Abstract
Background
RUNX1/AML1, which is a Runt family transcription factor critical for normal hematopoiesis, is frequently mutated or translocated in a broad spectrum of hematopoietic malignancies.
Findings
We describe here the case of a 54-year-old female developed acute myeloid leukemia with a t(5;21)(q21;q22). Transcriptome sequencing identified the chromodomain-helicase-DNA-binding protein 1 gene, CHD1, as a novel partner gene of RUNX1. Furthermore, the patient was found to harbor FLT3-ITD mutation, which might collaborated with CHD1-RUNX1 in the development of acute myeloid leukemia.
Conclusions
We have identified CHD1 as the RUNX1 fusion partner in acute myeloid leukemia with t(5;21)(q21;q22).
Similar content being viewed by others
Findings
The RUNX1 (previously AML1) gene encodes a DNA binding subunit of the core binding factor (CBF), which is critical for normal hematopoiesis. It is reported that RUNX1 frequently mutated or translocated with at least 61 different chromosomal loci in a broad spectrum of hematopoietic malignancies. To date, more than 20 distinct RUNX1 gene fusions have been reported in a variety of hematologic malignancies [1-4]. However, about half of RUNX1 translocations remain uncharacterized at the molecular level. Identification of those unknown fusion partners of RUNX1 will provide more clues about the molecular and pathogenic mechanisms of these translocations. Recently, whole transcriptome sequencing (also known as RNA-Sequencing, RNA-seq) has been shown as an efficient tool to identify uncharacterized fusion genes [5]. We describe here the identification of a novel fusion gene involving RUNX1 by case of a 54-year-old female developed acute myeloid leukemia with a t(5;21)(q21;q22) by transcriptome sequencing.
A 54-year-old female was admitted to our hospital in January 2011 because of fever and fatigue. Examination of peripheral blood indicated a platelet count of 103 × 109/L, hemoglobin level of 60 g/L, and a white blood cell count of 9.37 × 109/L with 22% circulating blasts. Bone marrow (BM) was hypercellular with 87.5%. Flow cytometry (FCM) immunophenotyping analysis showed positivity for CD34, CD14, CD13, CD33, CD117, CD15, CD11b and HLA-DR, as well as negativity for CD19, CD10, CD22, CD20, CD7, CD2, CD5 and CD3. The patient’s clinical picture was consistent with a diagnosis of AML-M4 according to the FAB classification, and AML not otherwise specified, acute myelomonocytic leukemia according to the World Health Organization (WHO) classification [6]. She was treated with induction chemotherapy of the IA regimen, including idarubicin and cytosine arabinoside. She achieved complete remission (CR) and received several courses of consolidation chemotherapy. However, her leukemia relapsed in April 2012. She was refractory to several courses of intensive combination chemotherapy and died in April 2013.
The BM cells of this patient at presentation showed an karyotype of 46,XX,t(5;21)(q21;q22) (Figure 1A). RT-PCR analysis failed to detect RUNX1-RUNX1T1 fusion transcripts from the bone marrow cells. For detection of RUNX1 rearrangements, dual color fluorescence in situ hybridization (FISH) experiments were performed with two contiguous BAC clones: RP11-177 L11, labeled with Spectrum Red-dUTP, and RP11-77I17, labeled with Spectrum Green-dUTP. FISH analysis showed one yellow signal corresponding to an intact RUNX1 gene, one separated red signal, and one separated green signal indicating a translocation involving RUNX1 gene (Figure 1B). Thus, 3’-rapid amplification of cDNA ends (3’RACE) and RT-PCR were performed to investigate the fusion partner of RUNX1 and failed to detect positive results.
Cytogenetic and fluorescence in situ hybridization analyses of the patient. The bone marrow cells were cultured for 24 h and were analyzed for karyotyping and fluorescence in situ hybridization (FISH) with standard procedures. Clonal karyotypic abnormalities were described according to the International System for Human Cytogenetic Nomenclature (ISCN, 2009). A) Karyotype was analyzed on R-banded metaphases showing a t(5;21)(q21;q22), suggesting the involvement of the RUNX1 gene located at 21q22. B) FISH analysis of the rearrangement of RUNX1 by using BAC probes RP11-177 L11 (red) and RP11-77I17 (green).The separated red and green signals indicated a translocation involving RUNX1 gene.
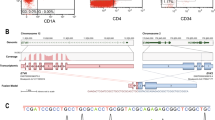
For the identification of the novel fusion gene in this patient, we performed whole-transcriptome sequencing of blast cells. Transcriptome sequence data were generated by high-throughput RNA sequencing performed on the Illumina HiSeq 2000. Candidate fusions were verified by RT-PCR amplification and bi-directional Sanger sequencing. Bioinformatic evaluation of the transcriptional sequencing data revealed 1 novel fusion, CHD1-RUNX1, in the patient. RT-PCR using primers for CHD1-RUNX1 fusion transcripts detected two bands (523 bp and 384 bp) corresponding to CHD1-RUNX1 fusion transcripts in this patient, while a normal individual had a negative result (Figure 2A). Sequence analysis indicated that the 523 bp PCR fragment was the product of a fusion event between exon 26 of CHD1 and exon 6 of RUNX1 (TypeI) (Figure 2B). In addition, we found that the 384 bp PCR fragment was the product of a fusion event between exon 25 of CHD1 and exon 6 of RUNX1 (TypeII) (Figure 2B). This suggested that the RUNX1 breakpoint was in intron 5 and had generated alternative fusion splice variants. The typeII fusion transcripts retained part of the “Runt homology domain” (RHD) as well as the whole Runx transcription activation and inhibition domain (TAD and TID). However, the typeI fusion led to a premature stop codon (TAG) forty-two amino acids downstream of the junction point. This out-of-frame fusion resulted in a truncated fusion protein presumably with nonfunctional RUNX1 domains (Figure 2C). For the detection of the reciprocal RUNX1-CHD1 transcript, a RUNX1 sense primer and a CHD1 antisense primer were used but no fusion transcript could be detected.
Characterization of the CHD1-RUNX1 fusion. A) RT-PCR confirmation for the CHD1-RUNX1 fusion. Lane M: 100 bp ladder; Lane 1: reagent control; Lane 2: negative control from a normal individual; Lane 3: CHD1-RUNX1 transcripts (523 bp and 384 bp) were detected in the patient discussed here. B) Sequencing analysis revealed two variant fusion transcripts between CHD1 and RUNX1.TypeI was a fusion between exon 26 of CHD1 and exon 6 of RUNX1, Type II was a fusion between exon 25 of CHD1 and exon 6 of RUNX1.The arrows indicated the fusion junction between CHD1 and RUNX1, the arabic numbers (1190, 1236, 169) indicated the amino acids position. C) Schematic structures of chimeric fusion proteins. The TypeII fusion protein retained the RUNX1 inhibition domain, however, the TypeI fusion created a frameshift and stop codon in the RUNX1 region, which resulted in a truncated protein without functional RUNX1 domain.
Internal tandem duplication (ITD) mutations of the FLT3 gene have been described in approximate 20-25% of AML [7]. In the present case, we identified an internal tandem mutation of the FLT3 gene (FLT3-ITD) by using Gene Scanning as previously described [8] (Additional file 1: Figure S1).
Most chimeric gene involving RUNX1 fuse the 5’ part of the RUNX1 gene with the 3’ part of the partner gene. These fusion proteins retain RHD domain of RUNX1 which is responsible for heterodimerization with the core-binding factor-β (CBF-β) and DNA binding, but loss the TAD and TID domains, such as RUNX1-RUNXT1, RUNX1-MECOM, and RUNX1-LPXN [9]. However, there are two chimeric genes, namely ETV6-RUNX1 and USP16-RUNX1, which fuse the 5’ region of partner gene with 3’ region of RUNX1. ETV6-RUNX1 retains RHD, TAD and TID domains of RUNX1. However, USP16-RUNX1 does not retain the RHD and no putative chimeric protein seems to be encoded due to loss of the open-reading frame [10,11]. Notably, our study identify a novel fusion gene CHD1-RUNX1, which is generated by 5’ region of CHD1 and 3’ region of RUNX1, retains the whole TAD and TID, and part of RHD. The incomplete RHD is likely to impair the DNA binding capacity of RUNX1 or its heterodimerization with CBF-β.
CHD1 locates in 5q15 and encodes a protein composed of 1710 amino acids. CHD1 is a chromatin-remodeling enzyme that belongs to the chromodomain family of proteins that play an important role in transcriptional regulation and developmental processes [12]. It has been reported that CHD1 is involved in assembly, shifting and removal of nucleosomes from the DNA double helix to keep them in an open and transcriptionally active state [13]. Two research groups have reported independently that CHD1 plays a tumor-suppressor role in prostate cancer [14,15]. However, the role of CHD1 in hematological malignancies remains unknown. By analyzing karyotypic results of over 6000 newly-diagnosed patients with acute leukemia admitted to our institute between January 1985 and February 2015, we detected t(5;21)(q21;q22) translocation in two AML patients. One was a 47-year-old male patient who was diagnosed with AML-M2 in April 1994. The other one (the present case, NO. 201100834) was a 54-year-old female diagnosed with AML-M4. We identified the CHD1-RUNX1 fusion transcript from the female case.
Animal models have revealed that RUNX1-related translocations or haploinsufficiency of RUNX1 are necessary but not sufficient for leukemogenesis [16,17], which suggests the requirement for additional genetic lesion for the development of leukemia. Internal tandem duplications (ITDs) in the juxtamembrane (JM) domain of FLT3 that lead to constitutive kinase activation in AML are associated with higher early relapse rate and inferior overall survival in patients with normal karyotype [18-20]. Furthermore, FLT3-ITD could cooperate strongly in leukemia induction with a variety of leukemia-initiating gene fusions such as AML1-ETO, MLL-AF9, or PML-RAR α [17,21,22]. We found the present patient harboring the FLT3-ITD mutation which might cooperate with CHD1-RUNX1 in the induction of AML.
Taken together, we have identified a novel CHD1-RUNX1 fusion consistent with the described t(5;21)(q21;q22) in a female patient with de novo AML (M4). Its role in the pathogenesis of AML still requires extensive investigation.
Reference
Ito Y. RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res. 2008;99:33–76.
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Abe A, Katsumi A, Kobayashi M, Okamoto A, Tokuda M, Kanie T, et al. A novel RUNX1-C11orf41 fusion gene in a case of acute myeloid leukemia with a t(11;21)(p14;q22). Cancer Genet. 2012;205:608–11.
Giguère A, Hébert J. Identification of a novel fusion gene involving RUNX1 and the antisense strand of SV2B in a BCR-ABL1-positive acute leukemia. Genes Chromosomes Cancer. 2013;52(12):1114–22.
Maher CA, Kumar-Sinha C, Cao X, Kalyana-Sundaram S, Han B, Jing X, et al. Transcriptome sequencing to detect gene fusions in cancer. Nature. 2009;458:97–101.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116:5089–102.
Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80.
De Braekeleer E, Douet-Guilbert N, Morel F, Le Bris MJ, Ferec C, De Braekeleer M. RUNX1 translocations and fusion genes in malignant hemopathies. Future Oncol. 2011;7:77–91.
Romana SP, Poirel H, Leconiat M, Flexor MA, Mauchauffe M, Jonveaux P, et al. High frequency of t(12;21) in childhood B-lineage acute lymphoblastic leukemia. Blood. 1995;86:4263–9.
Gelsi-Boyer V, Trouplin V, Adélaïde J, Aceto N, Remy V, Pinson S, et al. Genome profiling of chronic myelomonocytic leukemia: frequent alterations of RAS and RUNX1 genes. BMC Cancer. 2008;8:299.
Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS. Characterization of the CHD family of proteins. Proc Natl Acad Sci U S A. 1997;94:11472–7.
Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–8.
Liu W, Lindberg J, Sui G, Luo J, Egevad L, Li T, et al. Identification of novel CHD1-associated collaborative alterations of genomic structure and functional assessment of CHD1 in prostate cancer. Oncogene. 2012;31:3939–48.
Huang S, Gulzar ZG, Salari K, Lapointe J, Brooks JD, Pollack JR. Recurrent deletion of CHD1 in prostate cancer with relevance to cell invasiveness. Oncogene. 2012;31:4164–70.
Van der Weyden L, Giotopoulos G, Rust AG, Matheson LS, van Delft FW, Kong J, et al. Modeling the evolution of ETV6-RUNX1-induced B-cell precursor acute lymphoblastic leukemia in mice. Blood. 2011;118:1041–51.
Schessl C, Rawat VP, Cusan M, Deshpande A, Kohl TM, Rosten PM, et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest. 2005;115:2159–68.
Nazha A, Cortes J, Faderl S, Pierce S, Daver N, Kadia T, et al. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica. 2012;97:1242–5.
Walker A, Marcucci G. Impact of molecular prognostic factors in cytogenetically normal acute myeloid leukemia at diagnosis and relapse. Haematologica. 2011;96:640–3.
Whitman SP, Maharry K, Radmacher MD, Becker H, Mrózek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloidleukemia: a Cancer and Leukemia Group Bstudy. Blood. 2010;116:3622–6.
Stubbs MC, Kim YM, Krivtsov AV, Wright RD, Feng Z, Agarwal J, et al. MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment. Leukemia. 2008;22:66–77.
Kelly LM, Kutok JL, Williams IR, Boulton CL, Amaral SM, Curley DP, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. 2002;99:8283–8.
Acknowledgements
This work was supported by grants from National Key Scientific Projects of China (2011CB933501), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Natural Science Foundation of China (81070416), Jiangsu Provincial Special Program of Medical Science (BL2012005), Jiangsu Province’s Key Medical Center (ZX201102), and Jiangsu Province Natural Science Fund for Distinguished Young Scholars (BK2012006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SC, AS, HY and YZ designed and performed the research, and drafted the manuscript. JP, CW and JX performed the bone marrow karotype and FISH studies. HS, QW, QW, LW and LM participated in the analysis of gene mutations. LW and NP assessed the clinical data of the patient. All authors read and approved the final manuscript.
Additional file
Additional file 1: Figure S1.
Genescan analysis of FLT3-ITDs using automated capillary gel electrophoresis. A) WT-FLT3 (328 bp) was shown in a normal individual. B) WT-FLT3 and FLT3-ITDs (358 bp) was shown in the present case.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yao, H., Pan, J., Wu, C. et al. Transcriptome sequencing reveals CHD1 as a novel fusion partner of RUNX1 in acute myeloid leukemia with t(5;21)(q21;q22). Mol Cancer 14, 81 (2015). https://doi.org/10.1186/s12943-015-0353-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-015-0353-x