targeting or manipulating stromal components, among which fibroblasts and the extracellular matrix (ECM) proteins predominate, would lead to a full response to current and developing therapies, including the recently approved autologous T cell vaccination (Sipuleucel-T) for the treatment of asymptomatic, or minimally symptomatic, metastatic castration-resistant (mCR) PC [4].
The prostatic stroma is the supportive tissue framework surrounding the glandular epithelium, which facilitates its function by supplying oxygen and nutrients, via blood vessels, and which regulates smooth muscle contraction during ejaculation, via sympathetic and parasympathetic nerve fibers [5]. Its cellular composition consists of fibroblasts embedded in the collagen and elastin rich extracellular matrix (ECM) they produce, which also contains smooth muscle cells, blood and lymphatic vessels, and nerves. The prostatic stroma harbors and regulates innate and adaptive immune cells, such as naive, tissue-resident memory and regulatory CD4+T cells, as well as cytotoxic and tissue-resident memory CD8+T-cells, CD16+ and CD16− NK cells, B cells, zinc transporter-expressing prostate-associated macrophages [6], mast cells and a paucity of immature, usually tolerogenic, dendritic cells [7]. All the stromal components are hormone sensitive and undergo substantial phenotypical and genotypical changes depending on aging, inflammation, and cancer, as discussed below.
Origin and functions of fibroblasts in the prostatic stroma
-
Prostatic stroma fibroblasts originate from several sources.
During embryonic development, mesenchymal progenitor cells migrate from the urogenital sinus mesenchyme (UGM) [8] to the developing prostate gland, and differentiate into different cell types, including fibroblasts, smooth muscle cells, and endothelial cells. The periprostatic mesenchyme surrounding the developing prostate also contributes to the pool of stromal fibroblasts. While the UGM induces prostatic epithelial development [9], signals from the developing glandular epithelium induce the mesenchymal cells of the surrounding tissue to differentiate into fibroblasts [8]. These mesenchymal-epithelial interactions are essential for prostate development. In addition to androgens, which regulate both epithelial and fibroblast proliferation and differentiation, trasforming growth factor beta (TGFβ) is a key regulator of epithelial-mesenchymal interactions during organogenesis. TGFβ signaling from the UGM regulates epithelial proliferation, differentiation, and apoptosis [10], and modulates androgen signaling, fine-tuning the response of epithelial cells to androgens.
In adult tissues, including the prostate gland, bone marrow-derived mesenchymal stem cells (MSCs) can migrate to sites of tissue injury or inflammation and differentiate into fibroblasts, under appropriate microenvironmental conditions [11, 12]. Once established in the prostatic stroma, resident fibroblasts can undergo local proliferation and differentiation to replenish the fibroblast population. This process contributes to the maintenance of fibroblast density and function within the prostate stroma.
Overall, fibroblasts of the prostatic stroma arise from multiple sources, including the embryonic mesenchyme, the periprostatic mesenchyme, bone marrow-derived MSCs, and from the proliferation and differentiation of resident fibroblasts. Resting fibroblasts resident in the normal prostate stroma, positively stain for vimentin and PDGFRα, and can be distinguished from other stromal cells by their surface expression of CD49a, CD49e, CD51/61, and CD30. They have been characterized into two phenotypically and functionally distinct subtypes, namely Sca-1+CD90+fibroblasts, which are located close to the epithelium and express growth factors and genes associated with developmental process and androgen-regulated epithelial cell survival, and Sca-1+CD90−/lowmyofibroblast-like cells, which highly express genes associated with the extracellular matrix and cytokine-mediated signaling pathways, indicating a role in tissue repair and immune responses [13].
-
Fibroblasts play key roles in the prostatic stroma.
1. Secretion of extracellular matrix (ECM) components such as collagen, elastin, and fibronectin, which form the framework providing structural support to the prostate tissue [14]. 2. Maintenance of tissue homeostasis by regulating the balance of cell proliferation and cell death within the prostate tissue. In response to injury or inflammation, fibroblasts become activated and convert into highly contractile myofibroblasts (MFBs), co-expressing vimentin and Alpha Smooth Muscle Actin (αSMA), which secrete ECM components, such as collagen type-I and type-III and are destined for apoptosis after promoting would healing [15]. They proliferate, migrate to the site of injury, and produce ECM components to facilitate tissue repair and restoration of normal tissue architecture. Fibroblasts and the ECM are key components of the stem cell niche and generate an interconnected network of signaling pathways, which allow epithelial stem cell survival and regulate the balance between self-renewal and differentiation, in both normal condition and following injury [16]. 3. Crosstalk with neighboring epithelial cells through the release of mediators that activate paracrine signaling pathways, such as tumor necrosis factor-alpha (TNFα), which exerts growth inhibitory effects on normal prostatic epithelia [17]. Prostatic stromal fibroblasts and epithelial cells engage in bidirectional communication. Fibroblasts secrete growth factors, such as basic fibroblast growth factor (FGF)/FGF-2, transforming growth factor-beta (TGFβ), insulin-like growth factors (IGFs) and keratinocyte growth factor (KGF), which can regulate epithelial cell proliferation and differentiation, and the release of ECM components, such as collagen and elastin, which sustain epithelial cells and maintain the overall architecture of the prostate gland. Conversely, epithelial cells produce AR-modulated growth factors, including TGFβ, IGF, FGF-2 and epidermal growth factor (EGF) [18], and signaling molecules, such as Wnt and Hedgehog proteins (e.g., Sonic hedgehog), which can drive fibroblasts towards a myofibroblast phenotype and up-regulate the expression of ECM components [19, 20]. Prostatic epithelia can also secrete enzymes, such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), to balance ECM degradation and remodeling. Stromal fibroblast-epithelial cell interactions are essential for the normal development, differentiation, and function of the glandular epithelium. 4. Immunomodulation and immune privilege maintenance. Through the production and release of cytokines, such as Interleukin-6 (IL-6), TNFα, TGFβ, IL-1β, IL-33, and CXC and CC chemokines [21,22,23,24,25], prostatic fibroblasts regulate immune cell recruitment and activity to the site of inflammation or infection, and mainly establish an immunosuppressive environment. IL-6 signaling inhibits the expression of MHC-II, CD80/86, and IL‐12 in dendritic cells (DCs) [26] and re‐programs the differentiation into IL‐10‐producing regulatory DCs [27], promotes differentiation into M2 macrophages [28] and hinders T‐cell mediated antitumor responses [29]. TNFα has demonstrated ambivalent functions, mediating both proinflammatory and paradoxical anti-inflammatory and immunomodulatory effects, such as inactivation of TCR signaling [30] or induction of T cell exhaustion [31], CD8+T cell killing and reduction of autoreactive T cells [32]. TNFα may also stimulate myeloid-derived suppressor cells (MDSCs) [33, 34] and may promote regulatory T cell (Treg) expansion and functions [35, 36]. IL-1β promotes the recruitment of immunosuppressive neutrophils, inhibits macrophage activation and accumulation of effector T cells [37]. IL-33 has revealed immunosuppressive functions by promoting M1 to M2 transition and inhibition of T lymphocyte-mediated tumor cell killing [38]. It also promotes a Th2 immune environment and potentiates the suppressive activity of Tregs [39].
Moreover, fibroblasts and stromal components may contribute to the immune privileged state of prostatic tissue, which exhibits limited immune responses compared to other tissues, by regulating the local immune environment and by suppressing excessive immune activation. In addition to creating a physical barrier that limit the infiltration of immune cells, stromal fibroblasts and smooth muscle cells can secrete immunosuppressive cytokines such as TGFβ and IL-10, which dampen the local immune response. TGFβ promotes the differentiation of naïve CD4+ T cells into Tregs, which are critical for maintaining immune tolerance and preventing autoimmune responses, it inhibits the proliferation and cytotoxic activity of CD8+ T cells and downregulates the maturation and antigen-presenting capacity of DCs [40]. IL-10 suppresses the production of pro-inflammatory cytokines, it promotes the development and function of Tregs, inhibits the expression of MHC class II and co-stimulatory molecules (CD80, CD86) on DCs and macrophages, and inhibits the differentiation and proliferation of T helper cells, particularly Th1 and Th17 cells, which are involved in pro-inflammatory responses [41]. Fibroblasts also can express co-inhibitory receptor ligands and checkpoints of T cell functions. Proinflammatory cytokine-induced expression of FasL on fibroblasts [42] might confer immune privilege by inducing apoptosis in infiltrating immune cells, thus suppressing the inflammatory response, and preventing autoimmune reaction, while favoring immune evasion by cancer cells [43, 44]. In response to IFNγ, stromal fibroblasts may express inhibitory PD-1 ligands, such as PD-L1 [45,46,47], that engage with immune checkpoints, further inhibiting immune activation [48], and can express Indoleamine 2,3-Dioxygenase (IDO). This tryptophan-catabolizing enzyme contributes to an immunosuppressive environment, by inhibiting T cell proliferation and by promoting Treg development, which favors tumor immune evasion [49, 50]. 5. Support of blood vessels and nerves by contributing to their maintenance and organization within the prostate stroma. Angiogenesis is crucial for wound healing and fibroblasts may produce angiogenic mediators such as VEGF, FGF-2, PDGF, TGFβ, and angiopoietins, such as Ang-1 and Ang-2, which regulate blood vessel formation and maturation. Fibroblasts can also produce interleukins (e.g., IL-8) [51] and chemokines (e.g., CXCL12 and CXCL5) [52,53,54] that contribute to angiogenesis by recruiting and activating endothelial cells and other cell types involved in the process [55]. Stromal fibroblasts also provide trophic support to nerve fibers and may produce several neurotrophic factors including Nerve Growth Factor (NGF) [56,57,58], a neurotrophic factor that promotes the growth, differentiation, and survival of nervous cells, including sensory neurons [59], glial cell line-derived neurotrophic factor (GDNF), which regulates the development and the maintenance of peripheral nerves, neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5), which can support the survival and function of different types of neurons [60], FGF-2, which exerts neuroprotective effects on peripheral nerves and promotes neuron survival during injury [61, 62], and TGFβ, which also regulates neuronal function and plasticity [63]. 6. Interplay with the bacterial flora, also known as microbiota, which contributes to the extracellular microenvironment. Increasing evidence suggests that changes in the healthy microbiota (i.e. microbial dysbiosis), including gut, urinary-tract and prostatic microbes, which are also age-related [64], play a role not only in triggering inflammation, but also in cancer development, progression, and/or treatment outcome. Bacteria produce proteases, including collagenase, elastase, and hyaluronidase, which degrade the ECM [65] and induce inflammation, which sustains ECM remodeling and affects fibroblast functions, as well as the generation of oxygen radicals leading to DNA damage, compensatory epithelial cell proliferation and mutations that drive tumor onset and recurrence [66]. The gut microbiota affects both the stroma and prostatic epithelium through their metabolites [67]. It has been reported that the abundance of short-chain fatty acid (SCFA) producing intestinal bacteria, namely Rikenellaceae, Alistipes, and Lachnospira, is associated with a high-risk of developing PC, and that these bacterial populations are considerably increased in men with high Gleason grade PC [68]. The prostatic microbial ecosystem, which has not been fully explored, also appears to be altered in the PC microenvironment, with reduced overall species diversity in malignant tissue samples compared to benign tissue samples. The Shewanella genera might be associated with malignant transformation, whereas decreased Vibrio parahaemolyticus counts have been associated with the development of treatment resistance, and the Microbacterium sp. appear to be related with advanced stage PC [69]. An increase in Propionibacterium acnes, Herpesviridae and Papillomaviridae families, and Mycoplasma genitalium, has been recently associated with PC development, although the data needs validation in a larger cohort study [70, 71].
Overall, fibroblasts are crucial for maintaining the structural integrity, homeostasis, functions and immune privilege of the prostatic tissue and dysregulation of their function has been implicated in the pathogenesis of prostatic diseases, including benign prostatic hypertrophy (BPH) and PC.
Prostatic stroma in ageing
With advancing age, the prostate undergoes changes, including stromal remodeling, which are implicated in the development of BPH [72] and PC [73]. A key feature of senescence in cells, including stromal fibroblasts, is a widespread change in epigenetic gene expression [74] leading to an increased production and secretion of proinflammatory cytokines, such as GM-CSF, TGFβ, IL-1β, IL-6, IL-8, IL-10, IL-33, and chemokines, such as CXCL12, CXCL1 and CXCL2, and growth factors, such as connective tissue growth factor (CTGF), and angiogenic factors, such as insulin-like growth factor-binding protein 7 (IGFBP7), vascular endothelial growth factor (VEGF), MMPs, plasminogen activator inhibitors (PAIs), tissue-type plasminogen activator (tPA), as well as reactive oxygen species (ROS) [75, 76], and other key signaling proteins that have powerful paracrine effects on both the glandular epithelia [77], with proliferation-stimulating effects, and the surrounding stromal cells leading to fibrosis and chronic inflammation [78].
Proliferation of the prostatic epithelium has been shown to increase up to three times due to paracrine-acting proteins, such as FGF-7, hepatocyte growth factor (HGF), and amphiregulin released by senescent fibroblasts, suggesting that aging-related changes in the prostate microenvironment contribute to the development of PC [79, 80].
Age-associated stromal fibrosis results from the accumulation of extracellular matrix proteins, primarily senescent collagen, endowed with a high content of the glycosaminoglycan hyaluronan (HA), which stimulate epithelial cell proliferation [81], and from the increased fibroblast release of the enzyme lysyl oxidase (Lox), which cross-links collagen fibers promoting collagen maturation, and contributes to extracellular matrix (ECM) remodeling, matrix stiffening and fibrosis [82].
Age-associated chronic inflammation has the hallmarks of immunosuppression and immune evasion and is characterized by MDSCs and Treg cell infiltrates, and by a switch towards M2 and N2, alternatively activated macrophages [83] and neutrophils [84], both endowed with tumor-promoting activity.
As men age and testosterone levels decline, the balance between testosterone and estrogens may shift towards higher estrogen levels, which stimulate the proliferation of prostatic fibroblasts and their production of inflammatory cytokines, resulting in endothelial adhesion molecule expression and immune cell recruitment and activation, which, in turn, impact on endothelial functions [85, 86]. Estrogens also promote prostatic fibroblast production of angiogenic factors, such as FGF-2, EGF, and IGF-1 [87], and collagen, both of which affect endothelial permeability, functions and vascular remodeling [88, 89]. Along with dihydrotestosterone derived from testosterone as a consequence of an age-associated increase in 5-alpha reductase activity [90], estrogens have been implicated in the development of BPH and PC, in which activated and proliferating fibroblasts play a critical role [72, 91, 92].
Androgens can influence the transcriptional programs and inflammatory profile of fibroblasts, leading to an altered inflammatory environment which impacts the functional state of endothelia. Age-associated testosterone deficiency promotes pro-migratory cytokine release by fibroblasts [93] and contributes to chronic inflammation, induces endothelial dysfunction [94, 95], decreases vascularity [96], impairs arterial elasticity and microvascular function [97], and reduces nitric oxide (NO) production [98]. Reduced testosterone levels can contribute to the premature senescence of stromal fibroblasts [99]. Senescent fibroblasts secrete inflammatory and matrix-degrading molecules and are characterized by increased production of reactive oxygen species (ROS) [100]. Following ROS production, the activities of several enzymes of the testosterone biosynthetic pathway are reduced, resulting in further decrease in testosterone synthesis and secretion [101]. Elevated ROS levels can cause oxidative damage to endothelial cells, impairing their function. Endothelial dysfunction [102], defined as a reduced capacity for NO production and decreased NO sensitivity [94] which leads to lower peripheral vasodilation, is a hallmark of vascular ageing [103, 104]. Therefore, inflammation and chronic oxidative stress are associated with vascular ageing [105] and testosterone deficiency [95, 106].
Prostatic stroma in tumor onset and progression
Stromal reactivity in the early stages of prostate carcinogenesis
Alterations of the prostatic stroma, at the cellular and molecular level, are found during prostate carcinogenesis from the early stages. Chronic inflammation [107], hormonal changes [108], high concentrations of ROS [109,110,111], typically associated with ageing, along with the genetic background [112] promote, in the peripheral zone of the prostatic gland, the development of high-grade prostatic intraepithelial neoplasia (HG-PIN), the first step toward carcinogenesis. This premalignant lesion, consisting of multilayered atypical epithelial cells, endowed with prominent nucleoli, which proliferate within the prostatic duct and acini, is surrounded by a phenotypically and genotypically subverted stroma. Soluble factors, such as serine protease kallikrein-related peptidase 4 (KLK4), which is produced by atypical epithelial cells, promote stromal reactivity [113, 114], by favoring fibroblasts switch to myofibroblasts, which over-express procollagen I and tenascin C [115, 116], that regulate cell adhesion, migration, and signaling, leading to ECM remodeling. KLK4 activates IGF and TGFβ signaling pathways, and protease-activated receptor 1, PAR1, expressed by stromal cells [117], which lead to increased production of pro-tumorigenic and pro-angiogenic factors, such as FGF1 and VEGF [113]. In turn, vimentin+αSMA+myofibroblasts present within the reactive stroma, interact with atypical epithelial cells of the HG-PIN and regulate their behavior, through the release of growth factors and cytokines, such as EGF, VEGF and TGFβ. TGFβ contributes to the establishment of an immunosuppressive microenvironment by promoting Treg cell development and differentiation, through inducing forkhead box p3 (Foxp3) expression [118, 119], and by inhibiting CD8+ T cell activity, thus sustaining immune-escape mechanisms [120].
Phenotypic and genotypic differences between prostatic stroma and prostate cancer stroma
The stroma of PC exhibits cellular and molecular differences compared to normal prostatic stroma, reflecting the altered microenvironment and interactions with cancer cells [92, 121, 122]. Some of the key distinctions are the following.
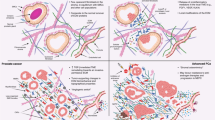
a. Cellular composition. PC stroma exhibits a loss of well-differentiated smooth muscle cells and increased numbers of mostly activated fibroblasts, namely cancer-associated fibroblasts (CAFs) [123], immune cells, and endothelial cells. Unlike “resting” fibroblasts harboring the normal stroma, CAFs which share co-expression of vimentin and αSMA with MFBs, remain in the “proliferative phase” of the wound healing response by releasing a range of growth factors and cytokines, such as TGFβ, FGFs, HGF, IL-6 [123], which foster tumor growth [124]. Compared to fibroblasts, CAFs overexpress fibroblast activation protein (FAP) (Fig. 1), PDGFRβ, fibroblast specific protein 1 (FSP-1) and αSMA [125] and can be distinguished into functionally distinct subsets of cells with dysregulated expression of genes associated with ECM remodeling, inflammation, angiogenesis, and immune modulation [126, 127]. Epigenetic alterations, including DNA methylation, histone modifications, and non-coding RNA dysregulation, contribute to the reprogramming of stromal cells towards a tumor-promoting and immunosuppressive phenotype. Promoter hypermethylation and silencing of the Ras GTPase-activating protein, RASAL3, which is further promoted by androgen deprivation therapy (ADT), result into Ras signaling activation in CAFs driving macropinocytosis-mediated glutamine synthesis, that provides the PC epithelia with abundant glutamine, which fuels its proliferation and neuroendocrine differentiation [128, 129] The epigenetic silencing of telomerases, due to inhibition of TGFβ signaling via TGFBR2 promoter methylation, leads to the increase in histone methyltransferase, SUV39H1 (which in turn affects histone methylation levels at the telomeric ends), and consequent telomere shortening in stromal CAFs, which is associated with PC progression and mortality [130, 131]. Accumulating evidence shows that noncoding RNAs (ncRNAs) play a critical role in the crosstalk between CAFs and tumor cells. MicroRNAs, which are small, noncoding RNAs, are pivotal regulatory factors for the formation and activation of CAFs and their metabolic reprogramming by tumor cells, whereas exosomal miRNAs, derived from CAFs, affect tumor cell proliferation, metabolism, angiogenesis, metastasis and chemoresistance, ultimately regulating tumor progression [132,133,134].
Research into the various subtypes of CAFs in PC is still ongoing, but several distinct populations have been identified based on their molecular and functional characteristics. 1. Myofibroblastic CD90highCAFs, characterized by the expression of αSMA, which are involved in ECM remodeling, enhanced matrix stiffness and secretion of growth factors stimulating cancer cell proliferation [135]. 2. Inflammatory CAFs, characterized by the secretion of CCL2, CXCL12, IL-6 and leukocyte inhibiting factor (LIF). They contribute to chronic inflammation within the tumor microenvironment (TME) and facilitate tumor progression by promoting angiogenesis, immune suppression, and epithelial-to-mesenchymal transition (EMT) [135]. 3. Senescent CAFs, which express high levels of αSMA, senescence-associated beta-galactosidase (SA-β-gal) and p16. They undergo cell cycle arrest and reveal a senescence-associated secretory phenotype (SASP), which includes factors that promote tumor cell proliferation, migration, and survival. Senescent CAFs can also induce therapy resistance in PC [136, 137]. 4. Neuroendocrine CD90lowCD105+CAFs, which promote PC progression and are associated with aggressive PC phenotypes, including resistance to ADT and neuroendocrine transdifferentiation [128, 138]. 5. Metabolic CAFs, which undergo metabolic reprogramming to support tumor growth and survival by promoting aerobic glycolysis (the Warburg effect), fatty acid metabolism, and amino acid metabolism. They supply energy substrates (lactate) and biosynthetic precursors to cancer cells, contributing to tumor progression and therapy resistance. The hallmark of this metabolic switch consists in high expression levels of lactic dehydrogenase (LDHA), pyruvate kinase M2 (PKM2) and monocarboxylate transporter 4 (MCT4) [139]. 6. Immunomodulatory CAFs, which inhibit anti-tumor immune responses, promote immune evasion, and contribute to immunotherapy resistance in PC. Immunomodulatory CAFs are represented by CCL2-secreting CAFs, which mainly recruit monocytes/macrophages and Tregs, and inhibit CD8+ T cell effector functions [140,141,142], and CXCL12-secreting CAFs, which in addition to contributing to tumor cell survival, angiogenesis, desmoplasia and chemoresistance [143], promote the recruitment of MDSCs, M2-phenotype macrophages, and Tregs [144, 145]. These subtypes of CAFs coexist in the PC stroma and exhibit phenotypic plasticity in response to stimuli from the TME [146, 147].
ECM remodeling. During PC development, the ECM undergoes significant changes in composition, organization, and function, which contribute to tumor growth, invasion, metastasis, and therapy resistance.
In the normal prostate tissue, the ECM primarily consists of proteins such as collagen, laminins, fibronectin, and proteoglycans, which provide structural support and regulate cellular functions. In PC, the ECM becomes disorganized and heterogeneous (Fig. 2) and shows an increase in the deposition of collagen, particularly collagen type I, III, IV and V, and of proteoglycans, such as versican, decorin, and perlecan, by CAFs, and alterations in collagen fibers alignment and density, that contribute to stromal stiffness and altered biomechanical properties [148]. Enhanced matrix stiffness promotes M2 phenotype polarization in macrophages, which in turn favor ECM deposition [149]. Higher collagen density has been associated with higher Gleason score suggesting its involvement in PC aggressiveness [115]. Proteomic signature of CAFs versus normal prostate fibroblasts revealed their prominent synthesis of multiple collagens, including the fibrillar types COL1A1/2 and COL5A1; increased activity and/or expression of the receptor tyrosine kinase discoidin domain-containing receptor 2 (DDR2), a receptor for fibrillar collagens; and lysyl oxidase-like 2 (LOXL2), an enzyme that promotes collagen crosslinking [150]. Additionally, there may be aberrant expression of ECM-modifying enzymes, such as MMPs, including upregulation of MMP-2, -7, -9, membrane-type (MT)1-MMP [151]; and downregulation of TIMPs, such as TIMP-1 [152], leading to a substantial ECM remodeling.
In the normal prostate tissue, the interactions between epithelial cells, stromal cells, and the ECM are tightly regulated and contribute to tissue homeostasis. In PC, neoplastic cells may exhibit enhanced adhesion to specific ECM proteins, allowing them to migrate and invade surrounding tissues. CAFs also subvert androgen biosynthesis in PC cells by secreting glucosamine that specifically upregulate epithelial 3β-Hydroxysteroid dehydrogenase-1 (3βHSD1) expression, and induce androgen synthesis, which leads to androgen receptor activation, development of CRPC and antiandrogen resistance [153]. The type II transmembrane FAP, which is enriched on the surface of CAFs in the PC stroma, has recently proven to be a useful biomarker for diagnosis, through 68Ga-FAP-targeted PET-CT254 imaging [154, 155], and a target for therapy, since FAP-directed ligands carrying therapeutic payloads have shown promising results in cancer patient trials [156, 157]. Increased stromal content of collagen, fibronectin, laminin, abundant expression of secreted protein acidic and rich in cysteine (SPARC/osteonectin) and tenascin C, in association with a downregulation of angiogenesis inhibitors, such as thrombospondin (TSP)-1 and TSP-2 [158,159,160] are all involved in ECM remodeling, endothelial cell recruitment and angiogenesis [161] and in the development of resistance to therapy [155].
b. Differences between fibrosis and desmoplasia. The term desmoplasia (from the Greek word desmos, to fetter or restrain; and plasis, formation) is used by pathologists to describe the formation of excessive connective tissue around invasive carcinoma [162]. It is characterized by alterations of the tumor stroma that can range from an abundance of cellular elements, such as fibroblasts, vascular cells, and immune cells with little ECM, to the presence of an abundant collagen-rich ECM with a minimum of cells, mainly fibroblasts and myofibroblasts [163]. It is considered as a response to the presence of invasive tumor cells, but the possibility that desmoplasia may precede the presence of malignant cells cannot be ruled out [164]. Fibrosis and desmoplasia are both terms used to describe the abnormal growth of fibrous tissue, however they have distinct characteristics and occur in different contexts. Fibrosis is a pathological process that can occur in response to various insults or injuries, such as inflammation resulting from ageing (IL-8, CXCL5, CXCL1, CXCL6, and CXCL12 secretion by senescent fibroblasts and epithelial cells), infection, or inflammation-associated metabolic diseases (for example, type 2 diabetes mellitus), and is characterized by the excessive accumulation of fibrous connective tissue, primarily collagen, in the stroma. Fibrosis is associated with the loss of normal tissue architecture and can contribute to lower urinary tract dysfunction and BPH [165]. Desmoplasia specifically refers to the growth of dense, fibrous tissue in response to cancer development and involves the deposition of collagen I and fibronectin, and other ECM components, such as proteoglycan syndecan-1, hyaluronic acid and tenascin-C around the tumor. TGFβ signaling from CAFs plays a key role in the structural and mechanical changes that lead to desmoplasia in PC. Desmoplasia and TGFβ induced translocation of SMAD2/3 to the nucleus of PC cells, amplifies their expression of mesenchymal markers, leading to EMT and favoring PC progression [166]. Desmoplasia creates a dense stroma that may help contain the tumor, but that can also create barriers that limit drug delivery and contribute to treatment resistance [167, 168], which ultimately depends on the delicate balance between the different microenvironmental components [169].
c. Immune cell context. In the normal healthy prostate, the immune cell atlas delineates a range of innate and adaptive immune cells, with several CD4+ and CD8+ T cell subsets, including naïve, tissue-resident memory, and regulatory CD4+ T cells, which help maintain immune tolerance and prevent autoimmune reactions, as well as cytotoxic and tissue-resident memory CD8+ T-cell clusters, two subsets of NK cells (CD16+ and CD16−), B cells, mostly mature non-naïve responsible for antibody production, CD1 and CD2 conventional DCs [170], mast cells, which can release histamine and other mediators regulating the local immune environment, monocytes and a prostate-specific metallothionein-expressing macrophage subset (MAC-MT), which regulates prostate zinc and plays homeostatic role that contribute to organ physiology and function [6, 171].
Stromal cells, mostly the CAF subsets, substantially contribute to the immune perturbation that characterize PC development, and exhibit increased expression of genes coding for cytokines, chemokines, and immune cell recruitment factors [172]. Expression of CXCL12 by CAFs, CCL2 by pericytes, along with CCL3,4, and 5 by cancer cells lead to CD16high monocyte and CD14high inflammatory macrophage recruitment [173]. Antigen presenting macrophages with a high “antigen processing and presentation gene signature”, as well as M2-macrophages with a high “M2-gene signature” have been found in the PC stroma and have been shown to suppress the anti-tumor immune response, as observed across a broad range of tumors [173]. High infiltration of M2-macrophages in PC tissue has been linked to tumor recurrence and metastasis [174].
Signals from both the stromal and the epithelial components of PC shape the immunosuppressive TME leading to T cell exhaustion, Treg cell recruitment, accumulation of monocytic (Mo)-MDSCs, endowed with iNOS activity and NO production [175] and granulocytic polymorphonuclear (PMN)-MDSCs that typically produce IL-1β and IL-23, and suppress T cell functions by NADPH-oxidase and ARG1 activities [176]. Expression of programmed death ligand-1/2 PD-L1 and PD-L2, by FAPhigh CAF subset has been recently described, in different tumor types, as well as PD-L1 induction on tumor cells by CXCL5 released by CAFs [177]. Based on its substantial content in CAFs, analogous mechanisms of anti-tumor T cell inhibition may take place in PC and contribute to its immunosuppressive microenvironment [178].
Prognostic value of the prostate cancer stroma
The PC stroma has emerged as a significant factor in predicting disease progression, treatment response, and overall patient outcomes [179]. Stromal cells and ECM components interact closely with cancer cells and influence tumor behavior promoting tumor growth, invasion, and metastasis through various mechanisms, including cytokine signaling, ECM remodeling, and angiogenesis. Deep learning methodologies in combination with mathematical modelling are currently being developed to quantify stromal stains and to allow digital multiplex analyses of cancer stroma components [180]. The key points regarding the prognostic value of the PC stroma are represented by, a. Elevated levels of stromal markers, such as αSMA, fibroblast-specific protein 1 (FSP1), and FAP, which have been associated with aggressive disease, metastasis, and poor prognosis in PC patients [181,182,183]; b. Increased stromal density, and alterations in stromal morphology and architecture, as assesses by histopathological methods [115], which have been associated with higher Gleason scores, advanced stage disease, and poorer prognosis; c. Copy number alterations and mutations of genes encoding ECM proteins and proteins modulating the ECM structure or function are frequent in cancer [184] and involve the PC stroma with an impact on tumor behavior and clinical outcome. Amplification of COL1A1, COL4A2, and COL6A1 genes and protein overexpression, have been observed in PC and are associated with tumor aggressiveness and metastasis. Aberrant expression of laminins, such as laminin-332, as well as dysregulation of integrins, including αvβ3 and αvβ6, and overexpression of MMPs, particularly MMP-2 and MMP-9, have been implicated in PC progression and metastasis [185]. Overexpression of versican, decorin, and periostin has been reported in PC and is associated with tumor aggressiveness.
Advanced imaging modalities, including magnetic resonance imaging (MRI) and multiparametric MRI (mpMRI), can provide insights into the stromal composition and its spatial distribution within the prostate gland and may complement traditional clinical staging methods. Integrating stromal features into prognostic models and nomograms can improve risk stratification and prediction of disease outcomes in PC patients. Multimodal approaches that incorporate both epithelial and stromal factors may enhance accuracy of prognostic assessments and guide personalized treatment decision-making.
Tumor stroma targeting strategies
As an essential component of the TME, the stroma is highly dynamic, heterogeneous and tumor-type specific. All of its components, which include ECM, CAFs, endothelial cells, pericytes and other mesenchymal cells, interact with each other in a coordinated fashion and collectively promote tumor onset, progression and therapeutic resistance [186, 187]. Clinical trials testing treatments targeting, specifically, the fibroblastic and matrix components of the PC stroma are currently lacking, whilst a study (NCT02452008) testing TGFβ pathway inhibition specifically in mCRPC is ongoing. However, several trials, aimed at subverting the tumor stroma components for anti-cancer purposes, are currently underway [188].
Active trials designed to target CAFs are the following.
•Fibroblast activation protein (FAP) is one of the most studied molecules in trials testing therapies aimed at targeting the tumor stroma, specifically CAFs. The clinical trials NCT05723640, NCT0541082 and NCT05963386 are currently testing the safety and tolerability of two novel FAP-targeted radiopharmaceuticals, 177Lu-LNC1004 and 177Lu-DOTA-EB-FAPI, in various solid tumors, whereas the LuMIERE study (NCT04939610) is evaluating the efficacy of 177Lu-FAP-2286 as a monotherapy in patients with pancreatic ductal adenocarcinoma, non-small cell lung cancer, and breast cancer [189].
•The NCT05626829 study is evaluating the safety and effectiveness of using Tranilast, an anti-allergic drug, as a radiotherapy sensitizer in nasopharyngeal carcinoma, since it was recently discovered, through in vivo and in vitro experiments, that it can inhibit the activity of CAFs and reduce their radiotherapy resistance [190,191,192].
•Similarly, the NCT06142318 trial is testing the efficacy of Pirfenidone, a drug approved for the treatment of idiopathic pulmonary fibrosis, as a radiosensitizer in head and neck squamous cell carcinoma, since it can enhance the radiosensitivity of CAFs, in vitro and in vivo [193,194,195].
Active trials designed to target the TGFβ pathway are the following.
•The NCT02452008 study is currently testing the efficacy of Galunisertib (an oral inhibitor of the TGFβ1 type I receptor kinase) in patients with mCRPC. This agent has provided evidence of significant antitumor activity in xenograft models of breast and hepatocellular carcinoma [196,197,198] and it has been demonstrated to reverse the TGFβ-mediated suppression of NK cell function [199].
•Similarly, the NCT05588648 study, is testing the antitumor activity of Vactosertib (a recently discovered TGFβ1 type I receptor kinase inhibitor) in patients with progressive osteosarcoma. Vactosertib is also being studied in two other trials, NCT05436990 and NCT03143985, which are evaluating its antitumor activity in patients with melanoma or multiple myeloma, respectively.
•Lastly, the NCT05821595 trial is evaluating the efficacy of JYB1907 (a humanized monoclonal antibody directed against the TGFβ activator Glycoprotein A Repetitions Predominant - GARP) in patients with solid tumors. The anti-GARP monoclonal antibody selectively targets and binds to GARP. This specifically blocks the GARP-mediated release of the cytokine TGFβ, thereby reversing the immunosuppressive nature of the tumor microenvironment [200].
A wide range of approaches aimed at targeting the cancer stroma to disrupt its supportive role in tumor growth and metastasis are being studied. Combinations of stroma-targeted therapies with conventional treatments such as chemotherapy, radiation therapy, or immunotherapy can synergistically disrupt stromal support and inhibit tumor progression improving patient outcome.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate Cancer. Cold Spring Harb Perspect Med. 2018;8(12):a030361. https://doi.org/10.1101/cshperspect.a030361.
Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, Gill IS. Trends in incidence of metastatic prostate Cancer in the US. JAMA Netw Open. 2022;5(3):e222246. https://doi.org/10.1001/jamanetworkopen.2022.2246.
Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P. Cancer immunotherapy: sipuleucel-T and beyond. Pharmacotherapy. 2011;31(8):813–28. https://doi.org/10.1592/phco.31.8.813.
Sanches BDA, Maldarine JS, Vilamaior PSL, Felisbino SL, Carvalho HF, Taboga SR. Stromal cell interplay in prostate development, physiology, and pathological conditions. Prostate. 2021;81(13):926–37. https://doi.org/10.1002/pros.24196.
Tuong ZK, Loudon KW, Berry B, Richoz N, Jones J, Tan X, Nguyen Q, George A, Hori S, Field S, Lynch AG, Kania K, Coupland P, Babbage A, Grenfell R, Barrett T, Warren AY, Gnanapragasam V, Massie C, Clatworthy MR. Resolving the immune landscape of human prostate at a single-cell level in health and cancer. Cell Rep. 2021;37(12):110132. https://doi.org/10.1016/j.celrep.2021.110132.
Sutherland SIM, Ju X, Horvath LG, Clark GJ. Moving on from Sipuleucel-T: new dendritic cell vaccine strategies for prostate Cancer. Front Immunol. 2021;12:641307. https://doi.org/10.3389/fimmu.2021.641307.
Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, Franco O, Baskin LS. Development of the human prostate. Differ 2018 Sep-Oct;103:24–45. https://doi.org/10.1016/j.diff.2018.08.005
Cunha GR, Cao M, Derpinghaus A, Baskin LS. Human urogenital sinus mesenchyme is an inducer of prostatic epithelial development. Am J Clin Exp Urol. 2021;9(4):329–36.
Timme TL, Truong LD, Merz VW, Krebs T, Kadmon D, Flanders KC, Park SH, Thompson TC. Mesenchymal-epithelial interactions and transforming growth factor-beta expression during mouse prostate morphogenesis. Endocrinology. 1994;134(3):1039–45. https://doi.org/10.1210/endo.134.3.8119140.
Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell Migration and tissue repair. Cells. 2019;8(8):784. https://doi.org/10.3390/cells8080784.
Nam D, Park A, Dubon MJ, Yu J, Kim W, Son Y, Park KS. Coordinated regulation of Mesenchymal Stem Cell Migration by various chemotactic stimuli. Int J Mol Sci. 2020;21(22):8561. https://doi.org/10.3390/ijms21228561.
Kwon OJ, Zhang Y, Li Y, Wei X, Zhang L, Chen R, Creighton CJ, Xin L. Functional heterogeneity of mouse prostate stromal cells revealed by single-cell RNA-Seq. iScience. 2019;13:328–38. https://doi.org/10.1016/j.isci.2019.02.032.
Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V, Ricard-Blum S, Schmelzer CEH, Duca L, Durbeej M, Afratis NA, Troeberg L, Franchi M, Masola V, Onisto M. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288(24):6850–912. https://doi.org/10.1111/febs.15776.
Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–3. https://doi.org/10.1002/path.1427.
Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24(5):645–51. https://doi.org/10.1016/j.ceb.2012.07.001.
Paland N, Kamer I, Kogan-Sakin I, Madar S, Goldfinger N, Rotter V. Differential influence of normal and cancer-associated fibroblasts on the growth of human epithelial cells in an in vitro cocultivation model of prostate cancer. Mol Cancer Res. 2009;7(8):1212–23. https://doi.org/10.1158/1541-7786.MCR-09-0073.
Wen S, Chang HC, Tian J, Shang Z, Niu Y, Chang C. Stromal androgen receptor roles in the development of normal prostate, benign prostate hyperplasia, and prostate cancer. Am J Pathol. 2015;185(2):293–301. https://doi.org/10.1016/j.ajpath.2014.10.012.
Wei X, Zhang L, Zhou Z, Kwon OJ, Zhang Y, Nguyen H, Dumpit R, True L, Nelson P, Dong B, Xue W, Birchmeier W, Taketo MM, Xu F, Creighton CJ, Ittmann MM, Xin L. Spatially restricted stromal wnt signaling restrains prostate epithelial progenitor growth through Direct and Indirect mechanisms. Cell Stem Cell. 2019;24(5):753–e7686. https://doi.org/10.1016/j.stem.2019.03.010.
Peng YC, Joyner AL. Hedgehog signaling in prostate epithelial-mesenchymal growth regulation. Dev Biol. 2015;400(1):94–104. https://doi.org/10.1016/j.ydbio.2015.01.019.
Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. https://doi.org/10.2741/a171.
Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine. 2003;24(6):266–76. https://doi.org/10.1016/j.cyto.2003.08.003.
Gharaee-Kermani M, Kasina S, Moore BB, Thomas D, Mehra R, Macoska JA. CXC-type chemokines promote myofibroblast phenoconversion and prostatic fibrosis. PLoS ONE. 2012;7(11):e49278. https://doi.org/10.1371/journal.pone.0049278.
Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–21. https://doi.org/10.1378/chest.06-2568.
Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. https://doi.org/10.1146/annurev-immunol-032713-120145.
Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, Murakami M, Hirano T. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23(5):491–502. https://doi.org/10.1016/j.immuni.2005.09.010.
Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like receptor 2 activation promotes Tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Cell Rep. 2015;13(12):2851–64. https://doi.org/10.1016/j.celrep.2015.11.053.
Zhou J, Qu Z, Sun F, Han L, Li L, Yan S, Stabile LP, Chen LF, Siegfried JM, Xiao G. Myeloid STAT3 promotes lung tumorigenesis by transforming Tumor Immunosurveillance into Tumor-promoting inflammation. Cancer Immunol Res. 2017;5(3):257–68. https://doi.org/10.1158/2326-6066.CIR-16-0073.
Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109(3):523–30. https://doi.org/10.1111/cas.13433.
Bazzaz JT, Amoli MM, Taheri Z, Larijani B, Pravica V, Hutchinson IV. TNF-α and IFN-γ gene variation and genetic susceptibility to type 1 diabetes and its microangiopathic complications. J Diabetes Metab Disord. 2014;13:46. https://doi.org/10.1186/2251-6581-13-46.
Beyer M, Abdullah Z, Chemnitz JM, Maisel D, Sander J, Lehmann C, Thabet Y, Shinde PV, Schmidleithner L, Köhne M, Trebicka J, Schierwagen R, Hofmann A, Popov A, Lang KS, Oxenius A, Buch T, Kurts C, Heikenwalder M, Fätkenheuer G, Lang PA, Hartmann P, Knolle PA, Schultze JL. Tumor-necrosis factor impairs CD4(+) T cell-mediated immunological control in chronic viral infection. Nat Immunol. 2016;17(5):593–603. https://doi.org/10.1038/ni.3399.
Ban L, Zhang J, Wang L, Kuhtreiber W, Burger D, Faustman DL. Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism. Proc Natl Acad Sci U S A. 2008;105(36):13644–9. https://doi.org/10.1073/pnas.0803429105.
Hu X, Li B, Li X, Zhao X, Wan L, Lin G, Yu M, Wang J, Jiang X, Feng W, Qin Z, Yin B, Li Z. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol. 2014;192(3):1320–31. https://doi.org/10.4049/jimmunol.1203195.
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122(11):4094–104. https://doi.org/10.1172/JCI64115.
Chen X, Bäumel M, Männel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4 + CD25 + T regulatory cells. J Immunol. 2007;179(1):154–61. https://doi.org/10.4049/jimmunol.179.1.154.
Chen X, Subleski JJ, Kopf H, Howard OM, Männel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4 + CD25 + FoxP3 + T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180(10):6467–71. https://doi.org/10.4049/jimmunol.180.10.6467.
Kiss M, Vande Walle L, Saavedra PHV, Lebegge E, Van Damme H, Murgaski A, Qian J, Ehling M, Pretto S, Bolli E, Keirsse J, Bardet PMR, Arnouk SM, Elkrim Y, Schmoetten M, Brughmans J, Debraekeleer A, Fossoul A, Boon L, Raes G, van Loo G, Lambrechts D, Mazzone M, Beschin A, Wullaert A, Lamkanfi M, Van Ginderachter JA, Laoui D. IL1β promotes Immune suppression in the Tumor Microenvironment Independent of the Inflammasome and Gasdermin D. Cancer Immunol Res. 2021;9(3):309–23. https://doi.org/10.1158/2326-6066.CIR-20-0431.
Wu J, Chen Z, Wickström SL, Gao J, He X, Jing X, Wu J, Du Q, Yang M, Chen Y, Zhang D, Yin X, Guo Z, Jensen L, Yang Y, Tao W, Lundqvist A, Kiessling R, Cao Y. Interleukin-33 is a Novel Immunosuppressor that protects Cancer cells from TIL killing by a macrophage-mediated shedding mechanism. Adv Sci (Weinh). 2021;8(21):e2101029. https://doi.org/10.1002/advs.202101029.
Siede J, Fröhlich A, Datsi A, Hegazy AN, Varga DV, Holecska V, Saito H, Nakae S, Löhning M. IL-33 receptor-expressing Regulatory T cells are highly activated, Th2 biased and suppress CD4 T cell proliferation through IL-10 and TGFβ release. PLoS ONE. 2016;11(8):e0161507. https://doi.org/10.1371/journal.pone.0161507.
David CJ, Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19(7):419–35. https://doi.org/10.1038/s41580-018-0007-0.
D’Orazio TJ, Niederkorn JY. A novel role for TGF-beta and IL-10 in the induction of immune privilege. J Immunol. 1998;160(5):2089–98.
Vaux DL. Immunology. Ways around rejection. Nature. 1995;377(6550):576–7. https://doi.org/10.1038/377576a0.
Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2(12):917–24. https://doi.org/10.1038/35103104.
Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8(1):74–80. https://doi.org/10.1038/nri2233.
Lizée G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12(16):4794–803. https://doi.org/10.1158/1078-0432.CCR-06-0944.
Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT, Li Y, Zhang M, Joseph RW, Bernatchez C, Ekmekcioglu S, Grimm E, Radvanyi LG, Davis RE, Davies MA, Wargo JA, Hwu P, Lizée G. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18(19):5329–40. https://doi.org/10.1158/1078-0432.CCR-12-1632.
Kawasaki K, Noma K, Kato T, Ohara T, Tanabe S, Takeda Y, Matsumoto H, Nishimura S, Kunitomo T, Akai M, Kobayashi T, Nishiwaki N, Kashima H, Maeda N, Kikuchi S, Tazawa H, Shirakawa Y, Fujiwara T. PD-L1-expressing cancer-associated fibroblasts induce tumor immunosuppression and contribute to poor clinical outcome in esophageal cancer. Cancer Immunol Immunother. 2023;72(11):3787–802. https://doi.org/10.1007/s00262-023-03531-2.
Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–30. https://doi.org/10.1126/science.1195300.
Sarkhosh K, Tredget EE, Karami A, Uludag H, Iwashina T, Kilani RT, Ghahary A. Immune cell proliferation is suppressed by the interferon-gamma-induced indoleamine 2,3-dioxygenase expression of fibroblasts populated in collagen gel (FPCG). J Cell Biochem. 2003;90(1):206–17. https://doi.org/10.1002/jcb.10593.
Curran TA, Jalili RB, Farrokhi A, Ghahary A. IDO expressing fibroblasts promote the expansion of antigen specific regulatory T cells. Immunobiology. 2014;219(1):17–24. https://doi.org/10.1016/j.imbio.2013.06.008.
Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–76. https://doi.org/10.4049/jimmunol.170.6.3369.
Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10(3–4):359–70. https://doi.org/10.1038/sj.mn.7800200.
Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43(2):194–9. https://doi.org/10.1016/j.cyto.2008.05.012.
Sorrentino C, D’Antonio L, Ciummo SL, Fieni C, Landuzzi L, Ruzzi F, Vespa S, Lanuti P, Lotti LV, Lollini PL, Di Carlo E. CRISPR/Cas9-mediated deletion of Interleukin-30 suppresses IGF1 and CXCL5 and boosts SOCS3 reducing prostate cancer growth and mortality. J Hematol Oncol. 2022;15(1):145. https://doi.org/10.1186/s13045-022-01357-6.
Cambier S, Gouwy M, Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol. 2023;20(3):217–51. https://doi.org/10.1038/s41423-023-00974-6.
Olgart C, Frossard N. Human lung fibroblasts secrete nerve growth factor: effect of inflammatory cytokines and glucocorticoids. Eur Respir J. 2001;18(1):115–21. https://doi.org/10.1183/09031936.01.00069901.
Chmilewsky F, Ayaz W, Appiah J, About I, Chung SH. Nerve growth factor secretion from pulp fibroblasts is modulated by complement C5a receptor and implied in Neurite Outgrowth. Sci Rep. 2016;6:31799. https://doi.org/10.1038/srep31799.
Manni L, Lundeberg T, Fiorito S, Bonini S, Vigneti E, Aloe L. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin Exp Rheumatol. 2003 Sep-Oct;21(5):617–24.
Rocco ML, Soligo M, Manni L, Aloe L. Nerve growth factor: early studies and recent clinical trials. Curr Neuropharmacol. 2018;16(10):1455–65. https://doi.org/10.2174/1570159X16666180412092859.
Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. https://doi.org/10.1146/annurev.neuro.24.1.677.
Kasai M, Jikoh T, Fukumitsu H, Furukawa S. FGF-2-responsive and spinal cord-resident cells improve locomotor function after spinal cord injury. J Neurotrauma. 2014;31(18):1584–98. https://doi.org/10.1089/neu.2009.1108.
Kang CE, Baumann MD, Tator CH, Shoichet MS. Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury. Cells Tissues Organs. 2013;197(1):55–63. https://doi.org/10.1159/000339589.
Meyers EA, Kessler JA. TGF-β Family Signaling in neural and neuronal differentiation, Development, and function. Cold Spring Harb Perspect Biol. 2017;9(8):a022244. https://doi.org/10.1101/cshperspect.a022244.
Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, Drake MJ. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. https://doi.org/10.3389/fcimb.2013.00041.
Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2(9):1051–60. https://doi.org/10.1016/s1286-4579(00)01259-4.
Kiraly O, Gong G, Olipitz W, Muthupalani S, Engelward BP. Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet. 2015;11(2):e1004901. https://doi.org/10.1371/journal.pgen.1004901.
Fujita K, Matsushita M, Banno E, De Velasco MA, Hatano K, Nonomura N, Uemura H. Gut microbiome and prostate cancer. Int J Urol. 2022;29(8):793–8. https://doi.org/10.1111/iju.14894.
Matsushita M, Fujita K, Motooka D, Hatano K, Fukae S, Kawamura N, Tomiyama E, Hayashi Y, Banno E, Takao T, Takada S, Yachida S, Uemura H, Nakamura S, Nonomura N. The gut microbiota associated with high-gleason prostate cancer. Cancer Sci. 2021;112(8):3125–35. https://doi.org/10.1111/cas.14998.
Salachan PV, Rasmussen M, Fredsøe J, Ulhøi B, Borre M, Sørensen KD. Microbiota of the prostate tumor environment investigated by whole-transcriptome profiling. Genome Med. 2022;14(1):9. https://doi.org/10.1186/s13073-022-01011-3.
Ward Grados DF, Ergun O, Miller CD, Gaburak P, Frimpong NA, Shittu O, Warlick CA. Prostate tissue microbiome in patients with prostate Cancer: a systematic review. Cancers (Basel). 2024;16(8):1549. https://doi.org/10.3390/cancers16081549.
Sarkar P, Malik S, Banerjee A, Datta C, Pal DK, Ghosh A, Saha A. Differential Microbial signature Associated with Benign Prostatic Hyperplasia and prostate Cancer. Front Cell Infect Microbiol. 2022;12:894777. https://doi.org/10.3389/fcimb.2022.894777.
Pollack AS, Kunder CA, Brazer N, Shen Z, Varma S, West RB, Cunha GR, Baskin LS, Brooks JD, Pollack JR. Spatial transcriptomics identifies candidate stromal drivers of benign prostatic hyperplasia. JCI Insight. 2024;9(2):e176479. https://doi.org/10.1172/jci.insight.176479.
Pederzoli F, Raffo M, Pakula H, Ravera F, Nuzzo PV, Loda M. Stromal cells in prostate cancer pathobiology: friends or foes? Br J Cancer. 2023;128(6):930–9. https://doi.org/10.1038/s41416-022-02085-x.
Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98(2):245–9. https://doi.org/10.1038/sj.bjc.6604087. Epub 2008 Jan 8. PMID: 18182995; PMCID: PMC2361445.
Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Brückner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185(9):5628–36. https://doi.org/10.4049/jimmunol.0903678.
Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol. 2010;21(1):93–102. https://doi.org/10.1681/ASN.2009020146.
Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98(2):245–9. https://doi.org/10.1038/sj.bjc.6604087.
Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. https://doi.org/10.1038/s41568-019-0222-9.
Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66(2):794–802. https://doi.org/10.1158/0008-5472.CAN-05-1716.
Berben L, Floris G, Wildiers H, Hatse S. Cancer and Aging: two tightly interconnected biological processes. Cancers (Basel). 2021;13(6):1400. https://doi.org/10.3390/cancers13061400.
Damodarasamy M, Vernon RB, Chan CK, Plymate SR, Wight TN, Reed MJ. Hyaluronan in aged collagen matrix increases prostate epithelial cell proliferation. Vitro Cell Dev Biol Anim. 2015;51(1):50–8. https://doi.org/10.1007/s11626-014-9800-z.
Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. https://doi.org/10.3389/fphar.2014.00123.
Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 2021;21(1):389. https://doi.org/10.1186/s12935-021-02089-2.
Wang X, Qiu L, Li Z, Wang XY, Yi H. Understanding the multifaceted role of neutrophils in Cancer and Autoimmune diseases. Front Immunol. 2018;9:2456. https://doi.org/10.3389/fimmu.2018.02456. PMID: 30473691; PMCID: PMC6237929.
Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6(8):2591–9. https://doi.org/10.1096/fasebj.6.8.1592209.
Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103(5):398–406. https://doi.org/10.1007/s00395-008-0733-0.
Hervé MA, Meduri G, Petit FG, Domet TS, Lazennec G, Mourah S, Perrot-Applanat M. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J Endocrinol. 2006;188(1):91–9.
Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9(7):781.
Lau KM, To KF. Importance of Estrogenic Signaling and its mediated receptors in prostate Cancer. Int J Mol Sci. 2016;17(9):1434. https://doi.org/10.3390/ijms17091434.
Welén K, Damber JE. Androgens, aging, and prostate health. Rev Endocr Metab Disord. 2022;23(6):1221–31. https://doi.org/10.1007/s11154-022-09730-z.
Madersbacher S, Sampson N, Culig Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: a Mini-review. Gerontology. 2019;65(5):458–64. https://doi.org/10.1159/000496289.
Bonollo F, Thalmann GN, Kruithof-de Julio M, Karkampouna S. The role of Cancer-Associated fibroblasts in prostate Cancer tumorigenesis. Cancers (Basel). 2020;12(7):1887. https://doi.org/10.3390/cancers12071887.
Cioni B, Nevedomskaya E, Melis MHM, van Burgsteden J, Stelloo S, Hodel E, Spinozzi D, de Jong J, van der Poel H, de Boer JP, Wessels LFA, Zwart W, Bergman AM. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol Oncol. 2018;12(8):1308–23. https://doi.org/10.1002/1878-0261.12327.
Hotta Y, Kataoka T, Kimura K. Testosterone Deficiency and endothelial dysfunction: nitric oxide, asymmetric dimethylarginine, and endothelial progenitor cells. Sex Med Rev. 2019;7(4):661–8. https://doi.org/10.1016/j.sxmr.2019.02.005.
Babcock MC, DuBose LE, Witten TL, Stauffer BL, Hildreth KL, Schwartz RS, Kohrt WM, Moreau KL. Oxidative stress and inflammation are Associated with Age-related endothelial dysfunction in men with low testosterone. J Clin Endocrinol Metab. 2022;107(2):e500–14. https://doi.org/10.1210/clinem/dgab715.
Hofer MD, Kapur P, Cordon BH, Hamoun F, Russell D, Scott JM, Roehrborn CG, Morey AF. Low testosterone levels result in decreased Periurethral Vascularity via an androgen receptor-mediated process: pilot study in Urethral stricture tissue. Urology. 2017;105:175–80. https://doi.org/10.1016/j.urology.2017.02.037.
Corrigan FE 3rd, Al Mheid I, Eapen DJ, Hayek SS, Sher S, Martin GS, Quyyumi AA. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol. 2015;194:94–9. https://doi.org/10.1016/j.ijcard.2015.05.065.
Vargas F, Moreno JM, Wangensteen R, Rodríguez-Gómez I, García-Estañ J. The endocrine system in chronic nitric oxide deficiency. Eur J Endocrinol. 2007;156(1):1–12. https://doi.org/10.1530/eje.1.02314.
Mori JO, Elhussin I, Brennen WN, Graham MK, Lotan TL, Yates CC, De Marzo AM, Denmeade SR, Yegnasubramanian S, Nelson WG, Denis GV, Platz EA, Meeker AK, Heaphy CM. Prognostic and therapeutic potential of senescent stromal fibroblasts in prostate cancer. Nat Rev Urol. 2024;21(5):258–73. https://doi.org/10.1038/s41585-023-00827-x.
Passos JF, Miwa S, von Zglinicki T. Measuring reactive oxygen species in senescent cells. Methods Mol Biol., Davalli P, Mitic T, Caporali A, Lauriola A. D’Arca D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127.
Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008 Oct-Dec;1(1):15–24. https://doi.org/10.4161/oxim.1.1.6843.
Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the Aging vasculature and role in Age-Related Disease. Circ Res. 2018;123(7):825–48. https://doi.org/10.1161/CIRCRESAHA.118.312563.
Perusquía M, Stallone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol. 2010;298(5):H1301–7. https://doi.org/10.1152/ajpheart.00753.2009.
da Silva FC, de Araújo BJ, Cordeiro CS, Arruda VM, Faria BQ, Guerra JFDC, Araújo TG, Fürstenau CR. Endothelial dysfunction due to the inhibition of the synthesis of nitric oxide: proposal and characterization of an in vitro cellular model. Front Physiol. 2022;13:978378. https://doi.org/10.3389/fphys.2022.978378.
Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70(4):660–7. https://doi.org/10.1161/HYPERTENSIONAHA.117.07802.
Phua TJ. Understanding human aging and the fundamental cell signaling link in age-related diseases: the middle-aging hypovascularity hypoxia hypothesis. Front Aging. 2023;4:1196648. https://doi.org/10.3389/fragi.2023.1196648.
De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–69. https://doi.org/10.1038/nrc2090.
Hsing AW. Hormones and prostate cancer: what’s next? Epidemiol Rev. 2001;23(1):42–58. https://doi.org/10.1093/oxfordjournals.epirev.a000795.
Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–36. https://doi.org/10.1016/j.canlet.2008.12.011.
Gupta-Elera G, Garrett AR, Robison RA, O’Neill KL. The role of oxidative stress in prostate cancer. Eur J Cancer Prev. 2012;21(2):155–62. https://doi.org/10.1097/CEJ.0b013e32834a8002.
Han C, Wang Z, Xu Y, Chen S, Han Y, Li L, Wang M, Jin X. Roles of reactive oxygen species in biological behaviors of prostate Cancer. Biomed Res Int. 2020;2020:1269624. https://doi.org/10.1155/2020/1269624.
Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev. 2018;32(17–18):1105–40. https://doi.org/10.1101/gad.315739.118.
Webber JP, Spary LK, Mason MD, Tabi Z, Brewis IA, Clayton A. Prostate stromal cell proteomics analysis discriminates normal from tumour reactive stromal phenotypes. Oncotarget. 2016;7(15):20124–39. https://doi.org/10.18632/oncotarget.7716.
Kryza T, Silva LM, Bock N, Fuhrman-Luck RA, Stephens CR, Gao J, Samaratunga H, Australian Prostate Cancer BioResource, Lawrence MG, Hooper JD, Dong Y, Risbridger GP, Clements JA. Kallikrein-related peptidase 4 induces cancer-associated fibroblast features in prostate-derived stromal cells. Mol Oncol. 2017;11(10):1307–29. https://doi.org/10.1002/1878-0261.12075.
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–23.
Ni WD, Yang ZT, Cui CA, Cui Y, Fang LY, Xuan YH. Tenascin-C is a potential cancer-associated fibroblasts marker and predicts poor prognosis in prostate cancer. Biochem Biophys Res Commun. 2017;486(3):607–12. https://doi.org/10.1016/j.bbrc.2017.03.021.
Ramsay AJ, Reid JC, Adams MN, Samaratunga H, Dong Y, Clements JA, Hooper JD. Prostatic trypsin-like kallikrein-related peptidases (KLKs) and other prostate-expressed tryptic proteinases as regulators of signalling via proteinase-activated receptors (PARs). Biol Chem. 2008;389(6):653–68. https://doi.org/10.1515/BC.2008.078.
Li Z, Li D, Tsun A, Li B. FOXP3 + regulatory T cells and their functional regulation. Cell Mol Immunol. 2015;12(5):558–65. https://doi.org/10.1038/cmi.2015.10.
Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3(3):230–8. https://doi.org/10.1038/mi.2010.7.
Zhang Q, Yang XJ, Kundu SD, Pins M, Javonovic B, Meyer R, Kim SJ, Greenberg NM, Kuzel T, Meagher R, Guo Y, Lee C. Blockade of transforming growth factor-{beta} signaling in tumor-reactive CD8(+) T cells activates the antitumor immune response cycle. Mol Cancer Ther. 2006;5(7):1733–43. https://doi.org/10.1158/1535-7163.MCT-06-0109.
Chiarugi P, Paoli P, Cirri P. Tumor microenvironment and metabolism in prostate cancer. Semin Oncol. 2014;41(2):267–80. https://doi.org/10.1053/j.seminoncol.2014.03.004.
Thiruvalluvan M, Bhowmick NA. Stromal-epithelial interactions in Cancer Progression and Therapy Response. Cancers (Basel). 2023;15(11):3014. https://doi.org/10.3390/cancers15113014.
Levesque C, Nelson PS. Cellular constituents of the prostate stroma: key contributors to prostate Cancer Progression and Therapy Resistance. Cold Spring Harb Perspect Med. 2018;8(8):a030510. https://doi.org/10.1101/cshperspect.a030510.
Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability. 2011;20(4):108–20. https://doi.org/10.1016/j.jtv.2009.11.004.
Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med., Vickman RE, Kakarla M, Hayward SW, Franco OE. Fibroblast heterogeneity in prostate carcinogenesis. Cancer Lett. 2022;525:76–83. doi: 10.1016/j.canlet.2021.10.028.
Owen JS, Clayton A, Pearson HB. Cancer-Associated Fibroblast Heterogeneity, activation and function: implications for prostate Cancer. Biomolecules. 2022;13(1):67. https://doi.org/10.3390/biom13010067.
Qian Y, Feng D, Wang J, Wei W, Wei Q, Han P, Yang L. Establishment of cancer-associated fibroblasts-related subtypes and prognostic index for prostate cancer through single-cell and bulk RNA transcriptome. Sci Rep. 2023;13(1):9016. https://doi.org/10.1038/s41598-023-36125-0.
Mishra R, Haldar S, Suchanti S, Bhowmick NA. Epigenetic changes in fibroblasts drive cancer metabolism and differentiation. Endocr Relat Cancer. 2019;26(12):R673–88. https://doi.org/10.1530/ERC-19-0347.
Mishra R, Haldar S, Placencio V, Madhav A, Rohena-Rivera K, Agarwal P, Duong F, Angara B, Tripathi M, Liu Z, Gottlieb RA, Wagner S, Posadas EM, Bhowmick NA. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J Clin Invest. 2018;128(10):4472–84. https://doi.org/10.1172/JCI99397.
Graham MK, Meeker A. Telomeres and telomerase in prostate cancer Heaphy CM, Yoon GS, Peskoe SB, Joshu CE, Lee TK, Giovannucci E, Mucci LA, Kenfield SA, Stampfer MJ, Hicks JL, De Marzo AM, Platz EA, Meeker AK. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death. Cancer Discov. 2013;3(10):1130-41. https://doi.org/10.1158/2159-8290.CD-13-0135
Mishra R, Haldar S, Biondi S, Bhari VK, Singh G, Bhowmick NA. TGF-β controls stromal telomere length through epigenetic modifications. 3 Biotech. 2022;12(11):290. https://doi.org/10.1007/s13205-022-03346-5.
Fang Z, Xu J, Zhang B, Wang W, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. The promising role of noncoding RNAs in cancer-associated fibroblasts: an overview of current status and future perspectives. J Hematol Oncol. 2020;13(1):154. https://doi.org/10.1186/s13045-020-00988-x.
Matsuda C, Ishii K, Nakagawa Y, Shirai T, Sasaki T, Hirokawa YS, Iguchi K, Watanabe M. Fibroblast-derived exosomal microRNA regulates NKX3-1 expression in androgen-sensitive, androgen receptor-dependent prostate cancer cells. J Cell Biochem. 2023;124(8):1135–44. https://doi.org/10.1002/jcb.30435.
Gandellini P, Giannoni E, Casamichele A, Taddei ML, Callari M, Piovan C, Valdagni R, Pierotti MA, Zaffaroni N, Chiarugi P. miR-205 hinders the malignant interplay between prostate cancer cells and associated fibroblasts. Antioxid Redox Signal. 2014;20(7):1045–59. https://doi.org/10.1089/ars.2013.5292.
Peiffer R, Boumahd Y, Gullo C, Crake R, Letellier E, Bellahcène A, Peulen O. Cancer-Associated Fibroblast Diversity shapes Tumor Metabolism in Pancreatic Cancer. Cancers (Basel). 2022;15(1):61. https://doi.org/10.3390/cancers15010061.
Mellone M, Hanley CJ, Thirdborough S, Mellows T, Garcia E, Woo J, Tod J, Frampton S, Jenei V, Moutasim KA, Kabir TD, Brennan PA, Venturi G, Ford K, Herranz N, Lim KP, Clarke J, Lambert DW, Prime SS, Underwood TJ, Vijayanand P, Eliceiri KW, Woelk C, King EV, Gil J, Ottensmeier CH, Thomas GJ. Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis. Aging. 2016;9(1):114–32. https://doi.org/10.18632/aging.101127.
Liu H, Zhao H, Sun Y. Tumor microenvironment and cellular senescence: understanding therapeutic resistance and harnessing strategies. Semin Cancer Biol. 2022;86(Pt 3):769–81. https://doi.org/10.1016/j.semcancer.2021.11.004.
Patel GK, Chugh N, Tripathi M. Neuroendocrine differentiation of prostate Cancer-An Intriguing Example of Tumor Evolution at Play. Cancers (Basel). 2019;11(10):1405. https://doi.org/10.3390/cancers11101405.
Li Z, Sun C, Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;11(17):8322–36. https://doi.org/10.7150/thno.62378.
Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9(7):556–62. https://doi.org/10.1593/neo.07307.
Hasegawa H, Inoue A, Muraoka M, Yamanouchi J, Miyazaki T, Yasukawa M. Therapy for pneumonitis and sialadenitis by accumulation of CCR2-expressing CD4 + CD25 + regulatory T cells in MRL/lpr mice. Arthritis Res Ther. 2007;9(1):R15. https://doi.org/10.1186/ar2122.
Peng L, Shu S, Krauss JC. Monocyte chemoattractant protein inhibits the generation of tumor-reactive T cells. Cancer Res. 1997;57(21):4849-54. PMID: 9354448.
Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R, Jain RK. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A. 2019;116(10):4558–66. https://doi.org/10.1073/pnas.1815515116.
Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–44. https://doi.org/10.1016/j.molmed.2010.01.003.
Vickman RE, Broman MM, Lanman NA, Franco OE, Sudyanti PAG, Ni Y, Ji Y, Helfand BT, Petkewicz J, Paterakos MC, Crawford SE, Ratliff TL, Hayward SW. Heterogeneity of human prostate carcinoma-associated fibroblasts implicates a role for subpopulations in myeloid cell recruitment. Prostate. 2020;80(2):173–85. https://doi.org/10.1002/pros.23929.
Xu Y, Li W, Lin S, Liu B, Wu P, Li L. Fibroblast diversity and plasticity in the tumor microenvironment: roles in immunity and relevant therapies. Cell Commun Signal. 2023;21(1):234. https://doi.org/10.1186/s12964-023-01204-2.
Zhang W, Wang J, Liu C, Li Y, Sun C, Wu J, Wu Q. Crosstalk and plasticity driving between cancer-associated fibroblasts and tumor microenvironment: significance of breast cancer metastasis. J Transl Med. 2023;21(1):827. https://doi.org/10.1186/s12967-023-04714-2.
Luthold C, Hallal T, Labbé DP, Bordeleau F. The Extracellular Matrix Stiffening: a trigger of prostate Cancer Progression and Castration Resistance? Cancers (Basel). 2022;14(12):2887. https://doi.org/10.3390/cancers14122887.
Xiong J, Xiao R, Zhao J, Zhao Q, Luo M, Li F, Zhang W, Wu M. Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy. J Transl Med. 2024;22(1):85. https://doi.org/10.1186/s12967-023-04810-3.
Nguyen EV, Pereira BA, Lawrence MG, Ma X, Rebello RJ, Chan H, Niranjan B, Wu Y, Ellem S, Guan X, Wu J, Skhinas JN, Cox TR, Risbridger GP, Taylor RA, Lister NL, Daly RJ. Proteomic profiling of human prostate Cancer-associated fibroblasts (CAF) reveals LOXL2-dependent regulation of the Tumor Microenvironment. Mol Cell Proteom. 2019;18(7):1410–27. https://doi.org/10.1074/mcp.RA119.001496.
Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel). 2014;6(3):1298–327. https://doi.org/10.3390/cancers6031298.
Brehmer B, Biesterfeld S, Jakse G. Expression of matrix metalloproteinases (MMP-2 and – 9) and their inhibitors (TIMP-1 and – 2) in prostate cancer tissue. Prostate Cancer Prostatic Dis. 2003;6(3):217–22. https://doi.org/10.1038/sj.pcan.4500657.
Cui D, Li J, Zhu Z, Berk M, Hardaway A, McManus J, Chung YM, Alyamani M, Valle S, Tiwari R, Han B, Goudarzi M, Willard B, Sharifi N. Cancer-associated fibroblast-secreted glucosamine alters the androgen biosynthesis program in prostate cancer via HSD3B1 upregulation. J Clin Invest. 2023;133(7):e161913. https://doi.org/10.1172/JCI161913.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, Adeberg S, Rathke H, Röhrich M, Winter H, Plinkert PK, Marme F, Lang M, Kauczor HU, Jäger D, Debus J, Haberkorn U, Giesel FL. 68Ga-FAPI PET/CT: Tracer Uptake in 28 different kinds of Cancer. J Nucl Med. 2019;60(6):801–5. https://doi.org/10.2967/jnumed.119.227967.
Samaržija I. The potential of Extracellular Matrix- and integrin adhesion complex-related molecules for prostate Cancer Biomarker Discovery. Biomedicines. 2023;12(1):79. https://doi.org/10.3390/biomedicines12010079.
Imlimthan S, Moon ES, Rathke H, Afshar-Oromieh A, Rösch F, Rominger A, Gourni E. New frontiers in Cancer Imaging and Therapy based on Radiolabeled Fibroblast activation protein inhibitors: a rational review and current progress. Pharmaceuticals (Basel). 2021;14(10):1023. https://doi.org/10.3390/ph14101023.
Fendler WP, Pabst KM, Kessler L, Fragoso Costa P, Ferdinandus J, Weber M, Lippert M, Lueckerath K, Umutlu L, Kostbade K, Mavroeidi IA, Schuler M, Ahrens M, Rischpler C, Bauer S, Herrmann K, Siveke JT, Hamacher R. Safety and efficacy of 90Y-FAPI-46 Radioligand Therapy in patients with Advanced Sarcoma and other Cancer entities. Clin Cancer Res. 2022;28(19):4346–53. https://doi.org/10.1158/1078-0432.CCR-22-1432.
Kwak C, Jin RJ, Lee C, Park MS, Lee SE. Thrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int. 2002;89(3):303–9. https://doi.org/10.1046/j.1464-4096.2001.01417.x.
Belotti D, Foglieni C, Resovi A, Giavazzi R, Taraboletti G. Targeting angiogenesis with compounds from the extracellular matrix. Int J Biochem Cell Biol. 2011;43(12):1674–85. https://doi.org/10.1016/j.biocel.2011.08.012.
Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and – 2. Cold Spring Harb Perspect Med. 2012;2(5):a006627. https://doi.org/10.1101/cshperspect.a006627.
Mongiat M, Andreuzzi E, Tarticchio G, Paulitti A. Extracellular matrix, a hard player in Angiogenesis. Int J Mol Sci. 2016;17(11):1822. https://doi.org/10.3390/ijms17111822.
Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001;3(3):143–5. https://doi.org/10.1186/bcr287.
DeClerck YA. Desmoplasia: a response or a niche? Cancer Discov. 2012;2(9):772–4. https://doi.org/10.1158/2159-8290.CD-12-0348.
DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu D, Marx JJ, Tjoe JA, Ziv E, Febbraio M, Kerlikowske K, Parvin B, Tlsty TD. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012;2(9):826–39. https://doi.org/10.1158/2159-8290.CD-12-0107.
Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;10(9):546–50. https://doi.org/10.1038/nrurol.2013.149.
Fernandes S, Oliver-De La Cruz J, Morazzo S, Niro F, Cassani M, Ďuríková H, Caravella A, Fiore P, Azzato G, De Marco G, Lauria A, Izzi V, Bosáková V, Fric J, Filipensky P, Forte G. TGF-β induces matrisome pathological alterations and EMT in patient-derived prostate cancer tumoroids. Matrix Biol. 2024;125:12–30. https://doi.org/10.1016/j.matbio.2023.11.001.
Chandler C, Liu T, Buckanovich R, Coffman LG. The double edge sword of fibrosis in cancer. Transl Res. 2019;209:55–67. https://doi.org/10.1016/j.trsl.2019.02.006.
Sato H, Hara T, Meng S, Tsuji Y, Arao Y, Saito Y, Sasaki K, Kobayashi S, Doki Y, Eguchi H, Ishii H. Multifaced roles of desmoplastic reaction and fibrosis in pancreatic cancer progression: current understanding and future directions. Cancer Sci. 2023;114(9):3487–95. https://doi.org/10.1111/cas.15890.
Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, Xie N. Adaptive mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front Cell Dev Biol. 2021;9:641469. https://doi.org/10.3389/fcell.2021.641469.
Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8. https://doi.org/10.1038/nri3712.
Henry GH, Malewska A, Joseph DB, Malladi VS, Lee J, Torrealba J, Mauck RJ, Gahan JC, Raj GV, Roehrborn CG, Hon GC, MacConmara MP, Reese JC, Hutchinson RC, Vezina CM, Strand DW. A Cellular anatomy of the normal adult human prostate and Prostatic Urethra. Cell Rep. 2018;25(12):3530–e35425. https://doi.org/10.1016/j.celrep.2018.11.086.
Harper J, Sainson RC. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin Cancer Biol. 2014;25:69–77. https://doi.org/10.1016/j.semcancer.2013.12.005.
Hirz T, Mei S, Sarkar H, Kfoury Y, Wu S, Verhoeven BM, Subtelny AO, Zlatev DV, Wszolek MW, Salari K, Murray E, Chen F, Macosko EZ, Wu CL, Scadden DT, Dahl DM, Baryawno N, Saylor PJ, Kharchenko PV, Sykes DB. Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses. Nat Commun. 2023;14(1):663. https://doi.org/10.1038/s41467-023-36325-2.
Hu W, Qian Y, Yu F, Liu W, Wu Y, Fang X, Hao W. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol Lett. 2015;10(3):1390–6. https://doi.org/10.3892/ol.2015.3400.
Hellsten R, Lilljebjörn L, Johansson M, Leandersson K, Bjartell A. The STAT3 inhibitor galiellalactone inhibits the generation of MDSC-like monocytes by prostate cancer cells and decreases immunosuppressive and tumorigenic factors. Prostate. 2019;79(14):1611–21. https://doi.org/10.1002/pros.23885.
Idorn M, Køllgaard T, Kongsted P, Sengeløv L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63(11):1177–87. https://doi.org/10.1007/s00262-014-1591-2.
Mhaidly R, Mechta-Grigoriou F. Fibroblast heterogeneity in tumor micro-environment: role in immunosuppression and new therapies. Semin Immunol. 2020;48:101417. https://doi.org/10.1016/j.smim.2020.101417.
Palicelli A, Bonacini M, Croci S, Magi-Galluzzi C, Cañete-Portillo S, Chaux A, Bisagni A, Zanetti E, De Biase D, Melli B, Sanguedolce F, Ragazzi M, Bonasoni MP, Soriano A, Ascani S, Zizzo M, Castro Ruiz C, De Leo A, Giordano G, Landriscina M, Carrieri G, Cormio L, Berney DM, Athanazio D, Gandhi J, Cavazza A, Santandrea G, Tafuni A, Zanelli M. What do we have to know about PD-L1 expression in prostate Cancer? A systematic literature review. Part 1: Focus on Immunohistochemical Results with discussion of pre-analytical and interpretation variables. Cells. 2021;10(11):3166. https://doi.org/10.3390/cells10113166.
Micke P, Strell C, Mattsson J, Martín-Bernabé A, Brunnström H, Huvila J, Sund M, Wärnberg F, Ponten F, Glimelius B, Hrynchyk I, Mauchanski S, Khelashvili S, Garcia-Vicién G, Molleví DG, Edqvist PH, O Reilly A, Corvigno S, Dahlstrand H, Botling J, Segersten U, Krzyzanowska A, Bjartell A, Elebro J, Heby M, Lundgren S, Hedner C, Borg D, Brändstedt J, Sartor H, Malmström PU, Johansson M, Nodin B, Backman M, Lindskog C, Jirström K, Mezheyeuski A. The prognostic impact of the tumour stroma fraction: a machine learning-based analysis in 16 human solid tumour types. EBioMedicine. 2021;65:103269. https://doi.org/10.1016/j.ebiom.2021.103269.
Burrows L, Sculthorpe D, Zhang H, Rehman O, Mukherjee A, Chen K. Mathematical modelling and deep learning algorithms to automate assessment of single and digitally multiplexed immunohistochemical stains in tumoural stroma. J Pathol Inf. 2023;15:100351. https://doi.org/10.1016/j.jpi.2023.100351.
Liu F, Qi L, Liu B, Liu J, Zhang H, Che D, Cao J, Shen J, Geng J, Bi Y, Ye L, Pan B, Yu Y. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS ONE. 2015;10(3):e0116683. https://doi.org/10.1371/journal.pone.0116683.
An J, Hou D, Wang L, Wang L, Yang Y, Wang H. Fibroblast activation protein-alpha knockdown suppresses prostate cancer cell invasion and proliferation. Histol Histopathol. 2022;37(6):597–607. https://doi.org/10.14670/HH-18-430.
Wu Z, Shi J, Lai C, Li K, Li K, Li Z, Tang Z, Liu C, Xu K. Clinicopathological significance and prognostic value of cancer-associated fibroblasts in prostate cancer patients. Urol Oncol. 2021;39(7):433e. 17-433.e23.
Izzi V, Davis MN, Naba A. Pan-cancer Analysis of the genomic alterations and mutations of the Matrisome. Cancers (Basel). 2020;12(8):2046. https://doi.org/10.3390/cancers12082046.
González LO, Eiro N, Fraile M, Beridze N, Escaf AR, Escaf S, Fernández-Gómez JM, Vizoso FJ. Prostate Cancer Tumor Stroma: responsibility in Tumor Biology, diagnosis and treatment. Cancers (Basel). 2022;14(18):4412. https://doi.org/10.3390/cancers14184412.
Xu M, Zhang T, Xia R, Wei Y, Wei X. Targeting the tumor stroma for cancer therapy. Mol Cancer. 2022;21(1):208. https://doi.org/10.1186/s12943-022-01670-1.
Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–81. https://doi.org/10.1038/s41571-018-0007-1.
ClinicalTrials.gov. online database of clinical research studies. https://clinicaltrials.gov/search?cond=Cancer&term=stroma. Accessed 12 April 2024.
ClinicalTrials.gov. online database of clinical research studies. https://clinicaltrials.gov/study/NCT04939610?cond=NCT04939610&rank=1. Accessed 12 April 2024.
Ochi K, Suzawa K, Thu YM, Takatsu F, Tsudaka S, Zhu Y, Nakata K, Takeda T, Shien K, Yamamoto H, Okazaki M, Sugimoto S, Shien T, Okamoto Y, Tomida S, Toyooka S. Drug repositioning of tranilast to sensitize a cancer therapy by targeting cancer-associated fibroblast. Cancer Sci. 2022;113(10):3428–36. https://doi.org/10.1111/cas.15502.
Osman S, Raza A, Al-Zaidan L, Inchakalody VP, Merhi M, Prabhu KS, Abdelaziz N, Hydrose S, Uddin S, Dermime S. Anti-cancer effects of Tranilast: an update. Biomed Pharmacother. 2021;141:111844. https://doi.org/10.1016/j.biopha.2021.111844.
Liu Q, Yao F, Wu L, Xu T, Na J, Shen Z, Liu X, Shi W, Zhao Y, Liao Y. Heterogeneity and interplay: the multifaceted role of cancer-associated fibroblasts in the tumor and therapeutic strategies. Clin Transl Oncol. 2024 Apr 11. https://doi.org/10.1007/s12094-024-03492-7. Epub ahead of print.
Mediavilla-Varela M, Boateng K, Noyes D, Antonia SJ. The anti-fibrotic agent pirfenidone synergizes with cisplatin in killing tumor cells and cancer-associated fibroblasts. BMC Cancer. 2016;16:176. https://doi.org/10.1186/s12885-016-2162-z.
Fujiwara A, Funaki S, Fukui E, Kimura K, Kanou T, Ose N, Minami M, Shintani Y. Effects of pirfenidone targeting the tumor microenvironment and tumor-stroma interaction as a novel treatment for non-small cell lung cancer. Sci Rep. 2020;10(1):10900. https://doi.org/10.1038/s41598-020-67904-8.
Zhang Y, Yu R, Zhao C, Liang J, Zhang Y, Su H, Zhao J, Wu H, Xu S, Zhang Z, Wang L, Zou X, Zhu Y, Zhang S, Lv Y. CAFs homologous biomimetic liposome bearing BET inhibitor and Pirfenidone synergistically promoting Antitumor Efficacy in Pancreatic Ductal Adenocarcinoma. Adv Sci (Weinh). 2024;11(1):e2305279. https://doi.org/10.1002/advs.202305279.
Bhola NE, Balko JM, Dugger TC, Kuba MG, Sánchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123(3):1348–58. https://doi.org/10.1172/JCI65416.
Serova M, Tijeras-Raballand A, Dos Santos C, Albuquerque M, Paradis V, Neuzillet C, Benhadji KA, Raymond E, Faivre S, de Gramont A. Effects of TGF-beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget. 2015;6(25):21614–27. https://doi.org/10.18632/oncotarget.4308.
Joseph JV, Balasubramaniyan V, Walenkamp A, Kruyt FA. TGF-β as a therapeutic target in high grade gliomas - promises and challenges. Biochem Pharmacol. 2013;85(4):478–85. https://doi.org/10.1016/j.bcp.2012.11.005.
Tran HC, Wan Z, Sheard MA, Sun J, Jackson JR, Malvar J, Xu Y, Wang L, Sposto R, Kim ES, Asgharzadeh S, Seeger RC. TGFβR1 blockade with Galunisertib (LY2157299) enhances Anti-neuroblastoma Activity of the Anti-GD2 antibody dinutuximab (ch14.18) with natural killer cells. Clin Cancer Res. 2017;23(3):804–13. https://doi.org/10.1158/1078-0432.CCR-16-1743.
Ge R, Huang GM. Targeting transforming growth factor beta signaling in metastatic osteosarcoma. J Bone Oncol. 2023;43:100513. https://doi.org/10.1016/j.jbo.2023.100513.
Acknowledgements
Not applicable.
Funding
The research leading to these results has received funding from: AIRC, under IG 2019 - ID. 23264 project – P.I. Emma Di Carlo; MUR (Ministero dell’Università e della Ricerca) and Programma Operativo Nazionale Ricerca e Innovazione 2014–2020 (PON R&I) to Carlo Sorrentino.
Author information
Authors and Affiliations
Contributions
EDC: Writing – original draft, Writing – review & editing. CS: Data curation, Writing – review & editing. All authors participated in the revision and gave final approval to the manuscript. Both authors reviewed and approved he manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Di Carlo, E., Sorrentino, C. The multifaceted role of the stroma in the healthy prostate and prostate cancer. J Transl Med 22, 825 (2024). https://doi.org/10.1186/s12967-024-05564-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05564-2