Abstract
Naloxone is an effective FDA-approved opioid antagonist for reversing opioid overdoses. Naloxone is available to the public and can be administered through intramuscular (IM), intravenous (IV), and intranasal spray (IN) routes. Our literature review investigates the adequacy of two doses of standard IM or IN naloxone in reversing fentanyl overdoses compared to newer high-dose naloxone formulations. Moreover, our initiative incorporates the experiences of people who use drugs, enabling a more practical and contextually-grounded analysis. The evidence indicates that the vast majority of fentanyl overdoses can be successfully reversed using two standard IM or IN dosages. Exceptions include cases of carfentanil overdose, which necessitates ≥ 3 doses for reversal. Multiple studies documented the risk of precipitated withdrawal using ≥ 2 doses of naloxone, notably including the possibility of recurring overdose symptoms after resuscitation, contingent upon the half-life of the specific opioid involved. We recommend distributing multiple doses of standard IM or IN naloxone to bystanders and educating individuals on the adequacy of two doses in reversing fentanyl overdoses. Individuals should continue administration until the recipient is revived, ensuring appropriate intervals between each dose along with rescue breaths, and calling emergency medical services if the individual is unresponsive after two doses. We do not recommend high-dose naloxone formulations as a substitute for four doses of IM or IN naloxone due to the higher cost, risk of precipitated withdrawal, and limited evidence compared to standard doses. Future research must take into consideration lived and living experience, scientific evidence, conflicts of interest, and the bodily autonomy of people who use drugs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Opioid use disorder affects more than 2.1 million individuals in the United States [1]. Persistent opioid use can leave individuals with opioid dependency resulting in daily opioid use, despite potential medical and social consequences, the most significant of which is overdose [2, 3]. Opioid overdose occurs when opioids bind to and activate opioid receptors and suppress breathing rate below that which is required to maintain consciousness [4]. If suppression is continued for an extended time, health complications including death can occur.
Opioid overdose, however, is reversible if a bystander identifies an overdose in progress and administers naloxone hydrochloride (hereafter, naloxone) quickly [1, 2]. Naloxone is an opioid antagonist medication with a stronger binding affinity for opioid receptors than heroin or fentanyl. When administered, it “knocks opioids off” the opioid receptors in the central or peripheral nervous system and binds without activation, thereby reversing both intentional (i.e. analgesia, euphoria) and unintentional (i.e. respiratory depression, coma) effects of the used opioid [3, 4]. Naloxone administered by community members (bystanders, friends, family, etc.) has proven successful in reversing opioid overdoses in 75–100% of cases [5]. It is considered safe at the recommended doses to opioid-naive persons as well [5].
Naloxone is primarily available to the public through free community-based programs, though it is also available through provider prescriptions and as an over-the-counter medication at pharmacies. Community health workers, pharmacists, and medical professionals distributing naloxone often also provide training on overdose recognition and response, however, some organizations also incorporate a train-the-trainer model to enable community members to share these skills in their networks.
Community based overdose response can occur anywhere individuals use substances including private homes and public spaces (restaurants, shopping centers, laundromat, etc.) [6]. The currently recommended response protocol is a five step process: (1) checking for signs of opioid overdose, such as unconsciousness, slow or absent breathing, pale and clammy skin, and slow or no heartbeat, (2) calls emergency medical services (EMS) to ensure timely medical attention, (3) administer naloxone, (4) clear the airways to perform rescue breathing to help provide oxygen to the body, (5) administer additional naloxone if the individual does not regain consciousness and respiration [7]. An additional strategy to support someone experiencing overdose is to administer oxygen, however oxygen should not be used as the sole treatment method especially if breathing has ceased [8,9,10]. Many factors determine the amount of naloxone needed including type, amount, half-life and method of opioid use, tolerance levels, health status, and naloxone administration route [11,12,13].
While overdose detection and response is straightforward, the general population has an ingrained fear of and stigma towards PWUD. Many social and environmental factors contribute to the fear including lack of understanding, political beliefs, personal experiences, dissemination of misinformation, criminalization, and the ‘war on drugs’ mentality [14,15,16]. Oftentimes, individuals who use recreationally for personal, constructive purposes, and in a manner characterized by safety and responsibility, remain largely invisible within media representation and public forums [17]. Instead, the focus tends to prioritize individuals with SUD, those who overdose, or those solely in abstinence-based recovery. This biased depiction results in an altered portrayal of the effects and risks associated with recreational drug use [18] and provides opportunities for reinforcing racial, gender, and class stereotypes pertaining to drug users [18, 19]. Misunderstandings and socially reinforced biases result in misunderstandings of and opposition towards harm reduction strategies [20, 21]. In the context of naloxone, the misunderstandings can be seen in the altered portrayal of the effects and risks associated with naloxone use for overdose reversal [21,22,23]. It also results in sidelining of people with lived and living experience when exploring new developments regarding appropriate naloxone dose and administration.
Opioid use is a complex issue that is influenced by a variety of factors, such as social, economic, environmental, and other determinants of health. People who use drugs (PWUD) should not be delineated solely by their drug consumption, but rather recognized as multifaceted individuals with distinct requirements and aspirations. Therefore, adopting an impartial and non-judgmental approach is imperative when addressing the subject of drug use and, consequently, the application of naloxone.
Another consequence of the criminalization of non-prescribed opioid use is that it forces individuals who are dependent on opioids to use the unregulated illicit drug supply. Lack of regulatory standards results in a market fraught with impurities including both filler and undesired illicit substances. Notably, fentanyl has overtaken the heroin supply as the predominantly available opioid and has been found in non-opioid substance samples [24,25,26]. Fentanyl and its analogs are short acting opioids with exceptionally high potency when compared to other opioids like heroin and morphine [24]. Consequently, individuals who are exposed to fentanyl unintentionally or intentionally or at a higher purity level than anticipated are particularly vulnerable to overdose [27].
According to provisional data from the Centers for Disease Control and Prevention (CDC), there were more than 100,000 drug overdose deaths in the United States in 2021[28]. This concern for high overdose rates and drug poisoning severity provides theoretical justification for the proposal of higher doses of naloxone formulations. Nevertheless, this theoretical foundation lacks input from the ground level experts: PWUD, harm reduction workers, and other relevant groups possessing firsthand experience and direct involvement with drug users. These groups possess unique experiences and knowledge that most researchers and medical providers do not regularly have access to, including in the development of high-dose naloxone formulations.
Two previous literature reviews have been conducted on this topic: Moe and colleagues conducted a systematic review of overdoses (n = 26,660) in North America and Europe through 2018. They found that although higher initial and cumulative naloxone doses were being used by lay and healthcare responders for overdoses presumed to be fentanyl or another synthetic opioid, a cumulative total dose of 4 mg of naloxone (e.g., two doses of standard IN) was sufficient in 97% of presumed fentanyl/potent opioid cases [29]. Abdelal et al. [30] recently examined the use of two or more naloxone doses, however the authors received consultancy fees or stock options from Hika Pharmaceuticals, which manufactures the high-dose naloxone formulations product Kloxxado. The implementation of naloxone programs remains an imprecise science despite the reliability of naloxone in reversing opioid overdose. The emergence of newer formulations necessitates close examination of scientific research. Accordingly, this literature review aims to improve our understanding of how often more than two doses of IM and IN naloxone are needed to reverse a fentanyl overdose and whether promoting high-dose naloxone formulations is an optimal and necessary solution for community-led overdose response.
Naloxone options in the U.S.
There are currently three U.S. Food and Drug Administration (FDA)-approved administration routes of naloxone that are available: injectable intramuscular (IM), intravenous (IV), and intranasal spray (IN). Only IM and IN formulations are currently used in lay person response. Subcutaneous auto-injectors were used previously but have been discontinued [31, 32]. IM naloxone solution has traditionally been provided in kits of two 1-mL vials containing a 0.4 mg/mL solution [33]. 10-mL vials containing 0.4 mg/mL are also available but are not readily accessible in IM naloxone kits provided to PWUD [34, 35]. IN naloxone is also provided in two-unit kits with a 4 mg/0.1 ml naloxone solution pre-loaded into an atomizer that is ready to use intranasally. Regardless of the exact route of administration, naloxone can be easily administered by a lay bystander as an intervention for overdose [16].
The FDA has continued to support high-dose naloxone formulations (high-dose naloxone formulations) such as Kloxxado (double the dosage of standard IN) and Zimhi (25 times higher dose than generic IM) [17], despite the voiced concerns of harm reduction workers and others with lived experience of using naloxone to reverse an overdose [36, 37]. This is in part due to concerns that the high potency of fentanyl could require higher doses of naloxone to be effective and the assumption that a single high dose formulation is preferred over multiple doses of the typical formulation. Having input on drug policy and research from people with lived and living experiences of drug use is invaluable because it provides a more comprehensive understanding of the complexities and realities of drug use. Inclusion results in policies and research that are informed by the perspectives and needs of those directly affected, leading to more effective and equitable outcomes [38]. However, the historical exclusion of these individuals can be attributed, in part, to the pervasive stigma surrounding drugs and PWUD [39].
The U.S. currently has seven overdose reversal products that contain naloxone described both below and in Table 1.
Generic injectable naloxone is one of the most popular formulations supplied to PWUD [40, 41]. It comes in 1 mL vials of 0.4 mg/mL concentration [33]. It can be utilized in any method of administration, including intramuscular (IM), and intravenous (IV) routes, as well as intranasally (IN) via an atomizer. NARCAN® Is the most well-known brand name for naloxone nasal spray. It comes with a 0.1 mL pre-packaged solution that contains 4 mg/0.1 mL of naloxone. This specific brand’s pre-packaged administration tool only allows for naloxone to be administered intranasally. The generic counterparts to NARCAN® are the Teva and Perrigo generic nasal sprays which have chemically identical active ingredients and concentrations. Kloxxado® is a newer naloxone nasal spray that also comes with double the dose of NARCAN. It comes with a 0.1 mL pre-packaged solution that contains 8 mg/0.1 mL of naloxone. Identical to NARCAN, this specific brand’s pre-packaged administration tool also only allows for naloxone to be administered intranasally. Zimhi is a brand name autoinjector that comes pre-loaded with 0.5 mL of 5 mg/0.5 mL naloxone. This syringe can only be administered intramuscularly (IM). Lastly, Amphastar® Prefilled Naloxone Syringes come in 2 mL, with 1 mg/mL naloxone. These prefilled syringes are also compatible with any method of administration, both nasal and injection routes. It is a recently approved IN naloxone formulation with a 4 mg/0.1 mL concentration. It is not yet widely available in the US.
In May 2023, the FDA approved Opvee®, a nasal spray version of nalmefene and the first alternative opioid antagonist indicated for opioid overdose reversal. The half-life of nalmefene is ~ 11 h, much longer than the 60- to 90-min half-life of naloxone [42]. Nalmefene may reverse opioid intoxication for longer than naloxone, which some view as a benefit over naloxone. However, its extended half-life presents the continued concern of placing opioid-dependent persons who overdose into precipitated opioid withdrawal for far longer than naloxone. Opvee was developed by Opiant Pharmaceuticals, which plans to release Opvee to the U.S. market as early as October 2023 [43]. Opiant also contributed to the development and manufacture of Narcan[44].
Bioavailability, referring to the proportion of a drug that is able to enter the body's circulation to have an active effect [45] and is a key consideration to take into account during discussions of naloxone dosing. It is used to approximate a drug’s effectiveness when taken by a patient. Factors that can affect bioavailability include the administration route (e.g. IN, IM, IV), drug form (e.g. tablet, liquid), and personal characteristics (e.g., age, weight, liver function).
The IN and IM administration routes are both commonly used by first responders in the community. They have bioavailability of 50% and 98% and a latency time to effectiveness of 15-min and 8-min, respectively [42, 45,46,47]. The IV route is only used in inpatient medical settings and has a bioavailability of 100% with a 2-min latency to effect. The IV route is preferred by medical professionals as the dose can be tailored to each patient allowing for sufficient overdose reversal while minimizing the risk of withdrawal.
Methods
This study was conducted using the principles outlined by researchers with lived experience [38, 48]. Tennessee Harm Reduction is a drug-user run community-based organization in rural West Tennessee focused on distributing naloxone, drug checking supplies, and safer use supplies to over 200 community members. The organization’s Director (second author) and Outreach Specialist (first author) initially connected with the third and senior authors at the Harm Reduction Innovation Lab to share their experiences, knowledge, and perspectives surrounding high-dose naloxone formulations. Through their work, DPG and PLM found that PWUD reported that IN naloxone seemed to cause worse precipitated withdrawal compared to IM naloxone and that it seemed to take longer to reverse the overdose. As a result, people giving or receiving naloxone became cautious about continuing to use it. Tennessee Harm Reduction’s client base have reported over 215 known overdose reversals, the majority of which (135) used IM naloxone first. About half of those only needed one dose and a further 40% required two doses with the remaining 10% having 3 or more doses administered. No unsuccessful overdose reversals have been reported. It is important to note that both time between doses and whether the administrator had proper training was not reported so it is unclear whether 3 or more doses were actually needed. High-dose naloxone formulations caused even worse symptoms of precipitated withdrawal. DPG also noted that newly available high-dose naloxone formulations cost between 2 to 10 times more than generic IM naloxone.
Given the limited resources allocated to harm reduction organizations, we saw a need to better understand whether adopting high-dose naloxone formulations would be beneficial. Through an unfunded partnership between Tennessee Harm Reduction and the Harm Reduction Innovation Lab, a search strategy was implemented between August 2022 and February 2023. Phrases included: “high-dose naloxone formulation”, “opioid overdose”, “naloxone dosage overdose”, “naloxone dosage”, “high dose naloxone”, “high dose naloxone opioid”, “naloxone dosing”, “naloxone formulation”, “high dose naloxone formulation”, “high-dose naloxone”, and “high-dosage naloxone”. A literature review was performed using search engines PubMed and Google Scholar to compile a collection of relevant scholarly works. We filtered for original peer-reviewed articles published between January 2012 to February 2023 when heroin and fentanyl became the leading cause of U.S. overdose death. We also conducted a Google search to gather information on each naloxone product and cited research. We reviewed the title and abstract of each article and excluded those that focused on unrelated concepts.
The remaining eligible articles were summarized by research assistants using a matrix developed by the study team. We extracted article characteristics, including support or opposition to high-dose naloxone formulations, any funding received, the authors’ employment/conflicts of interest, main findings, if more than two doses of naloxone were administered, and the stated advantages/disadvantages of high-dose naloxone formulations. The findings were discussed and organized into three topics as described below.
Given the number of articles funded by naloxone manufacturers or consultants paid by pharmaceutical companies, we decided to remove those articles and discuss them in a separate section in order to minimize bias.
Results
We identified 23 articles eligible for inclusion (Fig. 1). Most articles were based on community-based response (N = 8) or medical response (N = 13) which included EMS response, hospitals, and outpatient settings. The 3 remaining articles focused on a combination of site types or police response. Most articles did not specify the brand of naloxone that was used but 2 explicitly focused on Narcan. The majority of the articles included multiple administration routes (N = 15) whereas 3 focused on intravenous, 1 on intramuscular, and 3 on intranasal. Two articles did not specify how naloxone was administered in the reported data. Additionally, no included articles noted whether oxygen was given to support overdose reversal and recovery despite that it has been shown to improve the success of naloxone [9].
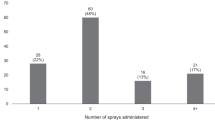
We coded each article as supportive of high-dose naloxone formulations (N = 7; 30%), unsupportive (N = 4; 17%), or neutral (N = 12; 52%) as seen in Table 2. Notably, very few articles elicited the perspectives of PWUD. Six articles (25%) directly interviewed or surveyed PWUD. Common points of discussion identified in the articles included frequency of more than two doses, arguments supporting high-dose naloxone, and arguments against high-dose naloxone. Specific details of our synthesis are organized under these three overarching questions below.
Part one: how often are more than two standard doses of im or in naloxone needed to reverse a fentanyl overdose?
Relatively few papers have been published examining the number of doses of IM or IN naloxone needed to reverse an overdose. Supporters of high-dose naloxone formulations often reference a single nationwide study conducted from 2012 to 2015 [49], which reported a trend of Emergency Medical Service (EMS) providers needing to administer more doses per patient each year. However, this study is limited in its methodology as it fails to consider administration route, dose volume, and dose concentration. The omission results in uncertainty that is driving the need for more doses, an important factor to understand before changing dosing practices.
Other researchers report that there was an increase in the average dose provided by EMS. One Ohio-based study from 2014 and 2016 reported the average IV dose increased from 2 to 5 mg [50]. However, a study conducted in Pittsburgh from 2013–2016 found that less than 5% of overdoses required three or more doses of IM naloxone [51]. This was corroborated by a national study from 2018, though heroin not fentanyl was involved in the majority of these cases [52]. Similarly, only two percent of EMS responders in New Jersey reported requiring a third dose of IN naloxone after having a second dose administered by an advanced life support team [53]. A large study of New York police officers from 2015–2020 noted that an average of two doses of naloxone were administered to rescue individuals [54]. One survey-based study reported that 30% of participants living in regions with fentanyl epidemics used 3 or more doses of IN naloxone [11]. A randomized double-dummy controlled trial found that the risk of receiving additional doses was 19.4% higher in those given IN naloxone (1.4 mg/0.1 mL) compared to IM (0.8 mg/2 mL), and that IN naloxone was less efficient in bringing overdose patients back to spontaneous breathing within 10 min in the prehospital setting[55]. However, heroin was the suspected drug in 196 of the 201 participants analyzed.
Interestingly, a Morbidity and Mortality Weekly Report (MMWR) reported 83% of patients in Massachusetts required 3 or more doses of nasal naloxone to reverse a suspected fentanyl overdose [56]. A 2021 survey-based study out of Maryland indicated that 79% of participants administered 3 or more doses at their last witnessed overdose [57]. Case studies and hospital chart reports have recorded high doses (12–15 mg) of naloxone being administered for synthetic opioid overdoses [58]. More than two doses of naloxone were required to reverse two carfentanil overdoses, likely owing to the greater affinity of carfentanil for μ-receptors than naloxone [59, 60]. μ-opioid receptors are one of the specific target sites in the body that naloxone binds to and blocks, effectively reversing the effects of opioid drugs [61, 62].
An aforementioned rigorous systematic review by Moe and colleagues of overdoses (n = 26,660) from North America and Europe through 2018 found that less patients with presumed fentanyl/ultra-potent opioid exposure were revived using initial low doses (≤ 0.4 mg/ml) versus when heroin was presumed (57% vs. 80%) but they concluded that a cumulative dose of 4 mg (e.g., two standard doses of IN) was sufficient in 97% of presumed fentanyl/potent opioid cases [29].
In conclusion, although there have been greater doses used in clinical and community settings, evidence suggests that the vast majority of fentanyl overdoses can be reversed with standard dosing. However, overdoses involving carfentanil or other similarly potent synthetic analogs may require three or more doses. Two doses of IM naloxone (0.8 mg) have also been insufficient in reversing some fentanyl overdoses though such data are subject to the amount of fentanyl exposure [63]. Additionally, depending on the half life of the opioid used, an individual may fall back into an overdose after being revived due to naloxone’s half life of 30–90 min [64]. Given these facts, and the observation that three or more doses are already being used in the community, our recommendation is that four doses of IN or IM naloxone be provided to community members with clear education on the length of latency to effectiveness to optimize coverage. To determine whether high-dose naloxone formulations are an optimal solution we weigh the advantages and disadvantages of these formulations next.
Part two: what are the potential advantages of high-dose formulations?
There are many perceived benefits of high-dose naloxone formulations, though, in practice, the evidence base is underdeveloped. Given that the formulations currently approved have been relatively comparable in their concentration to others of the same administration route, much of the literature has focused on administering naloxone slowly over time (“titration”) rather than administering a single high-dose naloxone formulation. According to the small number of papers [46, 65] on this topic, a high-dose naloxone formulation could theoretically result in a faster response and reduce the magnitude of the harmful non-fatal impacts of drug toxicity, including cognitive and physiologic issues, although this was not proven with real-world data. A high-dose naloxone formulation would improve reversal rates for overdoses involving carfentanil and other opioids that have a stronger μ-receptor affinity than fentanyl [59], though such experiences are relatively rare and localized. Interestingly, recent national study showed that almost half (48%) of people who had reversed an overdose with naloxone held no preference for, or were against, high-dose naloxone formulations, while over one third (36%) preferred a high-dose naloxone formulations to be made available [37].
Owing to the preconceived biases around drug use and naloxone by association, the need to carry fewer doses may help reduce experiences of stigma [11], while simultaneously providing more convenience in portability. Despite these potential benefits and community interest in high-dose naloxone formulations [37], we see many potential pitfalls of relying solely on them.
Part three: what are the potential disadvantages of high-dose formulations?
As discussed previously, prior literature suggests that, despite fentanyl poisoning becoming more prevalent, a standard dose can still be equally effective in many cases [36, 51, 66,67,68]. Below, we discuss further reasoning to not recommend high-dose naloxone formulations over standard dosing.
There is no pharmacological basis for high dose naloxone when it comes to fentanyl
In vivo, in the human brain, researchers have used a positron emission tomography (PET) scanner’s non-tomography positron detecting system to measure the dose–response curve of naloxone and found that ~ 13 μg/kg (0.013 mg/kg) of naloxone per kg of patient bodyweight was required to produce an estimated 50% receptor occupation when given intravenously[69]. In general, a drug typically needs to occupy a sufficient number of its target receptors to initiate the desired biological response. Studies have indicated that achieving approximately 50% receptor occupancy by naloxone is associated with its desired therapeutic effects in reversing opioid overdose [69, 70]. This suggests that higher doses of naloxone may not be needed as long as 50% of the μ-opioid receptors are occupied. However, there are many factors that could affect level of occupancy such as route of administration and bioavailability [71].
A second pharmacokinetic consideration is the variable binding affinities of various opioids relative to the antagonistic effects of naloxone. Each opioid has a unique binding affinity (Ki) towards the μ-opioid receptors. Naloxone must have a lower Ki, indicating a stronger binding affinity, to successfully reverse an overdose. Notably, morphine and fentanyl have similar Ki values despite their vastly different potency levels demonstrating that potency does not always correlate with binding affinity [72, 73]. Therefore, stronger analogs are not an indication of the need for high-dose naloxone formulations in the absence of binding affinity assessment.
While naloxone exhibits a relatively rapid and strong binding affinity to opioid receptors, the short duration of action means it is relatively quick to dissociate from the receptors [70, 74]. For longer acting opioids, this means opioids may rebind causing a recurrence of respiratory depression. high-dose naloxone formulations have been found to make the effect last longer but doesn't change how quickly it works to reverse an overdose[75]. The duration of a drug's effects can vary depending on several factors, including the individual's tolerance, the method of administration, the dosage, and the purity of the drug. high-dose naloxone formulations may have an application for these longer-acting opioids and circumstances, but evidence is limited.
The risk of withdrawal from high-dose naloxone
As with any medication, there are potential risks associated with taking too much naloxone. The main risk of excessive naloxone dosing is that it can cause rapid-onset naloxone-induced withdrawal symptoms if a person has a high dependence to opioids [76,77,78]. Naloxone is effective at reversing overdose as it displaces opioids from the receptors without activating their sedative and respiratory depressant effects. The displacement effectively reverses the effects of the opioids and causes withdrawal even if opioids remain in the person's system [79, 80]. This can include the well-known symptoms such as severe pain, agitation, muscle cramps, and nausea [81]. Additionally, precipitated withdrawal has serious symptoms, such as diarrhea, vomiting, myalgia, anxiety, and autonomic hyperactivity [82]. Additionally, in rare cases, naloxone can cause an allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat [5]. Sequelae such as death, coma, and encephalopathy have been documented in association with these occurrences. Notably, such events have predominantly manifested in patients with pre-existing cardiovascular disorders or those concurrently administered medications with comparable adverse cardiovascular effects. However, establishing a definitive cause-and-effect relationship requires further investigation [83].
Due to these risks, the recommended dose for opioid reversal remains controversial. The aforementioned withdrawal risk can lead to hesitation among PWUD when encountering a potential overdose. They must quickly balance the potentially life threatening consequence of withholding the narcan with the ensuing implications of withdrawal. Namely, that the intense discomfort and cravings following withdrawal can lead to subsequent increased use and opioid seeking behaviors. Additionally, negative experiences related to overdose reversal may result in avoidance of treatment due to fears of having similar experiences in an already stressful medical setting. Finally, withdrawal can temporarily impair an individual's ability to carry out acts of daily living such as caring for oneself, attending work, or engaging in social activities. high-dose naloxone formulations are likely to intensify these drawbacks as administration to someone with high opioid dependence can lead to more intensified symptoms then the typical dose would as more opioids will be displaced with naloxone.
The need for respect, consent, and a voice in drug policy: ethical considerations
From a literal perspective, consent is the act of voluntarily agreeing to participate in something, such as a medical procedure, sexual activity, or research study. It is important because it ensures that individuals know and understand what they are agreeing to. Consent is a fundamental aspect of respecting individual autonomy and personal freedom and it is crucial for maintaining ethical standards in healthcare, research and interpersonal relationships.
In the context of an overdose, obtaining consent is not possible because the victim is unconscious. Unless the responder and person experiencing the overdose had discussed their preferences on how to handle such a situation before the overdose occurred, standard guidance should aim to do as little harm as possible. In such cases, it's also important to provide clear and accurate information and to respect their autonomy as much as possible after administration of naloxone when conscious.
Listening to PWUD is crucial in making informed decisions about increasing the dose formulation of naloxone. Those with lived and living experience have unique insight into the complexities of overdose and the effectiveness of naloxone. They can provide valuable information on how a higher dose formulation may impact their ability to respond to an overdose. Additionally, they can offer insight into other factors that may contribute to overdose, such as polysubstance use or lack of access to harm reduction services. By listening to those with lived and living experience, we can gain a better understanding of the challenges and barriers faced by PWUD and make more informed decisions about how to address overdose in a way that is effective, equitable, and inclusive.
Collaborating with PWUD is also an important aspect of practicing informed consent. By actively hearing their experiences and concerns, we can better understand their needs and preferences allowing us to provide care with respect and consideration of their unique circumstances. Individuals who use drugs have the right to make informed decisions about their healthcare and incorporating their preferences ensures they are empowered to make informed decisions about their healthcare. This can help build trust between healthcare providers and PWUD, leading to better health outcomes and more effective overdose prevention strategies. Conrarily, making decisions on naloxone dose, route of administration, and cost without including those who are directly impacted in the decision process violates their right to consent, erodes their trust and perpetuates the overdose epidemic.
Cost considerations
As shown in Table 1, the costs of available naloxone formulations vary widely from $15-$40 per unit for the most affordable generic IM formulation to $131-$145 per unit for Zimhi high-dose IM auto injector and Kloxxado high-dose IN. As expected, generic formulations cost less than branded formulations with the IM and IN costing $15 and $20 at the lower cost range respectively. Notably, the two highest single dose formulations, Zimhi and Kloxxado, are also the most costly. Zimhi is 25 times stronger than generic IM naloxone and costs over 8 times the generic equivalent. Kloxxado is twice as strong as the generic IN formulation and costs about 5 times as much. Given that the majority of fentanyl overdoses studied only require two or three doses of standard IM or IN, high dose naloxone formulations with more than three times the dose within the same administration route category may not be a cost effective solution.
Discussion
We aimed to understand whether two doses of IM/IN naloxone can effectively reverse fentanyl overdoses and whether newer high-dose formulations are an optimal and necessary solution. Our findings indicate that although two or more standard doses of naloxone have been administered in clinical and community settings, most fentanyl overdoses can be successfully reversed using two standard dosages of IN or IM. Overdoses involving carfentanil, a highly potent fentanyl analog, necessitate three or more doses for effective reversal; this may be due to carfentanil having a slower rate of opioid receptor dissociation [84]. However, carfentanil overdoses are relatively rare compared to fentanyl overdoses throughout the United States.
Although comparing formulations was beyond the scope of our review, we did note that in some cases, the administration of two IM naloxone doses (0.8 mg) has been insufficient in reversing a fentanyl overdose. However, the accuracy of this conclusion is contingent upon the quantity of fentanyl present in the drug samples consumed and the individual’s tolerance. For this reason, community-based programs that solely distribute IM naloxone could pre-emptively begin distributing four or more doses to all program participants. Given the well-established knowledge that overdose symptoms may recur after resuscitation, depending on the half-life of the specific opioid, keeping additional doses of naloxone on hand can be useful regardless of the formulation distributed.
Considering these findings and the current community practice of using multiple doses of standard IM and IN, we recommend providing, at minimum, four standard doses of IN or IM naloxone to each individual (i.e., two two-dose kits). This guarantees that administration can continue until the recipient achieves stability, ensuring appropriate intervals between each dose, and extra doses are on hand in case of carfentanil exposure or symptom recurrence. Given that some people who use fentanyl use multiple times per day, and some bystanders know multiple people who use fentanyl, providing an ample number of kits to potential bystanders is critical.
Higher-dosage formulations are unnecessary for fentanyl overdoses, and may also cause harm as evidenced by the risk of precipitated opioid withdrawal. While there is little evidence that high-dose naloxone formulations will be more effective for responding to fentanyl overdoses, high-dose naloxone formulations may elicit a faster overdose reversal rate for carfentanil overdoses compared to standard doses.
One barrier that remains in scaling up IM and IN naloxone is that only one brand of over-the-counter IN naloxone (Emergent) has been FDA approved. Approving generic naloxone and standard IM formulations will help speed up community-level naloxone coverage. Another barrier to carrying IM naloxone is that syringe possession remains illegal in some states.
Data limitations
Much of the literature supporting the use of high-dose naloxone formulations fails to take into consideration the expressed needs, barriers, and consent of PWUD, which may have significant implications for the ethical and effective implementation of such interventions. For these reasons, we encourage scientists, medical providers, and pharmaceutical companies to speak to PWUD and service providers (such as harm reduction workers or others working directly with drug users) when developing and testing new high-dose naloxone products. Providing the context to epidemiological and clinical data through lived experience is important because it allows for a more accurate interpretation of the results as well as a more realistic understanding of how naloxone formulary changes would impact PWUD. Without context, assumptions may be based on bias, or draw the wrong conclusions. We also noted that some studies were either conducted or funded by pharmaceutical companies who may have a conflict of interest in the study’s outcome.
Future studies and conclusions
The majority of the research conducted in the field of substance use has not been done in settings that accurately reflect the contexts in which PWUD experience an overdose and withdrawal symptoms. For example, there has been scientific debate on the role of non-opioid sedatives such as xylazine (a tranquilizer commonly used in veterinary medicine) and benzodiazepines (a central nervous system depressant) in overdose response[85,86,87]. We must communicate to the public that naloxone will not reverse the effects of these sedatives and additional medical intervention may be required to assist PWUD even after naloxone is administered. More studies that center the perspectives of PWUD are needed to optimize community bystander reversals especially in the era of xylazine and other contaminants.
In conclusion we did not find rigorous evidence to support the distribution of high-dose naloxone formulations compared to standard doses. Community programs should provide at least four doses of standard IM or IN (and more if possible) to each program participant to optimize naloxone coverage without sacrificing the physical and psychological wellbeing of PWUD.
Availability of data and materials
Data sharing is not applicable to this article as this is a literature review. However, Table 2 has been provided that lists all published research articles involved in the literature search for this review. The data referenced by Tennessee Harm Reduction are not publicly available because it was used only as observation. However, a summary of this data is available on the Tennessee Harm Reduction website and data from the corresponding author on reasonable request.
Abbreviations
- CPR:
-
Cardiopulmonary resuscitation
- EMS:
-
Emergency medical services
- FDA:
-
Federal drug administration
- IM:
-
Intramuscular
- IN:
-
Intranasal
- IV:
-
Intravenous
- Ki :
-
Binding affinity
- MMWR:
-
Morbidity and mortality weekly report
- PET:
-
Positron emission tomography
- PWUD:
-
People who use drugs
- SAMHSA:
-
Substance abuse and mental health services administration
- SUD:
-
Substance use disorder
References
Dydyk AM, Jain NK, Gupta M. Opioid use disorder. StatPearls Publishing; 2024.
Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13–20.
Azadfard M, Huecker MR, Leaming JM. Opioid addiction. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–72.
Buajordet I, Naess A-C, Jacobsen D, Brørs O. Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur J Emerg Med. 2004;11:19–23.
Dahlem CH, Scalera M, Chen B, McCabe SE, Boyd CJ. Impact of the take ACTION Train-the-Trainer model of opioid overdose education with naloxone distribution- who benefits? Subst Abus. 2020;41:485–92.
Wermeling DP. Review of naloxone safety for opioid overdose: practical considerations for new technology and expanded public access. Ther Adv Drug Saf. 2015;6:20–31.
Schiller EY, Goyal A, Mechanic OJ. Opioid overdose. StatPearls. Treasure Island: StatPearls Publishing; 2022.
Rowe A, Chang A, Lostchuck E, Lin K, Scheuermeyer F, McCann V, et al. Out-of-hospital management of unresponsive, apneic, witnessed opioid overdoses: a case series from a supervised consumption site. CJEM. 2022;24:650–8.
How to respond to an overdose [Internet]. [cited 2023 Jun 24]. https://www.acton-ma.gov/619/Responding-to-an-Overdose
Abdelal R, Raja Banerjee A, Carlberg-Racich S, Darwaza N, Ito D, Shoaff J, et al. Real-world study of multiple naloxone administration for opioid overdose reversal among bystanders. Harm Reduct J. 2022;19:49.
Mahonski SG, Leonard JB, Gatz JD, Seung H, Haas EE, Kim HK. Prepacked naloxone administration for suspected opioid overdose in the era of illicitly manufactured fentanyl: a retrospective study of regional poison center data. Clin Toxicol. 2020;58:117–23.
Marco CA, Trautman W, Cook A, Mann D, Rasp J, Perkins O, et al. Naloxone use among emergency department patients with opioid overdose. J Emerg Med. 2018;55:64–70.
Barry CL, Sherman SG, Stone E, Kennedy-Hendricks A, Niederdeppe J, Linden S, et al. Arguments supporting and opposing legalization of safe consumption sites in the US. Int J Drug Policy. 2019;63:18–22.
Stewart RE, Cardamone NC, Loscalzo E, French R, Lovelace C, Mowenn WK, et al. “There’s absolutely no downside to this, I mean, except community opposition:” A qualitative study of the acceptability of vending machines for harm reduction. Harm Reduct J. 2023;20:25.
Davis S, Wallace B, Van Roode T, Hore D. Substance use stigma and community drug checking: a qualitative study examining barriers and possible responses. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph192315978.
Taylor S. Outside the outsiders: media representations of drug use. Probation J. 2008;55:369–87.
Sumnall HR, Atkinson A, Montgomery C, Maynard O, Nicholls J. Effects of media representations of drug related deaths on public stigma and support for harm reduction. Int J Drug Policy. 2023;111: 103909.
Celebrity TR, Scandals D. Media double standards. Contexts. 2013;12:36–41.
Scher BD, Neufeld SD, Butler A, Bonn M, Zakimi N, Farrell J, et al. “Criminalization Causes the Stigma”: perspectives from people who use drugs. Contemp Drug Probl. 2023;50:402–25.
Mahon LR, Hawthorne AN, Lee J, Blue H, Palombi L. Assessing pharmacy student experience with, knowledge of and attitudes towards harm reduction: illuminating barriers to pharmacist-led harm reduction. Harm Reduct J. 2018;15:57.
Garett R, Young SD. The role of misinformation and stigma in opioid use disorder treatment uptake. Subst Use Misuse. 2022;57:1332–6.
Moses TE, Chammaa M, Ramos R, Waineo E, Greenwald MK. Incoming medical students’ knowledge of and attitudes toward people with substance use disorders: implications for curricular training. Subst Abus. 2021;42:692–8.
McKnight C, Weng CA, Reynoso M, Kimball S, Thompson LM, Jarlais DD. Understanding intentionality of fentanyl use and drug overdose risk: findings from a mixed methods study of people who inject drugs in New York City. Int J Drug Policy. 2023;104063.
Gardner EA, McGrath SA, Dowling D, Bai D. The Opioid Crisis: Prevalence and Markets of Opioids. Forensic Sci Rev. 2022;34:43–70.
Tomassoni AJ, Hawk KF, Jubanyik K, Nogee DP, Durant T, Lynch KL, et al. Multiple fentanyl overdoses: New Haven, Connecticut, June 23, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:107–11.
Abuse S. Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit.; 2016. 2018.
Spencer M, Miniño A, Warner M. Drug overdose deaths in the United States, 2001–2021 [Internet]. National Center for Health Statistics (U.S.); 2022 Dec. https://stacks.cdc.gov/view/cdc/122556
Moe J, Godwin J, Purssell R, O’Sullivan F, Hau JP, Purssell E, et al. Naloxone dosing in the era of ultra-potent opioid overdoses: a systematic review. CJEM. 2020;22:178–86.
Abdelal R, Banerjee AR, Carlberg-Racich S, Cebollero C, Darwaza N, Kim C, et al. Real-world study of multiple naloxone administrations for opioid overdose reversal among emergency medical service providers. Subst Abus. 2022;43:1075–84.
Office of the Commissioner. Statement on continued efforts to increase availability of all forms of naloxone to help reduce opioid overdose deaths [Internet]. U.S. Food and Drug Administration. FDA; [cited 2022 Dec 7]. https://www.fda.gov/news-events/press-announcements/statement-continued-efforts-increase-availability-all-forms-naloxone-help-reduce-opioid-overdose
Tylleskar I, Gjersing L, Bjørnsen LP, Braarud A-C, Heyerdahl F, Dale O, et al. Prehospital naloxone administration—what influences choice of dose and route of administration? BMC Emerg Med. 2020;20:71.
Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63–88.
Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. Prescribing naloxone to actively injecting heroin users: a program to reduce heroin overdose deaths. J Addict Dis. 2006;25:89–96.
Naloxone Hydrochloride 0.4mg/mL MDV 10mL vial [Internet]. Lexicon Medical Supply. [cited 2023 Jul 5]. https://www.lexiconsupply.com/Products/Naloxone-Hydrochloride-04mgmL-MDV-10mL-vial__17478-0042-10.aspx
Hill LG, Zagorski CM, Loera LJ. Increasingly powerful opioid antagonists are not necessary. Int J Drug Policy. 2022;99: 103457.
Strickland JC, Marks KR, Smith KE, Ellis JD, Hobelmann JG, Huhn AS. Patient perceptions of higher-dose naloxone nasal spray for opioid overdose. Int J Drug Policy. 2022;106: 103751.
Salazar ZR, Vincent L, Figgatt MC, Gilbert MK, Dasgupta N. Research led by people who use drugs: centering the expertise of lived experience. Subst Abuse Treat Prev Policy. 2021;16:70.
Tsai AC, Kiang MV, Barnett ML, Beletsky L, Keyes KM, McGinty EE, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med. 2019;16: e1002969.
Kerensky T, Walley AY. Opioid overdose prevention and naloxone rescue kits: what we know and what we don’t know. Addict Sci Clin Pract. 2017;12:4.
Wheeler E, Jones TS, Gilbert MK, Davidson PJ. Centers for Disease Control and Prevention (CDC) opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:631–5.
Wang DS, Sternbach G, Varon J. Nalmefene: a long-acting opioid antagonist. Clinical applications in emergency medicine. J Emerg Med. 1998;16:471–5.
The. A new nasal spray to reverse fentanyl and other opioid overdoses gets FDA approval. NPR [Internet]. 2023 May 22 [cited 2023 Jul 5]; https://www.npr.org/2023/05/22/1177597319/fda-approves-opvee-naloxone-opioid-overdose-fentanyl
Document [Internet]. [cited 2024 Mar 6]. https://www.sec.gov/Archives/edgar/data/1385508/000138550822000130/opnt_3q22financialresultsx.htm
Vraníková B, Gajdziok J. Bioavailability and factors influencing its rate. Ceska Slov Farm. 2015;64:7–13.
McDonald R, Lorch U, Woodward J, Bosse B, Dooner H, Mundin G, et al. Pharmacokinetics of concentrated naloxone nasal spray for opioid overdose reversal: phase I healthy volunteer study. Addiction. 2018;113:484–93.
Dietze P, Jauncey M, Salmon A, Mohebbi M, Latimer J, van Beek I, et al. Effect of intranasal vs intramuscular naloxone on opioid overdose: a randomized clinical trial. JAMA Netw Open. 2019;2: e1914977.
Simon C, Brothers S, Strichartz K, Coulter A, Voyles N, Herdlein A, et al. We are the researched, the researchers, and the discounted: The experiences of drug user activists as researchers. Int J Drug Policy. 2021;98: 103364.
Faul M, Lurie P, Kinsman JM, Dailey MW, Crabaugh C, Sasser SM. Multiple naloxone administrations among emergency medical service providers is increasing. Prehosp Emerg Care. 2017;21:411–9.
Birmingham LE, Nielson JA. An increase in per-patient naloxone requirements in an opioid epidemic. Am J Emerg Med. 2017;35:1958–9.
Bell A, Bennett AS, Jones TS, Doe-Simkins M, Williams LD. Amount of naloxone used to reverse opioid overdoses outside of medical practice in a city with increasing illicitly manufactured fentanyl in illicit drug supply. Subst Abus. 2019;40:52–5.
Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34:573–6.
Klebacher R, Harris MI, Ariyaprakai N, Tagore A, Robbins V, Dudley LS, et al. Incidence of naloxone redosing in the age of the new opioid epidemic. Prehosp Emerg Care. 2017;21:682–7.
Pourtaher E, Payne ER, Fera N, Rowe K, Leung SYJ, Stancliff S, et al. Naloxone administration by law enforcement officers in New York State (2015–2020). Harm Reduct J. 2022;19:102.
Skulberg AK, Tylleskär I, Valberg M, Braarud A-C, Dale J, Heyerdahl F, et al. Comparison of intranasal and intramuscular naloxone in opioid overdoses managed by ambulance staff: a double-dummy, randomised, controlled trial. Addiction. 2022;117:1658–67.
Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, et al. Characteristics of fentanyl overdose - Massachusetts, 2014–2016. MMWR Morb Mortal Wkly Rep. 2017;66:382–6.
Schneider KE, Urquhart GJ, Rouhani S, Park JN, Morris M, Allen ST, et al. Practical implications of naloxone knowledge among suburban people who use opioids. Harm Reduct J. 2021;18:47.
Skolnick P. Treatment of overdose in the synthetic opioid era. Pharmacol Ther. 2022;233: 108019.
Bardsley R. Higher naloxone dosing may be required for opioid overdose. Am J Health Syst Pharm. 2019;76:1835–7.
Leen JLS, Juurlink DN. Carfentanil: a narrative review of its pharmacology and public health concerns. Can J Anaesth. 2019;66:414–21.
Wang S. Historical review: opiate addiction and opioid receptors. Cell Transplant. 2019;28:233–8.
Pasternak GW, Pan Y-X. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65:1257–317.
Carpenter J, Murray BP, Atti S, Moran TP, Yancey A, Morgan B. Naloxone dosing after opioid overdose in the era of illicitly manufactured fentanyl. J Med Toxicol. 2020;16:41–8.
Alaei S, Omidian H. Opioid overdose, interventions, and challenges. Bioimpacts. 2022;12:179–81.
Mundin G, McDonald R, Smith K, Harris S, Strang J. Pharmacokinetics of concentrated naloxone nasal spray over first 30 minutes post-dosing: analysis of suitability for opioid overdose reversal. Addiction. 2017;112:1647–52.
Jones JD, Sherwin E, Martinez S, Comer SD. Naloxone-induced withdrawal in individuals with and without fentanyl-positive urine samples. Am J Addict. 2020;29:51–6.
Farah T. How much naloxone is needed to reverse an opioid overdose? New high-dose treatments are raising questions [Internet]. STAT. 2021 [cited 2022 Dec 7]. https://www.statnews.com/2021/12/15/naloxone-opioid-overdose-zimhi-kloxxado/
Krotulski AJ, Chapman BP, Marks SJ, Ontiveros ST, Devin-Holcombe K, Fogarty MF, et al. Sentanyl: a comparison of blood fentanyl concentrations and naloxone dosing after non-fatal overdose. Clin Toxicol. 2022;60:197–204.
Melichar JK, Nutt DJ, Malizia AL. Naloxone displacement at opioid receptor sites measured in vivo in the human brain. Eur J Pharmacol. 2003;459:217–9.
Johansson J, Hirvonen J, Lovró Z, Ekblad L, Kaasinen V, Rajasilta O, et al. Intranasal naloxone rapidly occupies brain mu-opioid receptors in human subjects. Neuropsychopharmacology. 2019;44:1667–73.
Clarke SFJ, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J. 2005;22:612–6.
Volz MR, Moosmann B. Development of a non-radioactive mass spectrometry-based binding assay at the μ-opioid receptor and its application for the determination of the binding affinities of 17 opiates/opioids as well as of the designer opioid isotonitazene and five further 2-benzylbenzimidazoles. Anal Chim Acta. 2022;1219: 339978.
Torralva R, Janowsky A. Noradrenergic mechanisms in fentanyl-mediated rapid death explain failure of naloxone in the opioid crisis. J Pharmacol Exp Ther. 2019;371:453–75.
Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44:398–401.
Olofsen E, van Dorp E, Teppema L, Aarts L, Smith TW, Dahan A, et al. Naloxone reversal of morphine- and morphine-6-glucuronide-induced respiratory depression in healthy volunteers: a mechanism-based pharmacokinetic-pharmacodynamic modeling study. Anesthesiology. 2010;112:1417–27.
Weisshaar S, Brandt L, Litschauer B, Sheik-Rezaei S, Moser L, Nirnberger G, et al. Dose-dependent naloxone-induced morphine withdrawal symptoms in opioid-dependent males-a double-blinded, randomized study. Br J Clin Pharmacol. 2020;86:1610–9.
Kanof PD, Handelsman L, Aronson MJ, Ness R, Cochrane KJ, Rubinstein KJ. Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. J Pharmacol Exp Ther. 1992;260:355–63.
Purssell R, Godwin J, Moe J, Buxton J, Crabtree A, Kestler A, et al. Comparison of rates of opioid withdrawal symptoms and reversal of opioid toxicity in patients treated with two naloxone dosing regimens: a retrospective cohort study. Clin Toxicol. 2021;59:38–46.
Skolnick P. On the front lines of the opioid epidemic: Rescue by naloxone. Eur J Pharmacol. 2018;835:147–53.
Ke Y-Y, Huang Y-H, Chien W-C, Loh HH, Chuang J-Y, Yeh S-H. Mapping the naloxone binding sites on the mu-opioid receptor using cell-based photocrosslinkers. Biochim Biophys Acta: Proteins Proteomics. 2017;1865:336–43.
Farrell M. Opiate withdrawal. Addiction. 1994;89:1471–5.
Shah M, Huecker MR. Opioid Withdrawal. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
Center for Drug Evaluation, Research. FDA approves naloxone injection to counteract opioid overdoses [Internet]. U.S. Food and Drug Administration. FDA; 2021 [cited 2023 Aug 2]. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-naloxone-injection-counteract-opioid-overdoses
Mann J, Samieegohar M, Chaturbedi A, Zirkle J, Han X, Ahmadi SF, et al. Development of a translational model to assess the impact of opioid overdose and naloxone dosing on respiratory depression and cardiac arrest. Clin Pharmacol Ther. 2022;112:1020–32.
Friedman J, Montero F, Bourgois P, Wahbi R, Dye D, Goodman-Meza D, et al. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend. 2022;233: 109380.
Thangada S, Clinton HA, Ali S, Nunez J, Gill JR, Lawlor RF, et al. Notes from the field: Xylazine, a veterinary tranquilizer, identified as an emerging novel substance in drug overdose deaths–connecticut, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1303–4.
Russell C, Law J, Bonn M, Rehm J, Ali F. The increase in benzodiazepine-laced drugs and related risks in Canada: the urgent need for effective and sustainable solutions. Int J Drug Policy. 2023;111: 103933.
Acknowledgements
We thank the volunteers from Tennessee Harm Reduction and the Harm Reduction Innovation Lab who supported this work.
Funding
MA is funded by the Karen T. Romer Undergraduate Teaching and Research Award at Brown University. ET and JNP are funded by the Center of Biomedical Research Excellence on Opioids and Overdose (P20GM125507) from the NIH. JNP serves as a technical consultant for a modeling project funded by the Food and Drug Administration (U01FD00745501) based at Harvard Medical School. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of our funders.
Author information
Authors and Affiliations
Contributions
PML and DPG conceptualized and drafted the first version of the paper. All authors contributed to writing subsequent versions and have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lemen, P.M., Garrett, D.P., Thompson, E. et al. High-dose naloxone formulations are not as essential as we thought. Harm Reduct J 21, 93 (2024). https://doi.org/10.1186/s12954-024-00994-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12954-024-00994-z