Abstract
Objectives
Recurrent Kidney stone formation is a main medical problem imposing a significant burden on both healthcare and the economy worldwide. Environmental and genetic factors have been linked to a bigger risk of kidney stone formation. We aim to assess the role of methylation on recurrent stone formation in three target genes.
Methods
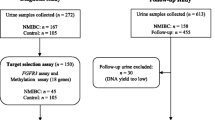
We aimed to check the association between promoter hypermethylation vitamin D receptor (VDR), calcium-sensing receptor (CaSR), and claudin 14 (CLDN14) genes in recurrent kidney stones. We enrolled 30 consecutive recurrent kidney stone formers (age 18–60 years) (cases) and 30 age and gender-matched controls.3. To identify promoter methylation, two target regions from each candidate gene were bisulfited after blood collection and DNA extraction. Methylation quantification was done through methylation-specific high resolution melting (MS-HRM).
Results
The mean age of the patients and controls (mean ± SD) was 49.58 ± 14.23 years and BMI 36.12 ± 2.72. The methylation status in all six target regions was meaningfully different between the stone-former group and controls when methylation was considered in three clusters of unmethylated, methylated, and hypermethylated. A higher effect in VDR and CLDN was observed compare to CasR (p-value < 0.001, and < 0.005 versus p-value < 0.256).
Conclusions
Methylation as an important epigenetic mechanism should be considered more in recurrent stone formations. Promoter hypermethylation of VRD and CLDN genes may have an essential role in recurrent kidney stones formations.
Similar content being viewed by others
Introduction
Genetic and epigenetic changes are two main elements that can increase the risk of kidney stones formation. Contrary to genetic change in which the DNA sequence changed, the gene expression pattern changed reversibly with no change in the DNA nucleotide sequences in epigenetic modifications like DNA methylation. Recurrent kidney stone formation is a multifactorial disease that can be the consequence of several environmental and lifestyle factors. Most genes involved in the condition are essential for transporting materials in and out of cells. Over the last decade, several epidemiological studies have shown high kidney stone disease in all age groups [1, 2]. Several risk factors are studied for recurrent urolithiasis like caffeine intake, dietary intake, smoking, alcohol, physical activity [3, 4] Epigenetic modifications like DNA methylation in CpG islands define spatial conformation of chromatin to regulate gene expression. DNA methylation change can be the consequence of environmental factors.
Some genes are the most highlighted ones in recurrent kidney stones, like Vitamin D Receptors (VitD R), Claudin 14 (CLDN14), and Calcium-sensing receptor (CaSR) [5, 6]. Despite the established importance of these three genes in kidney stone formation, their promoter methylations have not evaluated yet. In this study, for the first time we aimed to determine the promoter methylation status of VitD R, Claudin, and CaSR in recurrent kidney stone formation.
Materials and methods
Study population and specimen's collection
The study was run under the Tehran University of Medical Sciences ethical committee (IR.TUMS.SINA HOSPITAL.REC.1399.033) after receiving the written informed consent from both patients and controls. All the methods were in accordance with the relevant institutional or in accordance with the declaration of Helsinki. A total number of sixty patients were recruited, of which thirty were recurrent stones patients, and the other thirty were individuals with no kidney stones nor a positive history of urolithiasis. A recurrent stone former was defined as an individual with at least two symptomatic episodes in less than six months intervals. Patients were aged between 18 and 90 years old with no systemic disorders. Patients with a history of known metabolic, gastrointestinal, hepatic, renal, or endocrinological diseases were excluded. A blood sample was sent for serum urea, creatinine, calcium, phosphate, magnesium, and uric acid concentrations, as well as vitamin D3, calcitonin, parathyroid hormone (PTH), and alkaline phosphatase levels.
DNA extraction and bisulfite treatment
A total amount of 4–6 ml blood was collected in EDTA tubes. Then genomic DNA was extracted from the patients with recurrent kidney stones and their age and gender-matched control counterparts by a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) based on the manufacturer's instruction. Then genomic DNA was treated for Bisulfite modification of DNA by the "EZ DNA Methylation‐Gold™ Kit" (Cat No: D5005, Zymo Research) according to the manufacturerʼs protocol. This step is essential for discrimination between methylated CpG island and unmethylated ones because bisulfite Conversion is a procedure in which DNA (gDNA) is denatured (made single-stranded) and after sodium bisulfite treatment, delamination of unmethylated Cytosines into Uracil's happens, whereas methylated cytosines (both 5-methylcytosine and 5-hydroxymethylcytosine) stay unaffected.
Methylation analysis by MS-HRM
For detecting the quantity of CpG methylated islands, the Methylation Specific High-Resolution Melting (MS‐HRM) was applied. Specific primers were designed to evaluate the promoter methylation of three target genes VitD R, Claudin, and CaSR (Table 1). The primer sets for all MS-HRM assays were prepared according to the principles recently set out to compensate for PCR bias [7]. These primers were used to amplify six target promoter regions indicated by "a" and "b ". The locus of the primers and target amplicons are presented in Fig. 1. The MS‐HRM analyses consisted of the following three main steps: holding step (95 degrees Celsius for 15 min), trailed by 38 cycles of 95 degrees Celsius for 20 s; annealing temperature (ranging from 47 degrees Celsius to 60 degrees Celsius) for 30 s; and extension time of 72 degrees Celsius for 30 s leading to the last step of the Melting curve. The melting curve includes heating up to 94 degrees Celsius for 15 s and 60 degrees Celsius for 45 s leading to 65 degrees Celsius for 20 s, then heating up uninterruptedly to 94 degrees Celsius with data recording for every 0.5 degrees Celsius temperature rise. All reaction combinations contained 10 µl of master mix (Cat No: A325406; Amplicon), 20 pmol forward and reverse primers, and 2 µl (almost 20 ng) of bisulfite modified DNA template in the closing volume of 20 µl. MS‐HRM experiments were performed by ABI Step One Plus System.
Statistical analysis
The methylation status in control and stone-former groups was compared using the chi-squared test. The odds ratio (OR) of methylation status in the case group was calculated for each promoter region of three targeted genes, VitD R, Claudin, and Cas R and were compared to the control. The description for outside normal serum calcium was also estimated for two groups and was expressed as mean and standard deviation (SD). A statistically significant p-value of less than 0.05 was considered. Statistical Package did all analyses for Science Software (SPSS, version 17.0; SPSS Inc., Chicago, IL).
Results
A total of 30 stone-forming patients and 30 age and sex-matched counterpart controls were recruited in the study. The mean age of the patients and controls (mean ± SD) was 49.58 ± 14.23 years and BMI 36.12 ± 2.72 (Table 2).
The findings of Table 2 show the patient's demographic and characteristics in the case group compared to the control group. Only the Creatinine variable was marginally significantly different between the two groups (p-value = 0. 051). The frequency of other variables did not show a significant difference between case and control groups. MS-HRM methylation was defined as the unmethylated (methylation < 9%), methylated (9–29%) and highly/ hypermethylated (> 29%) [8].
MS-HRM was analyzed based on comparing melt profiles of experimental samples to profiles from DNA with known methylation levels. Universally (or 100%) methylated DNA is commercially available. For a source of unmethylated DNA, scientists often isolate DNA from blood mononuclear cells. For this study, the Sigma-Aldrich CpGenome™ Human Methylated & Non-Methylated DNA Standard Set (Cat# S8001) were used as the 0% and 100% methylated DNA. 100% methylated and 0% methylated DNA of equal concentration were then mixed in different ratios to mimic DNA samples with defined DNA methylation levels. The methylation quantification of each test sample was evaluated in triplets (Fig. 2).
The resulting graph of MS-HRM for 50%, 100%, 75% methylated DNAs. A Indicated to 50% methylation because half of amplicon are melted (double stranded DNA converts to single stranded DNA) at lower temperature which indicated to unmethylated cytosine replacement by uracil. B indicated to 100% unmethylated because all DNAs are melted at lower temperature. C Represent the 75% methylated promoter because ¼ of DNA are melted at lower temperature and ¾ are melted at higher temperature. D showed all runs over each other which should consider one by one like A, B, and C
The methylation status in all six target regions was statistically significantly different between the stone-former group and controls when methylation was considered in three clusters of unmethylated, methylated, and hypermethylated (Table 3). As we shown in Fig. 1, the VDRa is the distant promoter, and VDRb is the proximal one to the translation initiation codon (ATG). For CLDNa and CLDNb is the same as the CasRa and CasRb, in which "a" means the target sequence is distal and "b" is the proximal ones to the translation initiation codon (ATG).
When we consider the methylation status as methylated (methylation < 9%) and unmethylated (methylation ≥ 9%), the methylation CLDNb, CasRa, and CasRb did not show the significant difference between the two groups of stone-former cases and controls (Table 4). Compared to the healthy controls, the VDR was the only gene in which hypermethylated promoter was significantly increased in the recurrent stone-former group. In the CLDN gene, the distal promoter region was statistically significantly more hypermethylated in recurrent stone-formers (Table 5). The promoter methylation of the Receptor of Vit D is the most critical gene in recurrent stone formation (Fig. 3).
The methylation status of six target promoter regions was correlated with each other. The correlation coefficients for this correlation were estimated by the kappa agreement test and shown in Table 6. The most correlated ones between the two target intragenic regions were CasRa and CasRb, then VDRa and VDRb, and between two different genes of VDR with CLDN.
Discussion
Methylation as an important epigenetic mechanism should be considered more in recurrent stone formations. Promoter hypermethylation of VRD and CLDN genes may have an essential role in recurrent kidney stones formations. We select vitamin D receptor (VDR), calcium-sensing receptor (CaSR), and claudin 14 (CLDN14) genes based on literature and meta-analysis. Only these three genes were studied in this work. This deficiency needs to be reflected. We select the genes that their gene expression regulation is controlled by promoter methylation. Because there is not previous study on methylation and just SNPs are discussed in Urolithiasis our study can be the good starting point for such studies. Renal stone formation risk factors have classically reflected environmental, geographic, and dietary issues, often based on population studies. Several treatment strategies are considered for the stone treatment depending on the stone size and skin-to-stone distance [9,10,11,12,13]. Moreover, the heritability of renal stones has also been proven. In twin studies, the estimated heritability of kidney stones was reported at 56%, and this has been supported by additional studies measuring the familial pattern of stones [14]. More recently, there has been a comprehensive exploration for rare monogenetic bases of renal stones by means of selected stone-forming populations [15,16,17].
Hypercalciuria is integral in the pathogenesis of stone formation that can be arise through calcium deposition in the renal papilla. The etiology of hypercalciuria is not well understood but includes augmented bone resorption, calcium hyperabsorption in the intestine, and lessen renal reabsorption. Calcium reabsorption happens mainly in the proximal tubule that about sixty percent of calcium filtered through the glomerulus is reabsorbed. The calcium carriage is done by claudins, an important components of the epithelial tight junctions found at tight junctions. Tight junctions are a surrounding cellular blockade that controls the flow of molecules in the intercellular space between the cells of an epithelium. The claudins perform selective transport and control the movement of solutes through the epithelium.
Some infrequent monogenic forms of stone formation can originally look a fine-print issue; lost detects of monogenetic reasons of nephrolithiasis might consequence to sub-optimal action, complications, and disappointment to screen at-risk family members [18,19,20]. Screening common pathogenic mutations by some novel high-throughput genomics-based strategies to assay GWAS SNPs, Sequencing by synthesis technology, Bead Array microarray technology, and is rapidly becoming an available diagnostic tool [15, 21]. In studies of hypercalciuric and hypocalciuric patients' monogenic origins and infrequent alleles were not clearly recognized [22]. CLCN5 variants were a reasonable candidate for idiopathic hypercalciuria. However, those variants were found to be rare [23].
Several studies analyzed the associations of VDR gene expression with urolithiasis risk in different ethnic groups [24]. A meta-analysis by Imani D et al. indicated that although their study did not emphasize the relationship of FokI, TaqI, BsmI, and ApaI in the general sampled examination, but it proposes that ApaI and TaqI SNPs are linked to the bigger risk of urolithiasis in East-Asian and Caucasians populations [5]. It was indicated that urinary calcium excretion has increased in response to vitamin D (VitD) supplements, at least in some groups of kidney stone formers. It has been proposed that predisposed individuals may develop hypercalciuria and kidney stones in response to vitamin D supplements [25]. The VDR FokI polymorphism may be a good candidate for calcium oxalate stone disease marker. The epidemic of Randall plaque-associated renal stones in young patients can be the implication of altered vitamin D response [26].
Regarding CLDN, our data suggested the lower gene expression of CLDN can be the result of the promoter hypermethylation of CLDN. Similarly, Curryn JN and colleagues also described that a family with a rare missense variant in the CLDN gene has noticeable hypercalciuria, and kidney stone disease findings may indicate that CLDN can be a crucial regulator of calcium excretion and a potential target for therapies to prevent kidney stones [27]. Also, it was shown that single nucleotide polymorphisms of CLDN are significant in hypercalciuria and kidney stone formation [28]. The CLDN expression is downregulated in many pathologies, like cancer, inflammation, and fibrosis. The CLDN play an important role in energy-efficient ion and water transport in the proximal tubules of the kidneys and in the intestines. Notably, substantial studies highlighted a critical role for this protein as a modulator of critical cellular pathways related to stone diseases. Cell signaling pathways and molecular mechanism that are over activated in different conditions can change the claudin-2 expression, and a respectable association occurs concerning disease stage and claudin-2 overexpression. Additional, loss- and gain-of-function research presented that direct alterations in claudin-2 expression influence critical metabolic pathways in human cells. These properties seem to be intermediated by changes in critical signaling pathways [29].
The CaSR gene expression change commonly highlighted in the parathyroid glands and in renal tubules that control Parathyroid hormone (PTH) secretion. The kidneys are the main route for the excretion of salt and water and have an important role in the control of body fluid osmolality and Intracellular fluid volume (ICFV) and extracellular fluid volume (ECFV). The kidney controls electrolyte and water excretion, fix different tubular segments' functions. In particular, CaSR decreases both passive and active calcium reabsorption in distal tubules, raises phosphate reabsorption in proximal tubules, and triggers proton and water excretion in collecting ducts. Therefore, it can be an important gene for causing calcium nephrolithiasis. Our data indicated a less critical role of Cas R epigenetic control in recurrent kidney stones formation. Some contradictory results showed an essential function of Cas R [30]. One possible explanation might be that Cas R cis's gene expression is not regulated by epigenetic mechanisms like methylation.
Finally, we can say promoter hypermethylation of VRD and CLDN genes has an essential role in recurrent kidney stones formations.
Availability of data and materials
All datasets used and/or analyzed during the current study are provided and has no additional data.
References
Sas DJ. An update on the changing epidemiology and metabolic risk factors in pediatric kidney stone disease. Clin J Am Soc Nephrol. 2011;6(8):2062–8.
Maddahi NS, Mirzaei K, Aghamir SMK, Modaresi SS, Yekaninejad MS Major Dietary Patterns and kidney stone formation among Iranian men. J Nutr Sci Diet. 2017;11–7.
Do Lifestyle Factors Including Smoking. Alcohol, and exercise impact your risk of developing kidney stone disease? Outcomes of a systematic review. J Endourol. 2021;35(1):1–7.
Aghamir SMK, Elmimehr R, Modaresi SS, Salavati A. Comparing bleeding complications of double and single access totally tubeless PCNL: is it safe to obtain more accesses? Urol Int. 2016;96(1):73–6.
Imani D, Razi B, Khosrojerdi A, Lorian K, Motallebnezhad M, Rezaei R, et al. Vitamin D receptor gene polymorphisms and susceptibility to urolithiasis: a meta-regression and meta-analysis. BMC Nephrol. 2020;21(1):1–18.
Allison SJ. Claudin 2: role in hypercalciuria and kidney stone disease. Nat Rev Nephrol. 2020;16(5):252.
Wojdacz TK, Lotte HL. Reversal of PCR bias for improved sensitivity of the DNA methylation melting curve assay. Biotechniques. 2006;41(3):274–8.
Gurrieri L, De Carlo E, Gerratana L, De Maglio G, Macerelli M, Pisa FE, et al. MGMT pyrosequencing-based cut-off methylation level and clinical outcome in patients with glioblastoma multiforme. Future Oncol. 2018;14(8):699–707.
Fakhr Yasseri A, Taheri D. Urinary stone management during COVID-19 pandemic. Transl Res Urol. 2020;2(1):1–3.
Shabaninia S, Yahyazadeh SR, Mousavi A, Kasaeian A. The characteristics and outcomes of patients treated with percutaneous nephrolithotomy according to the preoperative renal function levels. Transl Res Urol. 2020;2(1):4–8.
Fakhr Yasseri A, Hamidi M, Aghamir SMK. Comparison of stone-free rates after ureteroscopic pneumatic lithotripsy in impacted vs. non-impacted upper ureteral stones. Transl Res Urol. 2019;1(2):79–83.
Mehrsai A, Hashemian Naeini E, Dadkhah Tehrani F, Noori JK. Impact of bone mineral density on the recurrent urolithiasis. Transl Res Urol. 2019;1(1):12–6.
Aghamir SMK, Hamidi M, Salavati A, Mohammadi A, Farahmand H, Meysamie AP et al. Is antibiotic prophylaxis necessary in patients undergoing ureterolithotripsy? Acta Med Iran. 2011;513–6.
Ljunghall S, Danielson B, Fellström B, Holmgren K, Johansson G. Family history of renal stones in recurrent stone patients. Br J Urol. 1985;57(4):370–4.
Braun DA, Lawson JA, Gee HY, Halbritter J, Shril S, Tan W, et al. Prevalence of monogenic causes in pediatric patients with nephrolithiasis or nephrocalcinosis. Clin J Am Soc Nephrol. 2016;11(4):664–72.
Daga A, Majmundar AJ, Braun DA, Gee HY, Lawson JA, Shril S, et al. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int. 2018;93(1):204–13.
Mohammadi A, Rakebi MM, Pishkuhi MA, Aghamir SMK. Does potassium citrate administration change the type and composition of encrusted material on Double-J stent compared to primary stone? Int Urol Nephrol. 2021. https://doi.org/10.1007/s11255-021-02891-x.
Ferraro PM, D’Addessi A, Gambaro G. When to suspect a genetic disorder in a patient with renal stones, and why. Nephrol Dial Transpl. 2013;28(4):811–20.
Jafari Shahdani MR, Fattahi B, Mohseni MG, Aghamir SMK. Comparison of Mini-perc and retrograde intrarenal surgery in residual stone fragments with hounsfield unit after percutaneous nephrolithotomy. Transl Res Urol. 2021;3(2):40–4.
Mirsadeghi SA, Tamehri Zadeh SS, Fakhr Yasseri A, Tabibi A, Radfar MH, Taheri D. Comparison of the tamsulosin and tolterodine effectiveness in stent-related symptoms reduction. Transl Res Urol. 2021;3(1):23–31.
Zia H, Zadeh SST, Khatami F, Aghamir SMK. Laparoscopic ureterolithotomy for large lower ureteral stone: a case series report. Afr J Urol. 2021;27(1):1–4.
Toka HR, Genovese G, Mount DB, Pollak MR, Curhan GC. Frequency of rare allelic variation in candidate genes among individuals with low and high urinary calcium excretion. PLoS ONE. 2013;8(8): e71885.
Scheinman SJ, Cox JP, Lloyd SE, Pearce SH, Salenger PV, Hoopes RR, et al. Isolated hypercalciuria with mutation in CLCN5: relevance to idiopathic hypercalciuria. Kidney Int. 2000;57(1):232–9.
Lin Y, Mao Q, Zheng X, Chen H, Yang K, Xie L. Vitamin D receptor genetic polymorphisms and the risk of urolithiasis: a meta-analysis. Urol Int. 2011;86(3):249–55.
Letavernier E, Daudon M, Vitamin D. hypercalciuria and kidney stones. Nutrients. 2018;10(3):366.
Letavernier E, Vandermeersch S, Traxer O, Tligui M, Baud L, Ronco P, et al. Demographics and characterization of 10,282 Randall plaque-related kidney stones: a new epidemic? Medicine. 2015;94(10):e566.
Curry JN, Saurette M, Askari M, Pei L, Filla MB, Beggs MR, et al. Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J Clin Investig. 2020;130(4):1948–60.
Gillion V, Devuyst O. Genetic variation in claudin-2, hypercalciuria, and kidney stones. Kidney Int. 2020;98(5):1076–8.
Venugopal S, Anwer S, Szászi K. Claudin-2: roles beyond permeability functions. Int J Mol Sci. 2019;20(22):5655.
Guha M, Bankura B, Ghosh S, Pattanayak AK, Ghosh S, Pal DK, et al. Polymorphisms in CaSR and CLDN14 genes associated with increased risk of kidney stone disease in patients from the eastern part of India. PLoS ONE. 2015;10(6): e0130790.
Acknowledgements
Special thanks to Urology research center.
Funding
The author disclose there is no funding information.
Author information
Authors and Affiliations
Contributions
SMKA is the principal investigator, MKH edited the manuscript, MAP and AKH analyzed the data, RM and AG provided data and data curation and ran laboratory tests, FKH and ANSH wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Tehran University of Medical Sciences ethical committee (IR.TUMS.SINA HOSPITAL.REC.1399.033. Written informed consent was obtained from both patients and controls. All the methods were in accordance with the relevant institutional or in accordance with the declaration of Helsinki.
Consent for publication
Written informed consent was obtained from patients. Written formal consent ensures that the publisher has the author's permission to publish research findings.
Competing interests
All authors declare there is no conflict of interest for this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khatami, F., Gorji, A., Khoshchehreh, M. et al. The correlation between promoter hypermethylation of VDR, CLDN, and CasR genes and recurrent stone formation. BMC Med Genomics 15, 109 (2022). https://doi.org/10.1186/s12920-022-01265-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01265-1