Abstract
Background
Pleiotropic effects have been implicated in clinical benefits of ticagrelor compared to thienopyridine P2Y12 antagonists. There are conflicting data regarding effects of ticagrelor vs. thienopyridine P2Y12 blockers on endothelial function. Our aim was to compare endothelial biomarkers and their relations with platelet reactivity in real-world patients after acute coronary syndrome (ACS) on maintenance dual antiplatelet therapy (DAPT) with ticagrelor or clopidogrel stratified by diabetes status.
Methods
Biochemical indices of endothelial dysfunction/activation and platelet reactivity by multiple electrode aggregometry were compared in 126 stable post-ACS subjects (mean age: 65 ± 10 years, 92 men and 34 women), including patients with (n = 61) or without (n = 65) coexistent type 2 diabetes (T2DM) on uneventful maintenance DAPT with either ticagrelor (90 mg b.d.) or clopidogrel (75 mg o.d.) in addition to low-dose aspirin. Exclusion criteria included a complicated in-hospital course, symptomatic heart failure, left ventricular ejection fraction < 40% and relevant coexistent diseases except for well-controlled diabetes, mild renal insufficiency or hypertension.
Results
Clinical characteristics were similar in patients on ticagrelor (n = 62) and clopidogrel (n = 64). The adenosine diphosphate-induced platelet aggregation and circulating soluble P-selectin (sP-selectin) were decreased in ticagrelor users irrespective of T2DM status (p < 0.001 and p < 0.01 for platelet reactivity and sP-selectin, respectively). Plasma levels of soluble vascular cell adhesion molecule-1 (sVCAM-1) were lower in T2DM subjects on ticagrelor vs. clopidogrel (758 ± 162 vs. 913 ± 217 µg/L, p < 0.01). In contrast, plasma sVCAM-1 was similar in non-diabetic patients on ticagrelor and clopidogrel (872 ± 203 vs. 821 ± 210 µg/L, p > 0.7). The concentrations of sE-selectin, monocyte chemoattractant protein-1 and asymmetric dimethylarginine did not differ according to the type of P2Y12 antagonist regardless of T2DM status. Platelet reactivity was unrelated to any endothelial biomarker in subjects with or without T2DM.
Conclusions
Our preliminary findings may suggest an association of ticagrelor-based maintenance DAPT with favorable endothelial effects compared to clopidogrel users in stable post-ACS patients with T2DM. If proven, this could contribute to more pronounced clinical benefits of ticagrelor in diabetic subjects.
Similar content being viewed by others
Background
Dual antiplatelet therapy (DAPT) is a standard of care in patients after acute coronary syndromes (ACS) and/or after coronary stent implantation [1, 2]. In addition to low-dose aspirin, potent P2Y12 antagonists, ticagrelor and prasugrel, are preferred over clopidogrel in post-ACS patients, whereas clopidogrel is recommended only if ticagrelor and prasugrel are unavailable, not tolerated or contraindicated, e.g. in patients with high bleeding risk [1, 2].
Beneficial clinical effects of ticagrelor compared to clopidogrel were first demonstrated in the PLATO trial, where ticagrelor was associated with a lower risk of myocardial infarction, cardiovascular (CV) and all-cause death as well as stent thrombosis over a median follow-up of 9 months regardless of ACS type or treatment strategy [3]. Additionally, in the PEGASUS-TIMI 54 trial benefits of prolonged DAPT including low-dose ticagrelor in high-risk patients with prior myocardial infarction 1–3 years earlier were shown [4]. Although relative clinical benefits of ticagrelor in these trials were consistent irrespective of diabetes status [3, 4], absolute risk reduction was greater in patients with coexistent diabetes [5, 6], known to associate with a higher risk of ischemic events via a corollary of mechanisms, including prothrombotic pathways [7, 8]. Moreover, in a GReek AntiPlatElet registry substudy, DAPT with newer P2Y12 antagonists, but not clopidogrel, attenuated the negative impact of diabetes on ischemic CV events after ACS [9]. Additionally, net clinical benefits of ticagrelor plus aspirin compared to aspirin alone were reported in participants of the THEMIS-PCI trial with type 2 diabetes (T2DM) and stable coronary artery disease (CAD) with a history of previous percutaneous coronary intervention (PCI) but without prior myocardial infarction or stroke, regardless of diabetes duration or glycated hemoglobin levels [10].
Therefore, keeping in mind a high THEMIS-like population European real-world prevalence [11], the investigation of mechanisms of the beneficial effects of ticagrelor is of relevance. These mechanisms include not only faster, more intense and constant platelet inhibition but also multiple pleiotropic effects, such as anti-inflammatory action, improved coronary flow reserve, downregulation of tissue factor expression, reduced myocardial remodeling and possible improvement of endothelial function, in part linked to enhanced accumulation of endogenous adenosine and vascular P2Y12 receptors blockade [12,13,14,15,16,17,18,19,20,21].
Nevertheless, both randomized and observational clinical studies focused on comparisons of endothelial effects of ticagrelor and thienopyridine P2Y12 antagonists brought conflicting results [14, 15, 22,23,24,25,26]. Importantly, although a recent meta-analysis of randomized controlled studies [27] suggested the ability of ticagrelor to improve brachial flow-mediated dilation (FMD) or reactive hyperemia index (by peripheral arterial tonometry) compared to clopidogrel or prasugrel, the putative effect was mostly driven by reports which had been published only as abstracts.
Importantly, some evidence indirectly suggests that effects of ticagrelor on endothelial function [22, 23, 25, 26] and clinical outcome [6, 28, 29] can be modulated by diabetes status.
In addition, interactions between endothelial function and platelet reactivity appear more complex as P2Y12 receptor blockade synergistically potentiates platelet-inhibitory effects of key endothelium-derived mediators, nitric oxide (NO) and prostacyclin (PGI2) [30,31,32].
Our aim was to compare circulating levels of biochemical indices of endothelial dysfunction or activation and their relations with platelet reactivity in real-world post-ACS patients on maintenance DAPT with ticagrelor or clopidogrel stratified by diabetes status.
Methods
Patients
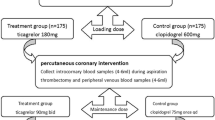
We studied hospitalized stable patients aged 30–75 years receiving uneventful maintenance DAPT with either ticagrelor (90 mg b.d.) or clopidogrel (75 mg o.d.) on the background of low-dose aspirin for 1–3 months after PCI for an ACS. The choice of ticagrelor or clopidogrel, initiated previously in the acute phase of ACS, was at the discretion of attending physician.
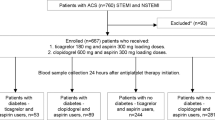
All the subjects were on angiotensin-converting enzyme inhibitors (ACEI) (or angiotensin receptor blockers [ARB]), high-dose statins, and a proton pump inhibitor (PPI). Exclusion criteria included a complicated in-hospital course, symptomatic heart failure, left ventricular ejection fraction ≤ 40%, congenital heart disease, more than mild valvular heart disease, and estimated glomerular filtration rate (eGFR) < 45 ml/min per 1.73 m2 of body surface area according to the Chronic Kidney Disease Epidemiology Collaboration formula. We had also excluded subjects with any relevant coexistent diseases except for well-controlled hypertension or T2DM (diagnosed at least 1 year prior to index hospitalization and treated with hypoglycemic agents), e.g. thyroid dysfunction, neoplastic or inflammatory disease patients, bleeding disorders, patients requiring oral anticoagulation for any indication, as well as those after major surgery or stroke within previous 6 months or with significant abnormalities in routine blood and urine analysis. Additionally, T2DM patients treated with sodium-glucose co-transporter-2 inhibitors or glucagon-like peptide-1 receptor agonists were excluded.
First, we pre-screened 291 consecutive post-ACS patients with regard to exclusion and inclusion criteria, so that similar numbers of diabetic and non-diabetic subjects were selected. Then, we aimed to ensure age- and sex-matching of patients treated with ticagrelor and clopidogrel, i.e. a patient on ticagrelor was matched to his/her counterpart on clopidogrel. Finally, 2 subjects (1 diabetic and 1 non-diabetic woman on ticagrelor) were excluded due to a history of post-discharge switch of P2Y12 antagonists, and 126 patients entered the final study.
Procedure
Blood sampling for the estimation of platelet reactivity and biochemical analyses was performed at routine blood sampling after overnight fast, before the administration of morning doses of drugs, including a maintenance dose of ticagrelor or clopidogrel. Blood for future biochemical assays was drawn from an antecubital vein into ethylenediaminetetraacetic acid-coated tubes, centrifuged, and then plasma was separated and stored in − 80 °C until assayed.
In accordance with the Declaration of Helsinki, the study protocol was approved by the Bioethical Committee of our university (Approval numbers: KBET/277/B/2013 and 1072.6120.143.2019 issued on November 28th, 2013 and May 30th, 2019, respectively) and informed consent was obtained from the study subjects.
Platelet reactivity
Platelet aggregatory responses to exogenous adenosine diphosphate (ADP) were assessed in whole blood by multiple electrode aggregometry (MEA) (Multiplate analyzer, Dynabyte, Münich, Germany), based on increased electrical impedance owing to platelet adhesion to the sensor wire surface, as previously described [33, 34]. In brief, a portion of thrombin inhibitor-anticoagulated blood was diluted (1:2) with 0.9% sodium chloride and stirred at 37 °C for 3 min. Then, ADP was added to achieve a final concentration of 6.4 µmol/L and the magnitude of the ADP-induced aggregation was reflected by a rise of impedance in the function of time over a 5-min continuous recording, represented by the area under the curve expressed in arbitrary units (AU) * min [33, 34].
Biochemical assays
Biochemical analyses included enzyme-linked immunosorbent assays (ELISA) for soluble forms of vascular cell adhesion molecule-1 (sVCAM-1), P-selectin (sP-selectin) and E-selectin (sE-selectin), as well as monocyte chemoattractant protein-1 (MCP-1) (R&D Systems, Minneapolis, MN, USA). According to the manufacturer’s instructions, the lower detection limit was 0.6 µg/L (sVCAM-1), 0.05 µg/L (P-selectin), 0.01 µg/L (sE-selectin) and 1.7 ng/L (MCP-1). For the range of concentrations measured in the study subjects, respective coefficients of variation corresponding to intra-assay (or inter-assay) precision were 2.3% (7.8%), 8.9% (11.9%), 5.1 (7.6%) and 4.9% (4.8%). Additionally, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) were measured in a subgroup of the study subjects by ELISA (Immundiagnostik AG, Bernsheim, Germany). As previously described [35], the detection limit was 0.04 μmol/L (ADMA) and 0.05 μmol/L (SDMA), while intra-assay (or inter-assay) coefficients of variation averaged 5.8 (7.6%) (ADMA) and 4.8 (7.0%) (SDMA),
Statistical analysis
Data are shown as means (± SD [standard deviation]), medians [interquartile range] or numbers (percentages). The concordance with a gaussian distribution was assessed by Kolmogorov–Smirnov test and data were natural logarithmically transformed prior to further analyses in case of a non-normal distribution.
Characteristics of patients on ticagrelor and clopidogrel were compared by Student’s t-test or Welch test for continuous variables, and Fisher test for dichotomous characteristics. Homogeneity of variance was checked by Levene’s test.
To estimate the effect of diabetes status on the differences in platelet reactivity, sP-selectin and endothelial biomarkers between ticagrelor and clopidogrel users, a 2-way analysis of variance (ANOVA) was performed followed by Tukey’s test. The analysis of covariance (ANCOVA) was used to control for potential confounding effects of age, body-mass index, glycated hemoglobin and low-density lipoproteins cholesterol. Relations between variables were estimated by Pearson’s (r) correlation coefficients.
A p-value below 0.05 was inferred significant. The Bonferroni correction for multiple comparisons was applied to adjust for possible mutual interrelations of endothelial biomarkers. Our study design had a power of 80% to detect an intergroup difference of about 0.73 SD at an alpha level of 0.05, which corresponds to about 145 µg/L, 125 ng/L and 0.07 µmol/L for the normally distributed endothelial biomarkers VCAM-1, MCP-1 and ADMA, respectively. All analyses were performed by the Statistica 64 (data analysis software system, version 13.3.704.0; TIBCO Software Inc. [2017], Palo Alto, CA, USA).
Results
Clinical characteristics were similar in our study subjects on ticagrelor and clopidogrel (Table 1).
The ADP-induced platelet aggregation was decreased in ticagrelor users compared to their counterparts treated with clopidogrel in patients with (136 ± 69 vs. 254 ± 111 AU * min, p < 0.001) and without T2DM (159 ± 83 vs. 260 ± 128 AU * min, p < 0.001) (Table 2, Fig. 1). Also, plasma sP-selectin levels were decreased on ticagrelor vs. clopidogrel regardless of T2DM status (Table 2).
A 2-way ANOVA revealed a significant interaction between ticagrelor use and diabetes status for plasma sVCAM-1 levels. In patients with T2DM the concentrations of sVCAM-1 were lower in subjects on ticagrelor compared to clopidogrel users (758 ± 162 vs. 913 ± 217 µg/L, p < 0.01) (Table 2; Fig. 2). In contrast, plasma sVCAM-1 was similar in non-diabetic subjects sVCAM-1 n ticagrelor and clopidogrel (872 ± 203 vs. 821 ± 210 µg/L, respectively, p > 0.7) (Table 2; Fig. 2).
The results did not substantially change upon adjustment for potential effects of age, body mass index, glycated hemoglobin and low-density lipoproteins cholesterol by ANCOVA or after exclusion of T2DM subjects taking insulin.
There were no significant differences in the concentrations of sE-selectin, MCP-1, ADMA or SDMA by the type of P2Y12 antagonist irrespective of diabetes status. (Table 2). Platelet reactivity was unrelated to any endothelial biomarker in patients with or without T2DM (p > 0.3).
Discussion
We observed lower circulating sVCAM-1 in post-ACS subjects with T2DM treated with ticagrelor compared to diabetic clopidogrel users, while plasma sVCAM-1 was similar in their non-diabetic counterparts on ticagrelor and clopidogrel. Platelet reactivity and plasma sP-selectin were decreased on ticagrelor vs. clopidogrel regardless of T2DM status. The concentrations of sE-selectin, MCP-1 and ADMA did not differ according to the type of P2Y12 antagonist used irrespective of T2DM status. Additionally, platelet aggregability was unrelated to any endothelial biomarker.
To the best of our knowledge, the present study, albeit observational, is the first report focused on comparing endothelial effects of DAPT with ticagrelor vs. clopidogrel in patients with and without T2DM.
Mechanistic considerations and comparisons with previous studies
Assuming that sVCAM-1, a biochemical index of endothelial activation can be perceived as a surrogate measure of endothelial function, our findings might result from the antiplatelet effect, stronger in patients on ticagrelor vs. clopidogrel. Accordingly, an improvement of endothelial function was previously demonstrated for various P2Y12 antagonists, presumably by interactions between platelets, leukocytes and endothelial cells [15, 21, 36, 37]. Additionally, platelet hyperactivity and resistance to DAPT with clopidogrel in T2DM [7, 38], partially dependent on a higher platelet turnover rate, associated with an increased proportion of immature platelets [39], could also favor ticagrelor (administered twice daily) over clopidogrel (dosed once daily). In particular, a gradually emerging subpopulation of juvenile platelets is constantly exposed to an active compound in ticagrelor users, in contrast to clopidogrel whose active metabolite is eliminated from the circulation several hours after prodrug intake, being absent in the systemic blood before the administration of the next dose [21]. Nevertheless, in our hands, a similar degree of platelet inhibition on ticagrelor or clopidogrel was observed in patients with and without T2DM, and on-treatment platelet reactivity was unrelated to any endothelial biomarker, including sVCAM-1 depressed only in diabetic subjects on ticagrelor.
Admittedly, the association of lower sVCAM-1 and ticagrelor use does not imply a cause-and-effect relationship. However, that sVCAM-1 levels were decreased only in T2DM patients on ticagrelor, might suggest a diabetes-specific interaction between endothelial function and ticagrelor use, There is some evidence from randomized crossover studies of CAD patients on DAPT that appears to support, albeit indirectly, this hypothesis [22, 23, 25]. In the CLOTILDIA study, Mangiacapra et al. [22] observed lower platelet reactivity and improved brachial FMD in T2DM patients with stable CAD randomized to ticagrelor vs. high-dose clopidogrel for 2 weeks. In patients with non-ST-elevation ACS and T2DM, Jeong et al. [23] described an improvement of brachial FMD, increased counts of circulating endothelial progenitor cells and decreases in interleukin-6 and tumor necrosis factor-α levels in patients with T2DM randomized to ticagrelor vs. prasugrel for 5 weeks, despite similar platelet reactivity on ticagrelor and prasugrel. In contrast, in stable post-ACS participants of the HI-TECH study (including only 20% of diabetic subjects), Ariotti et al. [25] observed no differences in either peripheral endothelial function or levels of vascular biomarkers (including ADMA, cell adhesion molecules and von Willebrand factor) between subjects on maintenance dose of ticagrelor, prasugrel and clopidogrel for 4 weeks, despite lower platelet reactivity on ticagrelor vs. clopidogrel or prasugrel. Additionally, in a randomized, blinded, parallel study of non-ST-elevation ACS subjects (including 24% of diabetic patients), Schnorbus et al. [26] reported more pronounced platelet inhibition, higher FMD and lower interleukin-6 on DAPT with prasugrel vs. ticagrelor and clopidogrel patients.
Therefore, the improved ability of ticagrelor vs. thienopyridine P2Y12 blockers to improve peripheral endothelial function in CAD patients on DAPT may be limited to subjects with coexistent diabetes (mostly T2DM) regardless of whether platelet inhibition on ticagrelor was stronger [22, 25] or not [23, 26] compared to thienopyridines.
As clinical characteristics were similar in our patients on ticagrelor and clopidogrel, intergroup differences are unlikely to account for decreased sVCAM-1 concentrations in our ticagrelor-treated subjects. In contrast to P-/E-selectin-dependent intercellular interactions, responsible for so-called leukocyte rolling along the endothelium, VCAM-1 (binding to very late antigen-4, an a4β1-integrin expressed on monocytes and lymphocytes) mediates more firm adhesion of leukocytes to endothelial cells and is linked to endothelial activation [40]. Importantly, higher sVCAM-1 (but not soluble forms of E-selectin or intercellular adhesion molecule-1) independently predicted fatal CV events over about 2.7 years in 1246 participants of the AtheroGene study with documented angiographic CAD [41]. Accordingly, it can be hypothesized that lower sVCAM-1 might reflect a probable ability of ticagrelor to modulate platelets-monocytes-endothelial interactions [15, 21] in our diabetic subjects with consequent correction of endothelial dysfunction, an antecedent of adverse CV events [42, 43], which could also translate into better long-term prognosis in T2DM subjects on ticagrelor.
Possible mechanisms underlying the association of ticagrelor use with lower sVCAM-1 in our diabetic stable post-ACS subjects remain speculative. Notably, in T2DM ACS patients Jeong et al. [23] reported higher plasma adenosine after ticagrelor loading dose and reduced sVCAM-1 levels after ticagrelor maintenance dose for 5 weeks compared to pre-treatment values. In contrast, Ariotti et al. [25] reported no effects of ticagrelor use on plasma adenosine or any endothelial biomarker (including sVCAM-1) after 4 weeks of drug maintenance dose in predominantly non-diabetic (80%) stable post-ACS subjects. As adenosine induced endothelial NO release [44, 45] and attenuated endothelial activation and VCAM-1 expression in various experimental models [46,47,48,49], the ticagrelor-induced enhancement of extracellular adenosine accumulation [16, 17] could account for lower sVCAM-1 levels in our diabetic ticagrelor users.
Second, previously reported differences in blood counts of platelet-derived microvesicles (PMVs) between subjects on DAPT with ticagrelor and clopidogrel might also be linked to intergroup differences in sVCAM-1 levels. Lower counts of PMVs, originating from activated platelets, were described in post-ACS patients on ticagrelor vs. clopidogrel by Gąsecka et al. [50] in a randomized AFFECT EV trial and by our group in an earlier observational study [51]. Additionally, in the former report [50], ticagrelor but not clopidogrel prevented an about twofold increase in the counts of circulating PMVs 6-month after ACS and PMVs counts correlated positively with C-reactive protein levels only in ticagrelor users. Notably, PMVs counts did not correlate with platelet reactivity in either of these studies [50, 51].
PMVs exhibit not only a pro-coagulant [52] but also pro-inflammatory and pro-atherosclerotic activity distantly from the site of platelet activation [53,54,55,56], expressing a variety of surface molecules, [53, 57, 58]. Notably, in post-ACS patients, Christersson et al. [59] described not only gradually increasing PMVs counts, but also the association of higher PMVs numbers (but not microvesicles of endothelial or monocytic origin) early after ACS with diabetes and adverse outcome over a 2-year follow-up.
Therefore, lower concentrations of sVCAM-1 in our diabetic patients receiving ticagrelor might reflect the proposed ability of ticagrelor to attenuate excessive PMVs generation, in part independently of the degree of platelet inhibition [50, 51, 60], with consequent lower endothelial activation.
Plasma sP-selectin was significantly lower in both diabetic and non-diabetic patients on ticagrelor vs. clopidogrel. Although P-selectin it is localized not only in platelet alpha-granules but also endothelial Weibel-Palade bodies, circulating sP-selectin mainly reflects platelet activation [61]. Thus, our finding could be ascribed to depressed platelet reactivity in ticagrelor users regardless of T2DM status.
With regard to ADMA, its similar concentrations in our diabetic ticagrelor and clopidogrel users, as opposed to lower sVCAM-1 on ticagrelor, may reflect distinct relationship between ADMA and prognosis in patients with and without T2DM. Accordingly, higher sVCAM-1 predicted adverse clinical outcome in both diabetics and non-diabetics in the population-based Hoorn study [62], while increased ADMA was associated with excessive CV risk in non-diabetic but not diabetic subjects in that [63] and other [64,65,66] studies.
Nonetheless, regardless of the mechanism involved, possible improvement of endothelial function in diabetic post-ACS patients on ticagrelor might translate into better prognosis.
Clinical implications: endothelial effects as a possible contributor to clinical benefits of DAPT with ticagrelor in CAD patients with diabetes and other comorbidities
T2DM is associated with worse 1-year outcome in revascularized ACS patients, mainly to excessive risk of ischemic events despite DAPT [7], similar to such comorbidities as chronic kidney disease (CKD) [67], chronic obstructive pulmonary disease (COPD) [68] or peripheral arterial disease [69]. In 2019, Knowles and Warner [70] hypothesized that a synergistic ability of P2Y12 blockade and endothelial mediators, PGI2 and NO (and their intraplatelet second messengers, cyclic adenosine 3’,5’-monophosphate (cAMP) and cyclic guanosine 3’,5’-monophosphate (cGMP), respectively) to inhibit platelet aggregation [30, 32, 71] might be of particular clinical relevance in post-ACS subjects with the above mentioned comorbidities, sharing widespread endothelial dysfunction and chronic inflammatory activation. Importantly, endothelial dysfunction extends also to peripheral and pulmonary microvessels where platelets undergo continuous and prolonged exposure to endothelial mediators due to a close proximity to numerous endothelial cells and slow blood flow velocity compared to large arteries [70].
Therefore, depressed generation of NO and PGI2 might also limit the efficacy of even potent P2Y12 antagonists despite DAPT in post-ACS patients with coexistent comorbidities, including T2DM, keeping in mind a three-way synergistic interaction between P2Y12 blockade and endothelial mediators, NO and PGI2, for adequate platelet inhibition [30,31,32, 71]. Moreover, a possible ability of ticagrelor to both inhibit P2Y12 receptors and improve endothelial function in T2DM might at least partially restore the synergistic antiplatelet effect with a subsequent reduction of ischemic CV events, more pronounced in CAD with comorbidities, including T2DM.
In agreement with the proposed hypothesis, in the ISAR-REACT 5 trial, a better ability of DAPT with prasugrel than ticagrelor [72] to reduce the risk of ischemic CV events or definite stent thrombosis was limited to non-diabetic ACS patients undergoing PCI [28], whereas in diabetic subjects an insignificant opposite tendency in favor of ticagrelor was observed [28].
Additionally, the hypothetical diabetes-specific endothelial effects of ticagrelor might have also contributed to stronger reductions of the risk of CV death and coronary death in diabetic vs. non-diabetic participants of the PEGASUS-TIMI 54 trial with a history of myocardial infarction 1–3 years earlier receiving ticagrelor (60 or 90 mg b.d.) with aspirin vs. aspirin alone [6], as well as to a lower risk of ischemic CV events with ticagrelor (60 mg b.d., most prevalent dose) as an addition to aspirin in T2DM subjects with stable CAD after prior PCI without previous myocardial infarction or stroke in the THEMIS-PCI trial [10, 73].
Also, in the NATHAN-NEVER study, a randomized, parallel trial including stable CAD patients with coexistent COPD, beneficial effects for 1-month DAPT with ticagrelor vs. clopidogrel with regard to a set of parameters related to endothelial function were reported, including lower circulating epidermal growth factor levels, reduced apoptosis rate and enhanced NO generation in cultured endothelial cells incubated with patients’ serum, as well as lower reactive oxygen species generation and increased SIRT1 and HES1 mRNA levels in peripheral leukocytes [24, 74, 75].
Future perspectives: can endothelial effects contribute to clinical benefits of ticagrelor monotherapy in CAD patients with diabetes ?
Notably, Tam et al. [76] have recently described improved FMD in patients with non-significant CAD (including only 25% of diabetics) randomized to low-dose ticagrelor (60 mg b.d.) for 12 weeks compared to low-dose aspirin despite comparable platelet activation markers. Hence, endothelial effects of ticagrelor can also extend to ticagrelor monotherapy. Ticagrelor monotherapy, following shortened DAPT, was recently proposed as an emerging strategy in patients after coronary stent implantation both after ACS and in chronic coronary syndromes [29, 77, 78].
In the GLOBAL LEADERS trial in the second year after angioplasty, ticagrelor monotherapy (90 mg b.d.) was more effective than aspirin monotherapy in lowering the risk of ischemic CV events in those with coexistent both diabetes and CKD [78, 79]. Thus, since an improvement of FMD on ticagrelor vs. aspirin was reported by Tam et al. [76] at a ticagrelor dose of 60 mg b.d., endothelial effects could possibly contribute to a superiority of ticagrelor monotherapy at a dose of 90 mg o.d. over aspirin alone for ischemic risk in some subgroups of CAD patients with comorbidities who had angioplasty 12–24 months earlier [79].
In the TWILIGHT trial, there was a marginal (p = 0.05) diabetes by treatment interaction, driven mostly by a lower risk of myocardial infarction at 15 months after high-risk PCI in diabetic patients on ticagrelor monotherapy (90 mg b.d.) vs. ticagrelor plus aspirin after 3-month DAPT with ticagrelor [29, 77]. This unexpected and counterintuitive finding is consistent with previous observations of only limited additional platelet inhibition by aspirin in the presence of strong P2Y12 blockade by ticagrelor or prasugrel active metabolite [70, 80, 81]. Moreover, a considerable aspirin-induced inhibition of non-platelet cyclooxygenase with consequent reduction of PGI2 generation was reported already at low aspirin doses [82]. Therefore, even low-dose aspirin might impair the synergism between intraplatelet cAMP and cGMP [71], second messengers of PGI2 and NO, respectively, responsible for continuous platelet inhibition [70].
It is intriguing why the above described aspirin-induced detrimental mechanistic effect [70, 82] could adversely affect prognosis exclusively in ticagrelor users with coexistent T2DM in the TWILIGHT trial [29, 77]. Nonetheless, it could be speculated that this finding might reflect a more pronounced dependence of clinical benefits of ticagrelor on an intact three-way synergism between P2Y12 blockade and endothelial mediators, PGI2 and NO [30, 32, 70, 71] in T2DM, owing to platelet hyperactivity in diabetic patients [7, 38, 39]. Additionally, this concept appears consistent with the ticagrelor-dependent enhanced accumulation of adenosine [16, 17] that increases endothelial NO release [44,45] and intraplatelet formation of cAMP [83], a PGI2 second messenger.
Limitations of the study
First, an observational, not randomized, study design and a low number of patients involved pose considerable limitations. Nevertheless, we applied a wide set of exclusion criteria, recruiting a relatively homogenous group of stable post-ACS subjects receiving uneventful maintenance DAPT without heart failure or coexistent diseases except for well-controlled diabetes, hypertension or mild eGFR reduction. Moreover, clinical characteristics were similar in balanced subgroups of patients on ticagrelor and clopidogrel and potential effects of selected confounders were excluded by ANCOVA. Additionally, ACEI (or ARB), a high-dose statin and PPI were used by all the study patients, while the proportions of subjects on beta-blockers, calcium channel blockers or diuretics were similar by ticagrelor use or diabetes status. Although we had previously reported lower sVCAM-1 levels in stable CAD patients with T2DM on metformin [35], the prevalence of metformin use was comparable in our T2DM subjects receiving ticagrelor and clopidogrel. Second, we have analyzed only biomarkers linked to endothelial dysfunction or activation, with no assessment of functional indices such as FMD or reactive hyperemia index by arterial tonometry. However, our in-house estimation has detected a relatively large individual variability of FMD and the need for long procedure in case of peripheral tonometry, which would limit its feasibility in clinical conditions. Third, we assessed platelet reactivity as ADP-induced platelet aggregation by MEA instead of a specific vasodilator-stimulated phosphoprotein phosphorylation (VASP-P) assay focused on the P2Y12-dependent effect. Nevertheless, platelet reactivity by MEA was a better predictor of stent thrombosis than the VASP-P assay in the PEGASUS-PCI study [84].
Conclusions
Our preliminary findings may suggest an association of ticagrelor-based maintenance DAPT with favorable endothelial effects compared to clopidogrel users in stable post-ACS patients. If proven, this could contribute to more pronounced clinical benefits of ticagrelor in diabetic subjects.
Availability of data and materials
The dataset supporting the principal conclusions of the current study is available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
Angiotensin-converting enzyme inhibitors
- ACS:
-
Acute coronary syndrome
- ADMA:
-
Asymmetric dimethylarginine
- ADP:
-
Adenosine diphosphate
- ANCOVA:
-
Analysis of covariance
- ANOVA:
-
Analysis of variance
- ARB:
-
Angiotensin receptor blockers
- AU:
-
Arbitrary units
- CAD:
-
Coronary artery disease
- cAMP:
-
Cyclic adenosine 3’,5’-monophosphate
- cGMP:
-
Cyclic guanosine 3’,5’-monophosphate
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CV:
-
Cardiovascular
- DAPT:
-
Dual antiplatelet therapy
- EF:
-
Ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- FMD:
-
Flow-mediate dilation
- LDL:
-
Low-density lipoproteins
- MCP-1:
-
Monocyte chemoattractant protein-1
- MEA:
-
Multiple electrode aggregometry
- NO:
-
Nitric oxide
- PPI:
-
Proton pump inhibitor
- PCI:
-
Percutaneous coronary intervention
- PGI2 :
-
Prostacyclin
- PMVs:
-
Platelet-derived microvesicles
- SD:
-
Standard deviation
- SDMA:
-
Symmetric dimethylarginine
- sE-selectin:
-
Soluble E-selectin
- sP-selectin:
-
Soluble P-selectin
- sVCAM-1:
-
Soluble vascular cell adhesion molecule-1
- T2DM:
-
Type 2 diabetes mellitus
- VASP-P:
-
Vasodilator-stimulated phosphoprotein phosphorylation
References
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM, ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–367.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, for the PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Bonaca MP, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;373(13):1274–5.
James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, Maya J, Nicolau JC, Spinar J, Storey RF, Stevens SR, Wallentin L, for the PLATO Study Group. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006–16.
Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF, Im K, Murphy SA, Held P, Braunwald E, Sabatine MS, Steg PG. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol. 2016;67(23):2732–40.
Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123(7):798–813.
Camm AJ, Sabbour H, Schnell O, Summaria F, Verma A. Managing thrombotic risk in patients with diabetes. Cardiovasc Diabetol. 2022;21(1):160.
Hamilos M, Petousis S, Xanthopoulou I, Goudevenos J, Kanakakis J, Sitafidis G, Vavouranakis M, Skalidis E, Kochiadakis G, Lekakis J, Vardas PE, Alexopoulos D. Antiplatelet treatment in diabetic patients with acute coronary syndrome undergoing percutaneous coronary intervention: a GReek AntiPlatElet registry substudy. Coron Artery Dis. 2018;29(1):53–9.
Leiter LA, Bhatt DL, McGuire DK, Teoh H, Fox K, Simon T, Mehta SR, Lev EI, Kiss RG, Dalby AJ, Bueno H, Ridderstråle W, Himmelmann A, Prats J, Liu Y, Lee JJ, Amerena J, Kosiborod MN, Steg PG, THEMIS Steering Committee and Investigators. Diabetes-related factors and the effects of ticagrelor plus aspirin in the THEMIS and THEMIS-PCI trials. J Am Coll Cardiol. 2021;77(19):2366–77.
Blin P, Darmon P, Henry P, Guiard E, Bernard MA, Dureau-Pournin C, Maizi H, Thomas-Delecourt F, Lassalle R, Droz-Perroteau C, Moore N. Patients with stable coronary artery disease and type 2 diabetes but without prior myocardial infarction or stroke and THEMIS-like patients: real-world prevalence and risk of major outcomes from the SNDS French nationwide claims database. Cardiovasc Diabetol. 2021;20(1):229.
Gurbel PA, Jeong YH, Tantry US. The dogged search for cryptic effects of ticagrelor: wishful thinking or real benefits beyond P2Y12 inhibition? Circulation. 2016;134(22):1720–3.
Reiner MF, Akhmedov A, Stivala S, Keller S, Gaul DS, Bonetti NR, Savarese G, Glanzmann M, Zhu C, Ruf W, Yang Z, Matter CM, Lüscher TF, Camici GG, Beer JH. Ticagrelor, but not clopidogrel, reduces arterial thrombosis via endothelial tissue factor suppression. Cardiovasc Res. 2017;113(1):61–9.
Torngren K, Ohman J, Salmi H, Larsson J, Erlinge D. Ticagrelor improves peripheral arterial function in patients with a previous acute coronary syndrome. Cardiology. 2013;124(4):252–8.
Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: from pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. 2018;8(5):568–80.
van Giezen JJ, Sidaway J, Glaves P, Kirk I, Björkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther. 2012;17(2):164–72.
Ohman J, Kudira R, Albinsson S, Olde B, Erlinge D. Ticagrelor induces adenosine triphosphate release from human red blood cells. Biochem Biophys Res Commun. 2012;418(4):754–8.
Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014;19(2):209–19.
Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63(23):2503–9.
Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C, Camoin-Jau L, Paganelli F, Dignat-George F, Guieu R. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63(9):872–7.
Nylander S, Schulz R. Effects of P2Y12 receptor antagonists beyond platelet inhibition–comparison of ticagrelor with thienopyridines. Br J Pharmacol. 2016;173(7):1163–78.
Mangiacapra F, Panaioli E, Colaiori I, Ricottini E, Lauria Pantano A, Pozzilli P, Barbato E, Di Sciascio G. Clopidogrel versus ticagrelor for antiplatelet maintenance in diabetic patients treated with percutaneous coronary intervention: results of the CLOTILDIA study (clopidogrel high dose versus ticagrelor for antiplatelet maintenance in diabetic patients). Circulation. 2016;134(11):835–7.
Jeong HS, Hong SJ, Cho SA, Kim JH, Cho JY, Lee SH, Joo HJ, Park JH, Yu CW, Lim DS. Comparison of ticagrelor versus prasugrel for inflammation, vascular function, and circulating endothelial progenitor cells in diabetic patients with non-ST-segment elevation acute coronary syndrome requiring coronary stenting: a prospective, randomized, crossover trial. JACC Cardiovasc Interv. 2017;10(16):1646–58.
Campo G, Vieceli Dalla Sega F, Pavasini R, Aquila G, Gallo F, Fortini F, Tonet E, Cimaglia P, Del Franco A, Pestelli G, Pecoraro A, Contoli M, Balla C, Biscaglia S, Rizzo P, Ferrari R. Biological effects of ticagrelor over clopidogrel in patients with stable coronary artery disease and chronic obstructive pulmonary disease. Thromb Haemost. 2017;117(6):1208–16.
Ariotti S, Ortega-Paz L, van Leeuwen M, Brugaletta S, Leonardi S, Akkerhuis KM, Rimoldi SF, Janssens G, Gianni U, van den Berge JC, Karagiannis A, Windecker S, Valgimigli M, HI-TECH Investigators. Effects of ticagrelor, prasugrel, or clopidogrel on endothelial function and other vascular biomarkers: a randomized crossover study. JACC Cardiovasc Interv. 2018;11(16):1576–86.
Schnorbus B, Daiber A, Jurk K, Warnke S, Koenig J, Lackner KJ, Münzel T, Gori T. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: a randomized, blinded, parallel study. Eur Heart J. 2020;41(33):3144–52.
Guan B, Zhao L, Ma D, Fan Y, Zhang H, Wang A, Xu H. The effect of ticagrelor on endothelial function compared to prasugrel, clopidogrel, and placebo: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:820604.
Ndrepepa G, Kastrati A, Menichelli M, Neumann FJ, Wöhrle J, Bernlochner I, Richardt G, Witzenbichler B, Sibbing D, Gewalt S, Angiolillo DJ, Hamm CW, Hapfelmeier A, Trenk D, Laugwitz KL, Schunkert H, Schüpke S, Mayer K. Ticagrelor or prasugrel in patients with acute coronary syndromes and diabetes mellitus. JACC Cardiovasc Interv. 2020;13(19):2238–47.
Angiolillo DJ, Baber U, Sartori S, Briguori C, Dangas G, Cohen DJ, Mehta SR, Gibson CM, Chandiramani R, Huber K, Kornowski R, Weisz G, Kunadian V, Oldroyd KG, Ya-Ling H, Kaul U, Witzenbichler B, Dudek D, Sardella G, Escaned J, Sharma S, Shlofmitz RA, Collier T, Pocock S, Mehran R. Ticagrelor with or without aspirin in high-risk patients with diabetes mellitus undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2020;75(19):2403–13.
Cattaneo M, Lecchi A. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate potentiates the antiplatelet effect of prostacyclin. J Thromb Haemost. 2007;5(3):577–82.
Kirkby NS, Lundberg MH, Chan MV, Vojnovic I, Solomon AB, Emerson M, Mitchell JA, Warner TD. Blockade of the purinergic P2Y12 receptor greatly increases the platelet inhibitory actions of nitric oxide. Proc Natl Acad Sci U S A. 2013;10(39):15782–7.
Chan MV, Knowles RB, Lundberg MH, Tucker AT, Mohamed NA, Kirkby NS, Armstrong PC, Mitchell JA, Warner TD. P2Y12 receptor blockade synergizes strongly with nitric oxide and prostacyclin to inhibit platelet activation. Br J Clin Pharmacol. 2016;81(4):621–33.
Sibbing D, Braun S, Jawansky S, Vogt W, Mehilli J, Schömig A, Kastrati A, von Beckerath N. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb Haemost. 2008;99(1):121–6.
Chyrchel B, Totoń-Żurańska J, Kruszelnicka O, Chyrchel M, Mielecki W, Kołton-Wróż M, Wołkow P, Surdacki A. Association of plasma miR-223 and platelet reactivity in patients with coronary artery disease on dual antiplatelet therapy: a preliminary report. Platelets. 2015;26(6):593–7.
Kruszelnicka O, Chyrchel B, Golay A, Surdacki A. Differential associations of circulating asymmetric dimethylarginine and cell adhesion molecules with metformin use in patients with type 2 diabetes mellitus and stable coronary artery disease. Amino Acids. 2015;47(9):1951–9.
Patti G, Grieco D, Dicuonzo G, Pasceri V, Nusca A, Di Sciascio G. High versus standard clopidogrel maintenance dose after percutaneous coronary intervention and effects on platelet inhibition, endothelial function, and inflammation results of the ARMYDA-150 mg (Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty) randomized study. J Am Coll Cardiol. 2011;57(7):771–8.
Rudolph TK, Fuchs A, Klinke A, Schlichting A, Friedrichs K, Hellmich M, Mollenhauer M, Schwedhelm E, Baldus S, Rudolph V. Prasugrel as opposed to clopidogrel improves endothelial nitric oxide bioavailability and reduces platelet-leukocyte interaction in patients with unstable angina pectoris: a randomized controlled trial. Int J Cardiol. 2017;248:7–13.
Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabaté M, Jimenez-Quevedo P, Hernández R, Moreno R, Escaned J, Alfonso F, Bañuelos C, Costa MA, Bass TA, Macaya C. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54(8):2430–5.
Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, Granada JF, Dong JF, Kleiman NS, Lev EI. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52(9):743–9.
Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82(5):521–33.
Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–42.
Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–54.
Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–906.
Vials A, Burnstock G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. Br J Pharmacol. 1993;109(2):424–9.
Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92(8):2135–41.
Bouma MG, van den Wildenberg FA, Buurman WA. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol. 1996;270(2 Pt 1):C522–9.
Walker G, Langheinrich AC, Dennhauser E, Bohle RM, Dreyer T, Kreuzer J, Tillmanns H, Braun-Dullaeus RC, Haberbosch W. 3-deazaadenosine prevents adhesion molecule expression and atherosclerotic lesion formation in the aortas of C57BL/6J mice. Arterioscler Thromb Vasc Biol. 1999;19(11):2673–9.
Hassanian SM, Dinarvand P, Rezaie AR. Adenosine regulates the proinflammatory signaling function of thrombin in endothelial cells. J Cell Physiol. 2014;229(9):1292–300.
Kutryb-Zając B, Mierzejewska P, Sucajtys-Szulc E, Bulińska A, Zabielska MA, Jabłońska P, Serocki M, Koszałka P, Milczarek R, Jasztal A, Bartoszewski R, Chłopicki S, Słomińska EM, Smoleński RT. Inhibition of LPS-stimulated ecto-adenosine deaminase attenuates endothelial cell activation. J Mol Cell Cardiol. 2019;128:62–76.
Gąsecka A, Nieuwland R, Budnik M, Dignat-George F, Eyileten C, Harrison P, Lacroix R, Leroyer A, Opolski G, Pluta K, van der Pol E, Postuła M, Siljander P, Siller-Matula JM, Filipiak KJ. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J Thromb Haemost. 2020;18(3):609–23.
Chyrchel B, Drożdż A, Długosz D, Stępień E, Surdacki A. Platelet reactivity and circulating platelet-derived microvesicles are differently affected by P2Y12 receptor antagonists. Int J Med Sci. 2019;16(2):264–75.
Tans G, Rosing J, Thomassen MC, Heeb MJ, Zwaal RF, Griffin JH. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77(12):2641–8.
Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123(1):8–23.
Badimon L, Suades R, Fuentes E, Palomo I, Padró T. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front Pharmacol. 2016;7:293.
Zaldivia MTK, McFadyen JD, Lim B, Wang X, Peter K. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med. 2017;4:74.
Rosińska J, Łukasik M, Kozubski W. The impact of vascular disease treatment on platelet-derived microvesicles. Cardiovasc Drugs Ther. 2017;31(5–6):627–44.
Barry OP, Praticò D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102(1):136–44.
Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25(7):1512–8.
Christersson C, Thulin Å, Siegbahn A. Microparticles during long-term follow-up after acute myocardial infarction. Association to atherosclerotic burden and risk of cardiovascular events. Thromb Haemost. 2017;117(8):1571–81.
Gąsecka A, Nieuwland R, van der Pol E, Hajji N, Ćwiek A, Pluta K, Konwerski M, Filipiak KJ. P2Y12 antagonist ticagrelor inhibits the release of procoagulant extracellular vesicles from activated platelets. Cardiol J. 2019;26(6):782–9.
Blann AD, Nadar SK, Lip GYH. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24(24):2166–79.
de Jäger J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol. 2006;26(5):1086–93.
Teerlink T, Heine RJ, Nijpels G, Bouter LM, Stehouwer CDA, Dekker JM. Asymmetric dimethylarginine (ADMA) is associated with incident cardiovascular disease in the general population. The Hoorn study. Atheroscler Suppl. 2006;7(3):23 (Abstract).
Anderssohn M, McLachlan S, Lüneburg N, Robertson C, Schwedhelm E, Williamson RM, Strachan MW, Ajjan R, Grant PJ, Böger RH, Price JF. Genetic and environmental determinants of dimethylarginines and association with cardiovascular disease in patients with type 2 diabetes. Diabetes Care. 2014;37(3):846–84.
Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119(12):1592–600.
Lu TM, Chung MY, Lin MW, Hsu CP, Lin SJ. Plasma asymmetric dimethylarginine predicts death and major adverse cardiovascular events in individuals referred for coronary angiography. Int J Cardiol. 2011;153(2):135–40.
Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, Wallentin L, Jernberg T, for SWEDEHEART. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120(10):851–8.
Salisbury AC, Reid KJ, Spertus JA. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am J Cardiol. 2007;99(5):636–41.
Morillas P, Quiles J, Cordero A, Guindo J, Soria F, Mazón P, Gonzalez-Juanatey JR, Bertomeu V, Prevalence of Peripheral Arterial Disease in Patients With Acute Coronary Syndrome (PAMISCA) Investigators. Impact of clinical and subclinical peripheral arterial disease in mid-term prognosis of patients with acute coronary syndrome. Am J Cardiol. 2009;104(11):1494–8.
Knowles RB, Warner TD. Anti-platelet drugs and their necessary interaction with endothelial mediators and platelet cyclic nucleotides for therapeutic efficacy. Pharmacol Ther. 2019;193:83–90.
Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92(3):639–46.
Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, Akin I, Bott-Flügel L, Fischer M, Landmesser U, Katus HA, Sibbing D, Seyfarth M, Janisch M, Boncompagni D, Hilz R, Rottbauer W, Okrojek R, Möllmann H, Hochholzer W, Migliorini A, Cassese S, Mollo P, Xhepa E, Kufner S, Strehle A, Leggewie S, Allali A, Ndrepepa G, Schühlen H, Angiolillo DJ, Hamm CW, Hapfelmeier A, Tölg R, Trenk D, Schunkert H, Laugwitz KL, Kastrati A, ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381(16):1524–34.
Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, Held C, Andersson M, Himmelmann A, Ridderstråle W, Chen J, Song Y, Diaz R, Goto S, James SK, Ray KK, Parkhomenko AN, Kosiborod MN, McGuire DK, Harrington RA, THEMIS Steering Committee and Investigators. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394(10204):1169–80.
Vieceli Dalla Sega F, Fortini F, Aquila G, Pavasini R, Biscaglia S, Bernucci D, Del Franco A, Tonet E, Rizzo P, Ferrari R, Campo G. Ticagrelor improves endothelial function by decreasing circulating epidermal growth factor (EGF). Front Physiol. 2018;9:337.
Aquila G, Vieceli Dalla Sega F, Marracino L, Pavasini R, Cardelli LS, Piredda A, Scoccia A, Martino V, Fortini F, Bononi I, Martini F, Manfrini M, Pannuti A, Ferrari R, Rizzo P, Campo G. Ticagrelor increases SIRT1 and HES1 mRNA levels in peripheral blood cells from patients with stable coronary artery disease and chronic obstructive pulmonary disease. Int J Mol Sci. 2020;21(5):1576.
Tam CF, Chan YH, Wong YK, Li Z, Zhu X, Su KJ, Ganguly A, Hwa K, Ling XB, Tse HF. Multi-omics signatures link to ticagrelor effects on vascular function in patients with acute coronary syndrome. Arterioscler Thromb Vasc Biol. 2022;42(6):789–98.
Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019;381(21):2032–42.
Chichareon P, Modolo R, Kogame N, Takahashi K, Chang CC, Tomaniak M, Botelho R, Eeckhout E, Hofma S, Trendafilova-Lazarova D, Kőszegi Z, Iñiguez A, Wykrzykowska JJ, Piek JJ, Garg S, Hamm C, Steg PG, Jüni P, Vranckx P, Valgimigli M, Windecker S, Onuma Y, Serruys PW. Association of diabetes with outcomes in patients undergoing contemporary percutaneous coronary intervention: Pre-specified subgroup analysis from the randomized GLOBAL LEADERS study. Atherosclerosis. 2020;295:45–53.
Gao C, Tomaniak M, Takahashi K, Kawashima H, Wang R, Hara H, Ono M, Montalescot G, Garg S, Haude M, Slagboom T, Vranckx P, Valgimigli M, Windecker S, van Geuns RJ, Hamm C, Steg PG, Onuma Y, Angiolillo DJ, Serruys PW. Ticagrelor monotherapy in patients with concomitant diabetes mellitus and chronic kidney disease: a post hoc analysis of the GLOBAL LEADERS trial. Cardiovasc Diabetol. 2020;19(1):179.
Armstrong PCJ, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, Warner TD. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9(3):552–61.
Kirkby NS, Leadbeater PD, Chan MV, Nylander S, Mitchell JA, Warner TD. Antiplatelet effects of aspirin vary with level of P2Y12 receptor blockade supplied by either ticagrelor or prasugrel. J Thromb Haemost. 2011;9(10):2103–5.
FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ 2nd, Lawson JA, Brash AR. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71(3):676–88.
Johnston-Cox HA, Ravid K. Adenosine and blood platelets. Purinergic Signal. 2011;7(3):357–65.
Siller-Matula JM, Delle-Karth G, Lang IM, Neunteufl T, Kozinski M, Kubica J, Maurer G, Linkowska K, Grzybowski T, Huber K, Jilma B. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost. 2012;10(4):529–42.
Funding
This work was supported in part by research grants (No. N41/DBS/000032, N41/DBS/000665, K/ZDS/003761, K/ZDS/006104 and K/ZDS/006105) from the Jagiellonian University Medical College (Cracow, Poland). The funding body had no role in the design of the study and collection, analysis, and interpretation of data, in writing the manuscript, and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
BC conceived and designed the study, collected, analyzed, and interpreted data, and wrote the manuscript. OK contributed to data collection and analysis. AS contributed to study design and supervised the study. BC, OK and AS contributed to revision of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Bioethical Committee of our university (Approval numbers: KBET/277/B/2013 and 1072.6120.143.2019 issued on November 28th, 2013 and May 30th, 2019, respectively) and written informed consent was obtained from the study subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chyrchel, B., Kruszelnicka, O. & Surdacki, A. Endothelial biomarkers and platelet reactivity on ticagrelor versus clopidogrel in patients after acute coronary syndrome with and without concomitant type 2 diabetes: a preliminary observational study. Cardiovasc Diabetol 21, 249 (2022). https://doi.org/10.1186/s12933-022-01685-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01685-4