Abstract
Background
Adverse atherogenic lipid profile is associated with an increased risk of major adverse cardiac events in patients after acute coronary syndrome (ACS). Knowledge regarding the impact of statins on lipid profile remains limited.
Methods
We retrospectively analysed multicenter, real-world data from the Chinese Cardiovascular Association Database-iHeart Project. Patients with a primary diagnosis of ACS from 2014 to 2021 during index hospitalisation and having at least one lipid panel record after discharge within 12 months were enrolled. We analysed target achievement of atherogenic lipid profile, including apolipoprotein B (< 80 mg/dL), low-density lipoprotein cholesterol (LDL-C) (< 1.8 mmol/L), lipoprotein(a) [Lp(a)] (< 30 mg/dL), triglycerides (< 1.7 mmol/L), remnant cholesterol (RC) (< 0.78 mmol/L), non-high-density lipoprotein cholesterol (< 2.6 mmol/L) at baseline and follow-up. Multivariate Cox regression models were employed to investigate the association between patient characteristics and target achievement.
Results
Among 4861 patients, the mean age was 64.9 years. Only 7.8% of patients had all atherogenic lipids within the target range at follow-up. The proportion of target achievement was for LDL-C 42.7%, Lp(a) 73.3%, and RC 78.5%. Patients with female sex, younger age, myocardial infarction, hypertension, and hypercholesteremia were less likely to control LDL-C, Lp(a), and RC. An increase in the burden of comorbidities was negatively associated with LDL-C and Lp(a) achievements but not with RC.
Conclusions
A substantial gap exists between lipid control and the targets recommended by contemporary guidelines. Novel therapeutics targeting the whole atherogenic lipid profile will be warranted to improve cardiovascular outcomes.
What is known?
1. Long-term prognosis of ACS remains challenging, underlining the need for optimisation of secondary prevention in these patients.
2. In addition to LDL-C, the residual lipid risk attributed to other atherogenic lipids is associated with increased risk after ACS.
What the study adds?
1. Only 7.8% of patients had all atherogenic lipids, including LDL-C, TG, ApoB, Lp(a), RC, and non-HDL-C, within the target range.
2. At follow-up, the proportions of the overall population and the very high-risk patients with LDL-C in the target range were 42.7% and 17.5%, respectively.
3. Patients with female sex, younger age, and comorbidities such as myocardial infarction, hypertension, and hypercholesterolemia were less likely to control their lipids.
Similar content being viewed by others
Introduction
Although substantial progress has been achieved over the last two decades in the acute management of patients presenting with acute coronary syndromes (ACS) with the widespread adoption of intensive medical therapy and timely percutaneous coronary intervention, long-term prognosis has remained poor after ACS, underlining the need for optimisation of secondary prevention in these patients [1, 2]. Among pharmacological treatments recommended by current practical guidelines, low-density lipoprotein cholesterol (LDL-C) remains the primary target of therapy, tailoring the level of optimal LDL-C reduction to the individual’s level of cardiovascular risk [3]. However, lowering LDL-C to very low levels does not eliminate cardiovascular risk [4, 5]. Other atherogenic lipoproteins, including lipoprotein(a) [Lp(a)] and triglyceride-rich lipoproteins (TRLs), contribute to residual cardiovascular risk. Accumulating body of evidence demonstrates that apolipoprotein B (ApoB) [6], non-high-density lipoprotein cholesterol (non-HDL-C) [7, 8], Lp(a) [9], and remnant cholesterol (RC) [10] are associated with increased risk independent of LDL-C. Hence, comprehensive management of atherogenic lipoproteins, rather than just lowering LDL-C, may provide an opportunity to address the growing and now global epidemic of the condition. Recent trials of non-statin lipid-lowering therapy (LLT) have shown reduction in cardiovascular events by lowering LDL-C through mechanisms like increasing LDL receptor expression or reducing cholesterol absorption [11]. New therapies targeting triglyceride (TG), Lp(a), with monoclonal antibodies, antisense oligonucleotide, and small-interfering RNA have emerged [12].
Despite these significant advancements in LLT, the management presents notable challenges in clinical practice. Primarily, the achievement of optimal LDL-C levels remains unacceptably inadequate. Among patients with atherosclerotic cardiovascular disease (ASCVD), 50% or less receive statins and less than 40% LDL-C goals below 1.8 mmol/L [13, 14]. While novel therapies are emerging as complementary to statins for the reduction of either LDL-C or other atherogenic lipoproteins, the identification of patients most likely to benefit from these therapies remains elusive.
Furthermore, a comprehensive characterisation of lipid profiles is crucial to fully understanding the effects of statin therapy and their potential prognostic implications. Specifically, there is still limited knowledge regarding the impact of statins on emerging targets such as Lp(a) and RC. We aimed to analyse the alterations of atherogenic lipoprotein profile upon statin treatment after discharge and identify the baseline characteristics of patients associated with optimal lipid levels during follow-up, harnessing real-world data from the multicenter database of patients hospitalised for ACS.
Methods
Study design and data sources
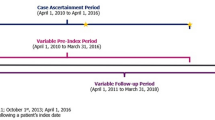
We conducted a multicenter retrospective cohort study using data from the iHeart Project, which was sponsored by the Chinese Cardiovascular Association (CCA) in December 2018 with the aim of developing a nationwide electronic health records (EHRs)-driven platform to collect real-world data from twenty tertiary hospitals across China. The project was overseen by the CCA steering committee. All patient data will be identified and analysed under a protocol that ensures privacy and confidentiality. Applications for research purposes should be reviewed and approved by the committee. Investigators must follow protocols and rules to ensure security before access to the patient data. Within this framework, a comprehensive dataset of patients admitted to the cardiology department between January 1, 2014, and December 31, 2021, was established. Patient information encompassing medical history, physical examinations, comorbid conditions, laboratory tests, imaging reports, prescribed medications, and procedure records were collected from local EHRs. Natural language processing (NLP) techniques were employed to extract clinical information from diverse sources of narrative text. Data quality control was conducted through the comparison of NLP with cardiologists. Briefly, two hundred EHRs were selected by random sampling. Medical texts were extracted using NLP models, and the corresponding information was analysed manually by two cardiologists for verification after structured text data processing. Performance metrics of NLP were 97.93% and 92.11% for accuracy and recall, respectively.
Study population
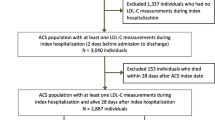
Adult patients with a primary diagnosis of ACS during hospitalisation from January 2014 to December 2021 were enrolled in the study. Patients with at least one lipid panel record within 12 months after discharge were included. Diagnoses were obtained from the hospital discharge record, based on the International Classification of Diseases −10 codes I20.0, I20.1, I21-I21.9, I22, and I23. ACS encompasses a spectrum of conditions, including ST-segment elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), and unstable angina (UA). Individual-level records with any missing data on age, sex, or total cholesterol (TC) were excluded. Patients with severe renal or hepatic dysfunction, reported intolerance to statin, life expectancy < 1 year, pregnant women, or severe haematological, metabolic or endocrine dysfunction were excluded. Finally, a total of 4861 ACS patients were enrolled for the final analyses (Fig. 1). The study was approved by the Ethics Committee of Shanghai Xuhui Central Hospital (Approval Number: 2023-014). Written informed consent was not required because all data were collected retrospectively and anonymously without unique patient identifiers.
Covariate
Demographic characteristics, smoking status, history of diseases, and medication use were collected. We used the medical history in the iHeart Project to ascertain the presence of comorbidities, including myocardial infarction (MI), heart failure, ischemic cerebrovascular disease, peripheral arterial disease, ischemic stroke, hypertension, diabetes, and chronic kidney disease (CKD). Hypertension was defined as systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg, self-reported diagnosis history of hypertension, or use of antihypertensive medication. Type 2 diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L or using any glucose-lowering medication or self-reported diagnosis history of diabetes. Atrial fibrillation was the occurrence of any atrial fibrillation present two weeks before admission. Prior MI was defined as any MI occurrence between birth and arrival at this facility, excluding a presenting MI.
Measurements of total cholesterol, triglyceride, LDL-C, high-density lipoprotein cholesterol (HDL-C), Apolipoprotein A (ApoA), Apolipoprotein B (ApoB), and Lp(a) at baseline and follow-up were collected and analysed. Non-HDL-C was calculated as TC minus HDL-C. RC was calculated as TC minus HDL-C minus LDL-C. In addition, we collected the medication utilisation from discharge records, including lipid-lowering, blood pressure-lowering (BP-lowering), glucose-lowering, anticoagulant and antiplatelet. For categorical data, the presence of a diagnostic code/medication prescription in the EHR was coded as “1”; the absence of a diagnostic code/medication prescription was coded as “0”.
Very high-risk patients were defined as those with history of multiple major ASCVD events or one major ASCVD event and multiple high-risk conditions [15]. Major ASCVD events include recent ACS, history of MI, history of ischemic stroke, and diagnostic peripheral arterial disease. High-risk conditions comprise individuals who were aged 65 years or older, had history of prior coronary artery bypass surgery or percutaneous coronary intervention, diabetes mellitus, hypertension, CKD (eGFR 15–59 mL/min/1.73 m2) and history of congestive heart failure.
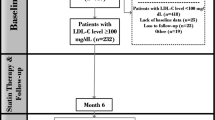
Follow-up and outcome definition
Baseline measurements were defined as laboratory tests during hospitalisation. After discharge, patients were recommended to visit the hospital in 4 to 6 weeks. Patients may undergo tests during these visits according to the follow-up protocol and the physician’s consideration. A follow-up measurement was defined as the last recorded test for lipid panel after the discharge within 12 months. The atherogenic lipid profile included TG, LDL-C, ApoB, non-HDL-C, Lp(a), and RC. We analysed the LDL-C levels within 12 months, as well as changes in other atherogenic lipid profiles. In accordance with the lipid management guidelines, the main outcome was the achievement of LDL-C < 1.8 mmol/L with concomitant reduction > 50%. In patients at very high-risk, an LDL-C reduction of > 50% from baseline and an LDL-C goal of < 1.4 mmol/L were recommended. The targets of other atherogenic lipids were defined as TG < 1.7 mmol/L, ApoB < 80 mg/dL, and non-HDL-C < 2.6 mmol/L [16], with elevated Lp(a) defined as ≥ 30 mg/dL [17] and high RC as ≥ 0.78 mmol/L [18].
Statistical analysis
Continuous covariates and patients with > 60% missing values were discarded, and the missing data was imputed using a random forest algorithm imputation based on the “missForest” package. Continuous variables were reported as mean ± standard deviation (SD) or medians (25th-75th). Categorical variables were reported as frequency and percent. The differences were compared using the Mann-Whitney test or chi-squared test between groups. Sankey plots were used to visualise the changes in LDL-C at baseline and at follow-up.
Unadjusted and adjusted Cox regression models were used to analyse the association between patient characteristics and lipid targets. The models were controlled for established risk factors in a stepwise fashion: Model 1 was crude, and Model 2 was adjusted for ACS subtype and LLT. Sensitivity analyses were performed at 6-month follow-up. All analyses were performed using R version 4.1.3. A 2-tailed P < 0.05 was considered significant.
Results
Baseline characteristics of patients
The baseline characteristics of the patients were shown in Table 1. Of 4861 enrolled patients, the mean age was 64.9 (12.4), 3562 (73.2%) were men. Participants comprised 31.4% diabetes, 70.5% hypertension, and 4186 (86.1%) participants receiving LLT at discharge. Overall, 4031 (82.9%) participants met very high-risk criteria.
Comprehensive management of atherogenic lipid profiles
Of the 4861 patients, 4639 (95.4%) had LDL-C records during index hospitalisation and after discharge. There were 1627 (33.4%) patients who had all TC, TG, LDL-C, ApoB, Lp(a), and RC measured at baseline and 1744 (35.8%) at follow-up. Only 2078 (42.7%) patients tested Lp(a) at baseline and 2294 (47.2%) at follow-up. Changes in lipid profiles between baseline and follow-up were shown in Table 2. TC, TG, LDL-C, ApoB, RC and non-HDL-C were significantly decreased (P < 0.001) within 12 months, whereas HDL-C and ApoA were increased (P < 0.001). Among the study population, 380 patients (7.8%) exhibited optimal levels for all six atherogenic lipids, while 326 patients (6.7%) did not meet the target range for any of these lipids.
Changes in LDL-C during follow-up after discharge
At baseline, the median LDL-C was 2.69 (2.06, 3.36) mmol/L. LDL-C levels of > 3.4 mmol/L, 2.6–3.4 mmol/L, 1.8–2.6 mmol/L, 1.4–1.8 mmol/L, and < 1.4 mmol/L accounted for 24.4%, 29.6%, 30.6%, 9.9%, and 5.6%, respectively. Longitudinal changes and distribution in LDL-C levels were shown in Fig. 2. As the baseline LDL-C levels decreased, an increase in the proportion of patients with LDL-C achieving the target during follow-up was observed.
Overall, at baseline, 15.5% of patients had LDL-C < 1.8mmol/L, and at follow-up, the proportion of patients increased to 42.7%. The percentage of patients who had both LDL-C > 50% reduction and < 1.8 mmol/L during follow-up was 13.2%. Paradoxically, target attainment decreased with increasing risk. The proportion of very high-risk patients within the target range of LDL-C was much lower than those at high risk (17.5% vs. 54.7%, P < 0.001) during follow-up. In addition, the percentage of very high-risk patients who had both LDL-C > 50% reduction and < 1.4 mmol/L during follow-up dropped to 7.4%.
Residual lipid risk beyond LDL-C
Median Lp(a) levels did not change. At baseline, there were 1527 (73.5%) with Lp(a) < 30 mg/dL, 259 (12.5%) with 30–50 mg/dL, and 292 (14.1%) with > 50 mg/dL, and at follow-up, there were 1681 (73.3%) with Lp(a) < 30 mg/dL, 285 (12.4%) with 30–50 mg/dL, and 328 (14.3%) with > 50 mg/dL. Among the participants with Lp(a) record, 938 (45.1%) had an elevated Lp(a) level compared to the baseline.
At baseline, 3219 patients (66.2%) had RC < 0.78 mmol/L, while this proportion increased to 3814 (78.5%) during follow-up. Of the 4861 patients, elevated RC levels were observed in 421 patients (8.7%). Among 1980 patients with follow-up LDL-C < 1.8 mmol/L, 245 patients (12.4%) had Lp(a) ≥ 30 mg/dL, and 379 (19.1%) had RC ≥ 0.78 mmol/L.
Predictors of target achievement of LDL-C, lp(a), and RC
Results of univariate and multivariable analysis (Model 1) were shown in S1 Table. After adjustment for ACS subtype and LLT (Model 2), we found that female sex, newly-diagnosed MI, prior MI, hypertension, and baseline LDL-C ≥ 3.4 mmol/L were negatively associated with LDL-C target. For RC, patients of younger age, newly diagnosed MI, prior MI, hypertension, and baseline LDL-C ≥ 3.4 mmol/L were less likely to maintain optimal. In addition, hypertension was also a predictor of elevated Lp(a) during follow-up (Fig. 3).
We further analysed the association between the burden of comorbidities and lipid control. We observed an increase in the number of comorbidities was negatively associated with LDL-C and Lp(a) in the target range (P < 0.001), whereas it was not associated with RC attainment (P > 0.05), as shown in Fig. 4.
Sensitivity analysis
Further sensitivity analyses were performed at 6-month follow-up. The results showed that patients with female sex and baseline LDL-C ≥ 3.4 mmol/L faced challenges with RC within the target range at follow-up. Similarly, patients with female sex, hypertension and baseline LDL-C ≥ 3.4 mmol/L were less likely to be within the LDL-C target range. In terms of Lp(a), patients with baseline LDL-C levels ≥ 3.4 mmol/L and CKD were less likely to meet the LDL-C target range, consistent with the findings at the 12-month follow-up (S2 Table).
Discussion
In the present study, we analysed the comprehensive management of atherogenic lipid profile within 12 months after discharge among patients with ACS. The main findings were summarised as follows: (1) LDL-C, non-HDL-C, ApoB, and RC were significantly decreased, while Lp(a) was unchanged; (2) Only 7.8% of patients had all atherogenic lipids within the target range; (3) At follow-up, the proportions of the overall population and the very high-risk patients with LDL-C in the target range were 42.7% and 17.5%, respectively; (4) Among patients with LDL-C level below target, 12.4% had Lp(a) ≥ 30 mg/dL, and 19.1% had RC ≥ 0.78 mmol/L; (5) Patients with female sex, younger age, and comorbidities such as MI, hypertension, and hypercholesterolemia were less likely to control their lipids.
ApoB serves as a crucial structural component of atherogenic lipoprotein particles, and its entrapment within the arterial wall represents the pivotal event that drives the entire process of atherosclerosis. Statin therapy does not optimally treat all lipoproteins that are causal in atherogenesis, such as TRLs and Lp(a). Hence, substantial patients with residual risk are unrecognised, highlighting the missed opportunity for additional benefits from LLT. In the present study, only 7.8% of patients had all atherogenic lipids within the target range. Among patients with LDL-C below the target level, 12.4% had Lp(a) ≥ 30 mg/dL, and 19.1% had RC ≥ 0.78 mmol/L. Moreover, the follow-up measurement revealed that 45.1% of the patients had an increase in Lp(a) compared to the baseline. Recent studies have reported conflicting effects of statins on plasma Lp(a) levels [19, 20]. A body of evidence indicates that elevated levels of Lp(a) are linked to a heightened risk of cardiovascular events in patients with documented cardiovascular disease, regardless of LDL-C levels. A recent study demonstrated approximately 6-fold atherogenicity of Lp(a) greater than that of LDL-C on a per-particle basis [21]. Nevertheless, whether and to what extent an increase in Lp(a) following a specific LLT is clinically relevant warrants further investigation. A mild reduction in median RC was observed in the present study, which was consistent with results from the TNT trial [22]. Notably, the elevation of RC level was noted in 22% of patients. Multiple epidemiological studies report statistically significant associations between TRLs and incident ASCVD [22, 23]. Unlike LDL-C particles, which require chemical modification to enter the arterial wall, TRLs can be taken up by peripheral macrophages via the VLDL receptor without modification, leading to a substantially greater atherogenicity than LDL-C [24]. As for per particle reduction, the beneficial effect will probably be greater for TRLs than for LDL-C. To this end, targeting Lp(a) and TRLs beyond LDL-C may potentially yield a more pronounced reduction in ASCVD risk, aligning with the objective of LLT.
Optimal lipid control depends on adequate testing and guidance of the lipid panels. Up to now, clinicians commonly consider LDL-C as the best marker of pro-atherogenic lipids and monitor this parameter most frequently while overlooking other lipids. In the present analysis, only about one-third of enrolled patients obtained complete lipid profiles, including TC, TG, LDL-C, ApoB, and Lp(a). LDL-C might not fully reflect the total atherogenic burden in individuals with conditions such as metabolic syndrome, elevated triglycerides, diabetes, obesity, or very low LDL-C levels. Therefore, the comprehensive phenotype of atherogenic lipids may offer profound insights into the response to LLT and optimise lipid management in very high-risk populations, thereby supporting and extending the recent recommendation, which underscores LDL-C measurement as a performance metric for lipid control [25]. Further investigations are warranted to explore the range of lipid components for risk evaluation, the incorporation of treatment targets, and cost-effectiveness comparisons.
The suboptimal control of LDL-C target in secondary prevention is ubiquitous. In the present study, only 42.7% of patients had LDL-C < 1.8mmol/L during the 12-month follow-up, in line with the findings from observational Dyslipidemia International Study II that 41.7% of patients with ACS had their LDL-C < 1.8mmol/L at four months post-discharge [26]. In a study of the National Health and Nutrition Examination Survey to estimate lipid control among US adults, 34.8% of patients with self-reported coronary artery disease receiving statin therapy have an LDL-C < 1.8 mmol/L [27]. In a retrospective cohort study from an Australian ACS registry, 45% of participants had not achieved lipid targets despite 73% being prescribed intensive LLT at discharge [28]. Notably, the simultaneous reduction of LDL-C by > 50% from baseline levels upon target value achievement poses a greater challenge. In the present study, only 13.2% of patients met the combined criteria, which is consistent with a recent subgroup analysis of an ACS study where 22.2% achieved an LDL-C goal of < 55 mg/dL but only 12.7% additionally reduced the LDL-C value > 50% from baseline levels [29]. A substantial gap exists between the actual control of LDL-C and the high proportion of LLT at discharge in our study, possibly attributed to several reasons. Firstly, the LDL-C target is determined based on risk stratification, and a “treat to target” approach is recommended by current Chinese guidelines. However, initiation with moderate-intensity statins may require up to three adjustment stages for target achievement, according to analyses from recent simulation studies [30, 31]. Patients often show a reluctance to adjust lipid-lowering regimens in real-world settings [32]. The need for multiple visits, blood draws, and medication adjustments may also contribute to significant impairment to physician engagement with guidelines. Secondly, to achieve at least a 50% reduction in LDL-C levels for secondary prevention, lifestyle modifications and treatment with high-intensity statins are recommended by European and American guidelines [3, 13]. However, a meta-analysis utilising individual patient data from 8 randomised controlled trials on statins revealed that even with high-dose statin therapy, 40.4% of patients did not achieve the LDL-C target of < 1.8 mmol/L and 78.3% did not reach the even lower target of < 50 mg/dL [33]. In the SWEDEHEART study conducted in the patients with AMI, 82.9% had not attained the target of an LDL-C level of < 1.4 mmol/L and a > 50% LDL-C level reduction despite 86.6% of enrolled patients receiving high-intensity statin. From a population perspective, the utilisation of high-intensity statins appeared insufficient in fulfilling guideline recommendations adequately and was found to significantly increase the risk of statin intolerance [34]. Accumulated evidence from studies has shown that PSCK9i (monoclonal antibodies and small interfering ribonucleic acid) significantly reduce LDL-C and the occurrence of major adverse cardiovascular events when these therapies were added to statins in patients with ACS [35, 36]. AT-TARGET-IT registry showed that 68.3% of patients receiving PCSK9i prescription achieved LDL-C target. [37, 38]. Therefore, new LLTs hold a potential promise when statin intolerance or inadequate lipid-lowering efficacy with high-intensity statins is encountered.
A significant impact of comorbidities on the control of LDL-C levels was observed. Our study showed that an increase in the number of comorbidities was negatively associated with LDL-C and Lp(a) in the target range, similar to findings from other studies [39, 40]. The presence of comorbidities not only indicates greater complexity and poorer prognosis but also suggests increased medication burden, administration fragmentation, and poor quality of management due to the involvement of other specialities in managing non-cardiovascular comorbidities. Notably, patients with baseline hypercholesterolemia (LDL-C ≥ 3.4 mmol/L) were less likely to achieve the target, considering a moderate intensity of statin may not be sufficient to achieve the target. The results of EUROASPIRE V suggest that the lower the pre-treatment LDL-C level, the smaller the percentage of LDL-C reduction for a given statin dose [41]. Therefore, baseline phenotype, including LDL-C, must be considered as a key factor in individualised LLT. The findings of our study indicated that a significantly higher proportion of patients (82.9%) were classified as being at very high-risk compared to the prevalence observed among individuals with ASCVD (55.3%) [42]. In contrast to the requirement for lower LDL-C levels, these patients exhibited a lower rate of achievement. Our findings demonstrated a positive correlation between an increasing number of comorbid conditions and a heightened risk of failing to attain lipid targets, thereby offering valuable insights into the impact of comorbidities on medical adherence and therapeutic response.
Clinical implications
The therapeutic paradigm shift aims to not only enhance patient adherence for long-term achievement of the LDL-C target but also address the residual lipid risk associated with other lipids. It can be postulated that a reduction of all atherogenic lipids with combined lipid-lowering drugs or medications targeting multiple lipids would confer greater advantages. The analysis from the VYMET study revealed that the combination of statins and cholesterol absorption inhibitors significantly augmented the achievement of triple targets of LDL-C, non-HDL-C, and ApoB in comparison to high-intensity statin therapy alone [43]. In addition, PCSK9 inhibitors exert pleiotropic effects by not only potent reduction of LDL-C [44] but also a substantial decrease of atherogenic lipids targeting non-HDL-C, Lp(a), and ApoB [45,46,47]. Moreover, they may offer additional advantages over high-intensity statins in terms of mitigating the elevated risk of diabetes.
By characterising predictors of goal attainment for the management of patients with ACS, the present study nevertheless provides important insights that may inform researchers and practitioners regarding which patients are less likely to achieve lipid control despite similar medical strategies. In patients with the potential of elevated Lp(a) or RC, surveillance following statin therapy may be useful in understanding residual risk or recurrent events in such patients. Our findings may help guide tailored efforts to improve goal attainment in clinical practice based on the individual lipid profile.
Taken together, LDL-C control remains suboptimal, especially in high-risk and multimorbid individuals. Moreover, patients undergoing statin therapy still exhibit a relatively high residual risk. Therefore, the comprehensive assessment and management of atherogenic lipids using innovative agents and medical strategies are imperative. Future research should involve longer-term, larger, and multi-ethnic prospective studies to validate our perspectives.
Limitation
Some limitations of the study merit consideration. First, due to the retrospective nature of the study, complete information regarding medication adjustment by physicians and patient adherence was not available. Hence, the determination of causality between low achievement rates and these factors is impossible. Second, data on hard CV endpoints such as MI and stroke are lacking. However, failing to achieve guideline-recommended targets implies an increased risk of recurrent cardiovascular events. Therefore, the use of achievement rates as surrogate endpoints is appropriate. Third, the follow-up records obtained were limited to the hospitals where the index treatment for ACS occurred and did not include information on patients’ records at other hospitals. Fourth, the study only included patients with follow-up records. Therefore, these results may not necessarily apply to those without measurement records. The conclusions of this study cannot be generalised to other ethnic groups or clinical settings. Hence, caution is warranted when interpreting and applying these findings.
Conclusion
The current management paradigm of atherogenic lipoproteins and lipids is suboptimal. Elevated Lp(a) and RC upon statin therapy may confer residual risk beyond LDL-C. Therefore, novel therapeutics targeting the whole atherogenic lipid profile are warranted to improve cardiovascular outcomes.
Data availability
The data that support the findings of this study are collected from the iHeart Project of the Chinese Cardiovascular Association, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission from the iHeart Project of the Chinese Cardiovascular Association.
Abbreviations
- ACS:
-
Acute coronary syndrome
- LDL-C:
-
Low-density lipoprotein cholesterol
- Lp(a):
-
Lipoprotein(a)
- RC:
-
Remnant cholesterol
- TRLs:
-
Triglyceride-rich lipoproteins
- ApoA:
-
Apolipoprotein A
- ApoB:
-
Apolipoprotein B
- non-HDL-C:
-
Non-high-density lipoprotein cholesterol
- LLT:
-
Lipid-lowering therapy
- ASCVD:
-
Atherosclerotic cardiovascular disease
- CCA:
-
Chinese Cardiovascular Association
- EHRs:
-
Electronic health records
- NLP:
-
Natural language processing
- MI:
-
Myocardial infarction
- STEMI:
-
ST-segment-elevation myocardial infarction
- NSTEMI:
-
Non-ST-elevation myocardial infarction
- UA:
-
Unstable angina
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High-density lipoprotein cholesterol
- BP-lowering:
-
Blood pressure-lowering
- CKD:
-
Chronic kidney disease
- SD:
-
Standard deviation
- FBG:
-
Fasting blood glucose
- HbA1c :
-
Hemoglobin A1c
References
Piironen M, Ukkola O, Huikuri H, Havulinna AS, Koukkunen H, Mustonen J, et al. Trends in long-term prognosis after acute coronary syndrome. Eur J Prev Cardiol. 2017;24(3):274–80.
Li L, Zhou X, Jin Z, Sun AG, Wang P. Z, Clinical characteristics and in-hospital management strategies in patients with acute coronary syndrome: results from 2,096 accredited chest Pain centers in China from 2016 to 2021. Cardiol Plus. 2022;7(4).
Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–826.
Schwartz GG, Szarek M, Bhatt DL, Bittner VA, Bujas-Bobanovic M, Diaz R, et al. Transiently achieved very low LDL-cholesterol levels by statin and alirocumab after acute coronary syndrome are associated with cardiovascular risk reduction: the ODYSSEY OUTCOMES trial. Eur Heart J. 2023;44(16):1408–17.
Gaba P, O’Donoghue ML, Park JG, Wiviott SD, Atar D, Kuder JF, et al. Association between Achieved Low-Density Lipoprotein Cholesterol Levels and Long-Term Cardiovascular and Safety outcomes: an analysis of FOURIER-OLE. Circulation. 2023;147(16):1192–203.
Hagstrom E, Steg PG, Szarek M, Bhatt DL, Bittner VA, Danchin N, et al. Apolipoprotein B, residual Cardiovascular Risk after Acute Coronary Syndrome, and effects of Alirocumab. Circulation. 2022;146(9):657–72.
Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk than LDL Cholesterol in statin-treated patients. J Am Coll Cardiol. 2021;77(11):1439–50.
Hansen MK, Mortensen MB, Warnakula Olesen KK, Thrane PG, Maeng M. Non-HDL cholesterol and residual risk of cardiovascular events in patients with ischemic heart disease and well-controlled LDL cholesterol: a cohort study. Lancet Reg Health Eur. 2024;36:100774.
Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk after Acute Coronary Syndrome. J Am Coll Cardiol. 2020;75(2):133–44.
Cordero A, Alvarez-Alvarez B, Escribano D, Garcia-Acuna JM, Cid-Alvarez B, Rodriguez-Manero M, et al. Remnant cholesterol in patients admitted for acute coronary syndromes. Eur J Prev Cardiol. 2023;30(4):340–8.
Lobo LM, Molinero G, Masson W, et al. Non-statin lipid-lowering therapy in coronary atherosclerosis regression: a meta-analysis and meta-regression. Eur Heart J. 2020;41:2981.
Tokgözoğlu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. Eur Heart J. 2022;43(34):3198–208.
Sud M, Han L, Koh M, Abdel-Qadir H, Austin PC, Farkouh ME, et al. Low-Density Lipoprotein Cholesterol and adverse Cardiovascular events after percutaneous coronary intervention. J Am Coll Cardiol. 2020;76(12):1440–50.
Munkhaugen J, Sverre E, Otterstad JE, Peersen K, Gjertsen E, Perk J, et al. Medical and psychosocial factors and unfavourable low-density lipoprotein cholesterol control in coronary patients. Eur J Prev Cardiol. 2017;24(9):981–9.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA et al. /ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e143.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European atherosclerosis society consensus statement. Eur Heart J. 2022;43(39):3925–46.
Castaner O, Pinto X, Subirana I, Amor AJ, Ros E, Hernaez A, et al. Remnant cholesterol, not LDL cholesterol, is Associated With Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;76(23):2712–24.
Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–84.
de Boer LM, Oorthuys AOJ, Wiegman A, Langendam MW, Kroon J, Spijker R, et al. Statin therapy and lipoprotein(a) levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(5):779–92.
Bjornson E, Adiels M, Taskinen MR, Burgess S, Chapman MJ, Packard CJ, et al. Lipoprotein(a) is markedly more atherogenic than LDL: an apolipoprotein B-Based genetic analysis. J Am Coll Cardiol. 2024;83(3):385–95.
Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, Hovingh GK, Kastelein JJ, Melamed S, et al. Triglyceride-Rich Lipoprotein Cholesterol and risk of Cardiovascular events among patients receiving statin therapy in the TNT Trial. Circulation. 2018;138(8):770–81.
Wadstrom BN, Wulff AB, Pedersen KM, Jensen GB, Nordestgaard BG. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study. Eur Heart J. 2022;43(34):3258–69.
Bjornson E, Adiels M, Taskinen MR, Burgess S, Rawshani A, Boren J, et al. Triglyceride-rich lipoprotein remnants, low-density lipoproteins, and risk of coronary heart disease: a UK Biobank study. Eur Heart J. 2023;44(39):4186–95.
Virani SS, Aspry K, Dixon DL, Ferdinand KC, Heidenreich PA, Jackson EJ, et al. The importance of low-density lipoprotein cholesterol measurement and control as performance measures: a joint clinical perspective from the National Lipid Association and the American Society for Preventive Cardiology. Am J Prev Cardiol. 2023;13:100472.
Poh KK, Ambegaonkar B, Baxter CA, Brudi P, Buddhari W, Chiang FT, et al. Low-density lipoprotein cholesterol target attainment in patients with stable or acute coronary heart disease in the Asia-Pacific region: results from the Dyslipidemia International Study II. Eur J Prev Cardiol. 2018;25(18):1950–63.
Aggarwal R, Chiu N, Libby P, Boden WE, Bhatt DL. Low-Density Lipoprotein Cholesterol Levels in adults with coronary artery disease in the US, January 2015 to March 2020. JAMA. 2023;330(1):80–2.
Alsadat N, Hyun K, Boroumand F, Juergens C, Kritharides L, Brieger DB. Achieving lipid targets within 12 months of an acute coronary syndrome: an observational analysis. Med J Aust. 2022;216(9):463–8.
Farmakis I, Zafeiropoulos S, Pagiantza A, Boulmpou A, Arvanitaki A, Tampaki A, et al. Low-density lipoprotein cholesterol target value attainment based on 2019 ESC/EAS guidelines and lipid-lowering therapy titration for patients with acute coronary syndrome. Eur J Prev Cardiol. 2020;27(19):2314–7.
Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid-lowering therapy intensification in a Population with Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2017;2(9):959–66.
Allahyari A, Jernberg T, Hagstrom E, Leosdottir M, Lundman P, Ueda P. Application of the 2019 ESC/EAS dyslipidaemia guidelines to nationwide data of patients with a recent myocardial infarction: a simulation study. Eur Heart J. 2020;41(40):3900–9.
Peterson BE, Bhatt DL, Ballantyne CM, de Lemos JA, Rosenson RS, Kosiborod MN, et al. Intensity of lipid-lowering therapy among patients with Polyvascular Disease. JAMA Netw Open. 2023;6(3):e234709.
Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485–94.
Bytyci I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43(34):3213–23.
Ray KK, Raal FJ, Kallend DG, Jaros MJ, Koenig W, Leiter LA, et al. ORION Phase III investigators. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44(2):129–38.
Marfella R, Prattichizzo F, Sardu C, Paolisso P, D’Onofrio N, Scisciola L, et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis. 2023;378:117180.
Gargiulo P, Basile C, Cesaro A, Marzano F, Buonocore D, Asile G, et al. Efficacy, safety, adherence and persistence of PCSK9 inhibitors in clinical practice: a single country, multicenter, observational study (AT-TARGET-IT). Atherosclerosis. 2023;366:32–9.
Gargiulo P, Basile C, Galasso G, Bellino M, D’Elia D, Patti G et al. AT-TARGET-IT Investigators. Strike early-strike strong lipid-lowering strategy with PCSK9i in ACS patients. Real-world evidence from AT-TARGET-IT registry. Eur J Prev Cardiol. 2024 May 24:zwae170. https://doi.org/10.1093/eurjpc/zwae170. Epub ahead of print. Erratum in: Eur J Prev Cardiol. 2024 Jun 27:zwae209.
Mitchell S, Roso S, Samuel M, Pladevall-Vila M. Unmet need in the hyperlipidaemia population with high risk of cardiovascular disease: a targeted literature review of observational studies. BMC Cardiovasc Disord. 2016;16:74.
Berteotti M, Profili F, Nreu B, Casolo G, Zuppiroli A, Mannucci E, et al. LDL-cholesterol target levels achievement in high-risk patients: an (un)expected gender bias. Nutr Metab Cardiovasc Dis. 2024;34(1):145–52.
Bacquer D, Smedt D, Reiner Z, Tokgozoglu L, Clays E, Kotseva K, et al. Percentage low-density lipoprotein-cholesterol response to a given statin dose is not fixed across the pre-treatment range: real world evidence from clinical practice: data from the ESC-EORP EUROASPIRE V study. Eur J Prev Cardiol. 2020;27(15):1630–6.
Colantonio LD, Shannon ED, Orroth KK, Zaha R, Jackson EA, Rosenson RS, et al. Ischemic event rates in very-high-risk adults. J Am Coll Cardiol. 2019;74(20):2496–507.
Robinson JG, Ballantyne CM, Hsueh W, Rosen J, Lin J, Shah A, et al. Achievement of specified low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol apolipoprotein B, and high-sensitivity C-reactive protein levels with ezetimibe/simvastatin or atorvastatin in metabolic syndrome patients with and without atherosclerotic vascular disease (from the VYMET study). J Clin Lipidol. 2011;5(6):474–82.
Szarek M, Bittner VA, Aylward P, Baccara-Dinet M, Bhatt DL, Diaz R, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41(44):4245–55.
Ray KK, Ginsberg HN, Davidson MH, Pordy R, Bessac L, Minini P, et al. Reductions in atherogenic lipids and Major Cardiovascular events: a pooled analysis of 10 ODYSSEY trials comparing Alirocumab with Control. Circulation. 2016;134(24):1931–43.
Lorenzatti AJ, Monsalvo ML, Lopez JAG, Wang H, Rosenson RS. Effects of evolocumab in individuals with type 2 diabetes with and without atherogenic dyslipidemia: an analysis from BANTING and BERSON. Cardiovasc Diabetol. 2021;20(1):94.
Ray KK, Stoekenbroek RM, Kallend D, Leiter LA, Landmesser U, Wright RS, et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: Prespecified secondary end points in ORION 1. Circulation. 2018;138(13):1304–16.
Acknowledgements
We appreciate very much the contributions made by the Chinese Cardiovascular Association in the construction of the iHeart Project. Thanks to all participating hospitals and colleagues.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2500500) and the Xuhui Healthcare System Peak Discipline Construction Funding Project (No. SHXHZDXK202305).
Author information
Authors and Affiliations
Contributions
JY had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The concept and study were designed by JY. JY, RZ, and ANW were responsible for the acquisition, analysis, or interpretation of data. JY and RZ drafted the manuscript. GHS and YW critically reviewed the manuscript for important intellectual content and supervision. BH, HL, JFW, YHX, XFY, SFG, CLD, HY, TBJ, HBC, SY, ZQZ, and YGD provided administrative, technical, or material support. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai Xuhui Central Hospital (Approval Number: 2023-014). Written informed consent was not required because all data were collected retrospectively and anonymously without unique patient identifiers.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, J., Zhang, R., Han, B. et al. Atherogenic lipid profile in patients with statin treatment after acute coronary syndrome: a real-world analysis from Chinese cardiovascular association database. Lipids Health Dis 23, 271 (2024). https://doi.org/10.1186/s12944-024-02244-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02244-4