Abstract
Background
Alcohol-based handrub (ABHR) is the gold standard for hand hygiene (HH) and is a cornerstone of infection prevention and control (IPC) strategies. However, several factors influence the efficient use of ABHR by health workers. This study evaluated the tolerability and acceptability of a locally produced ABHR product and HH behaviour among health workers.
Methods
A longitudinal hospital-based intervention study was conducted in accordance with the WHO’s standardized protocol for evaluating ABHR tolerability and acceptability (Method 1). Sixty health workers across 4 hospitals in Sierra Leone were observed over a 30-day period at three separate visits (days 1, 3–5, and 30) by trained observers. The outcomes of interest included skin tolerability and product acceptabilityevaluated using subjective and objective measures.
Results
Objective and subjective evaluations demonstrated strong skin tolerability and high acceptability with the product. At all three visits, the skin tolerability score assessed by trained observers was < 2 in ≥ 97% of participants, exceeding the WHO benchmark score (BMS = < 2 in ≥ 75%). Participants’ self-evaluations of overall skin integrity were 97% (visit 2) and 98% (visit 3) for scores > 4 (BMS = > 4 in ≥ 75%). The primary acceptability criteria increased up to 95% (colour) and 88% (smell) at visit 3 (BMS = > 4 in ≥ 50%). Despite high acceptability, the product’s drying effect remained low at 52% and 58% during visits 2 and 3, respectively (BMS = > 4 in ≥ 75%). There were positive HH behaviours (n = 53, 88%), with more than half (n = 38, 63%) of them exhibiting HH at almost every HH moment. The mean ABHR was notably high (76.1 ml, SD ± 35), especially among nurses (mean = 80.1 ml) and doctors (mean = 74.0 ml).
Conclusion
The WHO-formulated, locally produced ABHR was well tolerated and accepted by health workers. These findings support the continuous utilization of evidence-based, cost-effective hand hygiene interventions in resource-limited settings. High handrub consumption and frequent HH practices were noticeable HH behaviours. Further research is recommended to optimize product formulations for skin dryness and investigate the association between ABHR consumption and hand hygiene compliance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An effective infection prevention and control (IPC) programme sustains its interventions and their benefits over time [1]. Hand hygiene (HH), for example, is a crucial IPC intervention that should be sustained in healthcare facilities for the prevention of healthcare-associated infections (HAIs) [2]. HAI remains a global health concern due to the resultant prolonged hospital stay, increased healthcare costs, long-term disability and avoidable deaths of patients and health workers [3,4,5].
A systematic review showed a disproportionately high burden of HAIs in low- and middle-income countries (LMICs), with a pooled incidence of 15.5 per 100 patients, which is approximately threefold greater than that in high-income countries [4, 6]. The hands of health workers are the main pathways for the transmission of germs that cause these HAIs [7, 8] including bacteria-resistant organism contaminating mobile phones [9]. There is sufficient evidence demonstrating that up to 70% of HAIs can be prevented through effective IPC interventions, including appropriate HH as the single most effective action [10]. Studies have shown that HH improvement intervention is cost-effective in healthcare settings, saving approximately $16.5 in healthcare spending for every $1.0 investment [3, 10]. Therefore, global efforts to reduce the burden of infectious diseases have focused on HH, and these include the implementation of the World Health Organization (WHO)’s multimodal HH strategy [11] and innovative intervention for locally producing HH products such as alcohol-based hand rubs using WHO-recommended formulations [12].
The alcohol-based hand rub (ABHR) is part of the WHO essential medicines list for adults and children (EML and EMLc) [13] and is the gold standard of care for HH practice in healthcare settings and the HH product of choice for all standard aseptic procedures [3]. It is known that either of the two WHO-approved ABHR formulations was successfully used as an effective IPC measure during viral outbreaks, such as those involving Zika, Ebola, and coronaviruses, and can also effectively kill other enveloped viruses [14]. The use of ABHR is also preferred over handwashing with soap and water in most clinical situations because of its wide microbiological efficacy, time efficiency, availability at the point of care, and improved skin tolerance [15]. Locally produced ABHRs based on WHO formulations were found to be less expensive than commercially produced ABHR products [16, 17]. A better infrastructure and reliable ABHR supply at the point of care have the potential to improve HH compliance [18, 19], which can prevent unnecessary morbidity, mortality and costs related to HAI [3, 7, 8] and other deadly diseases, such as Ebola and Lassa fever. Local ABHR production is feasible globally, and particularly important in LMICs such as Sierra Leone, where healthcare facilities are challenged with infrastructural problems related to performing HH, including a lack of sinks with a constant water supply, soap, and disposable hand towels [20]. A recent study conducted in two major referral hospitals in Sierra Leone showed that only 9.5% of hospital units had running tap water, with Veronica buckets being the main resource for hand washing in 90.5% of the hospital units in the capital city hospitals [21]. Local ABHR production based on either of two approved formulations is encouraged [15] for low-income countries using the WHO guide for local production of ABHR [22]. This has been undertaken in several African countries, such as Uganda [17, 23], Kenya [24] and Ethiopia [25].
In 2019, through the national IPC program with technical and operational support from the WHO country office in Sierra Leone, the Ministry of Health of Sierra Leone instituted the local production of ABHR as a strategic intervention for the provision of affordable, sustainable, and safe HH products according to local needs and resources. ABHR production was based on WHO formulation 1 [12]. This has facilitated better distribution of ABHR products at the point of care across all public health facilities. This intervention also paid off at the peak of the COVID-19 pandemic. As of 31 December 2023, more than 1,239,060 L (L) of ABHR had been produced and distributed to healthcare facilities across the country. This quantity exceeded the estimated demand for ABHRs during the COVID-19 pandemic. Additionally, there was a considerable increase in the number of available and functioning HH stations with locally produced ABHRs in healthcare facilities [26].
Although the use of ABHRs improves HHs, health workers often report skin irritation and dryness when using these products frequently [27]. This poor tolerability and poor acceptance of ABHRs are common causes of poor HH compliance [28]. Therefore, user acceptance and good skin tolerability are considered among the most important criteria for the selection of ABHRs according to the WHO Guidelines on Hand Hygiene in Health Care (2009) [3]. A product that is pleasant to use and has no harmful effects on the hands is a major asset for the promotion of optimal HH practices [22]. A PubMed search conducted on January 4, 2024, using the term ‘ABHR tolerability and acceptability’, reported only two studies with information on ABHR tolerability and acceptability using the WHO single-product evaluation protocol (Method 1) in Poland and Switzerland [28, 29]; however, no studies have reported data on locally manufactured ABBRs based on the WHO formulation. Even though in-country local production is highly recommended by the WHO and is being undertaken by countries, there are very limited data on the user acceptability and tolerability of locally produced handrub products that also detail HH behaviour among health workers at the same time. No study has been performed in Africa to evaluate the tolerability and acceptability of locally produced ABHRs. In this study, we evaluated the user tolerability and acceptability of a WHO-formulated locally produced AHBR and HH behaviour among health workers using a WHO single-product evaluation protocol.
Methods
Study design
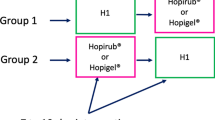
We conducted a longitudinal hospital-based observational study adhering to the standardized WHO protocol for evaluation of tolerability and acceptability of ABHR in use or planned to be introduced: Method 1” [22]. The objective was to assess the skin tolerability and user acceptance of a WHO-recommended, locally produced ABHR among health workers in Sierra Leone. This single-group study was designed to provide insights into the efficacy and practicality of implementing WHO-formulated HH solutions within healthcare settings, focusing on both immediate and prolonged use outcomes.
Study setting
This study was conducted over 30 days, from December 2022 to January 2023, within four healthcare facilities in Sierra Leone. The study included two tertiary hospitals and two secondary hospitals, providing a comprehensive overview of the two different levels of healthcare delivery. The tertiary facilities included Connaught Hospital (CH), a pivotal healthcare provider with a capacity of 300 beds, and Princess Christian Maternity Hospital (PCMH), specialising in maternal health with 160 beds. The Kingharman Maternal and Child Hospital (KMCH) and the 34 Military Hospital (M34) were used as complementary secondary facilities with bed capacities of 77 and 181, respectively. These facilities were purposively chosen based on their functional IPC programs and the propensity for high utilisation of the locally produced ABHR, as evidenced during routine IPC supportive site visits.
Study population
The study involved a cohort of 60 healthcare professionals, each of whom was actively engaged in full-time clinical duties within the participating healthcare facilities. Eligibility for inclusion was strictly limited to individuals aged 18 years and above, ensuring that all participants had the legal capacity to provide informed consent obtained after a comprehensive briefing on the study’s aims, procedures, potential benefits, and risks. Participation was voluntary, and informed consent to participate was obtained from all of the participants. In our meticulous selection process, we excluded individuals who presented with significant dermatological or systemic conditions that could unduly influence the assessment of ABHR skin tolerability or skew perceptions of product acceptability. This exclusion criterion was critical for isolating the effects of the ABHR under investigation, ensuring that the findings would be attributable to the product’s properties rather than to preexisting health issues.
Intervention
The core of this study was the evaluation of a locally produced ABHR formulated in strict adherence to the WHO formulation 1. This formulation comprises 96% ethanol, 3% hydrogen peroxide, 98% glycerol, and sterile distilled water, resulting in a final product characterised by 80% ethanol (v/v), 1.45% glycerol (v/v), and 0.125% hydrogen peroxide (v/v). The ingredients were carefully managed locally, ensuring the highest standards of quality and adherence to good manufacturing practices (GMPs). This endeavour was supported by a multidisciplinary team of pharmacists, physicians, and nurses who received comprehensive training in production techniques to uphold the integrity of the ABHR.
Participants were engaged in three evaluation sessions—initially on Day 1, subsequently one day between Days 3 and 5, and finally on Day 30. Table 1 summarises the contact sessions and what was done. These sessions were designed to monitor the use of ABHRs and conduct both objective and subjective assessments of skin tolerability and product acceptability. On the first day, each participant was provided with an unlabeled 500 ml bottle of the test product, accompanied by instructions to integrate the ABHR into their routine HH practices over the ensuing 30-day period. Concurrently, participants completed the “Part 2 questionnaire” of the WHO protocol for evaluating the tolerability and acceptability of ABHRs [22], which was intended for subjective evaluation. The inaugural session also included the first objective evaluation of skin tolerability conducted by trained observers. On day three or five, participants returned the completed “part 2 questionnaire” and the bottles for the quantification of the amount of the product utilized. The second objective skin evaluation was conducted by trained observers. At the conclusion of the 30-day period, ABHR bottles were collected for quantification of product consumption, and part 1 and part 2 questionnaires were completed and returned. Additionally, the last objective skin evaluation was conducted by trained observers.
During the study, when the quantity of ABHR for each participant was reduced to approximately 50 ml, participants alerted the trained observer and later returned the empty bottles for recording of the quantity and replacement with a new unlabeled 500 ml ABHR bottle. The use of unlabeled ABHR bottles throughout was important to reducing demand characteristics bias and improving the objectivity of participants.
The WHO protocol included questionnaires divided into four parts; Part 1 (demographic information, influencing skin tolerance and hand hygiene practices), Part 2 (product evaluation for tolerability and acceptability, additional hand hygiene practices), Part 3 (objective skin evaluation for tolerability) and a control form for each sessions and amount of ABHR used.
Outcomes
The endpoints of this study were the acceptability and tolerability of the test product, evaluated through a combination of subjective and objective measures according to the WHO single-product evaluation protocol (Method 1) [22]. In summary, using a seven-point Likert scale, participants evaluated the products:
-
Color (“unpleasant”, “pleasant”).
-
Smell (“unpleasant”- “pleasant”),
-
Texture (“sticky”, “nonsticky”),
-
Irritation (“very irritating”- “not irritating”).
-
Drying effect (“very much”, “not at all”),
-
Ease of use (“very difficult”- “very easy”).
-
Speed of drying (“very slow”, “very fast”),
-
Application (“unpleasant”, “pleasant”).
-
Overall evaluation (“dissatisfied”- “satisfied”).
Similarly, participants used a seven-point Likert scale to evaluate the condition of their skin on their hands:
-
appearance (“abnormal”- “normal”);
-
Intactness (“abnormal”- “normal”);
-
Moisture content (“abnormal”- “normal”);
-
Sensation (“abnormal”- “normal”);
-
Overall integrity of the skin (“very altered”-“perfect”).
The skin condition was also evaluated by an observer in the following manner:
-
Redness (0–4, no redness-very bright with edema);
-
Scaliness (0–3, no scale-very pronounced separation from skin);
-
Fissures (0–3, no fissure-extensive cracks with bleeding or seeping).
-
Overall visual scoring of the skin condition:
-
0: no observable scale or irritation of any kind.
-
1: occasional scale that is not necessarily uniformly distributed;
-
2: dry skin and/or redness;
-
3: Very dry skin with a whitish appearance, rough to touch, and/or redness but without fissures;
-
4: Cracked skin surface without bleeding/seeping;
-
5: Extensive cracking of the skin surface with bleeding/seeping).
-
The criteria for skin tolerability and product acceptability were as follows:
-
criteria for skin tolerability
-
Subjective (self) evaluation of the state of skin on hands: ≥75% of participants had to score > 4.
-
Objective evaluation of the state of skin on hands by the observer: 75% of participants had a score < 2.
-
-
criteria for product acceptability
-
Product evaluation for “Color” & “Smell” ≥50% of participants score > 4.
-
Product evaluation for “Other items”: ≥75% of participants score > 4.
-
Study variables
To investigate the factors influencing skin tolerance, we collected sociodemographic data and detailed information on the factors that might affect skin health. These data were gathered through Part 1 and Part 2 of our questionnaire, which included questions on age, sex, skin color, and the current climate and season. We also considered nonwork-related activities that could damage the skin, the use of hand lotions outside the study period, and a history of skin conditions or allergies such as irritative dermatitis, atopic dermatitis, allergic rhinitis or conjunctivitis, and asthma. Participants were asked about any known intolerance to ABHRs. We also inquired about participants’ employment status (full-time or part-time), duration of ABHR use, perceptions of improving hand hygiene compliance, and potential barriers to using ABHR products, such as forgetfulness, lack of time, or skin damage.
The evaluation of HH practice frequency involved recording the number of consecutive working days the test product was used, the number of direct patient contacts per working day, HH compliance rates, and whether participation in the present study influenced participants’ HH behaviours. Additionally, we assessed how often participants practiced HH during an average working hour.
Statistical analysis
The study utilized the WHO Multimodal Hand Hygiene Improvement Strategy toolkit [30] to guide the data capture, analysis, and interpretation. We performed double entry and data validation using Epi Info™ software (version 3.5.1) files developed specifically for the WHO single-product ABHR evaluation protocol to ensure quality-assured data capture. Categorical data are summarized using frequencies and proportions. The Shapiro‒Wilk test was used to determine the type of distribution of the numerical data, with a significance level of 0.05. Normally distributed data are summarized using the mean with standard deviation (SD) as the central variable, and nonnormally distributed data are summarized using the median and the interquartile range.
The percentage score was derived for the items in the WHO benchmark criteria for product tolerability and acceptability, and the mean quantity of ABHR used was derived for each professional category and expressed in millilitres (ml) per shift in days. Shifts in days were determined by the daily shift duration (hours) and working day census of health workers during the one-month study period.
The data were analyzed appropriately using Microsoft Excel (Microsoft Corporation, 2018) and R Statistical Software (version 4.3.0).
Results
Demographic characteristics of the study participants
In this study, we analyzed the demographic and professional characteristics of 60 health workers. The cohort comprised predominantly female health workers, 43 (72%) females and 17 (28%) males. The mean age of the participants was 35.5 years (SD = 7.5). Nurses composed the predominant professional cadre, representing 73% (n = 44), followed by medical doctors, at 10% (n = 6); students, at 3% (n = 2); and other healthcare professions, including laboratorians, pharmacists, and midwives, accounting for 13% (n = 8). The distribution of workplace settings among the participants varied, with the highest proportion working in medical wards (28%, n = 17), followed by maternity wards (20%, n = 12) and surgical wards (12%, n = 17). Regarding the use of ABHR products, a majority (53%, n = 32) reported utilizing these products for more than five years in their professional practice. Furthermore, 33% (n = 20) had been using ABHR products for between one and five years, while a minority, 13% (n = 8), had utilized these products for less than one year.
User skin tolerability of the WHO-formulated locally produced ABHR
Objective assessments revealed that the skin tolerability criterion score was less than 2 in at least 97% of participants across all three visits and for all assessment items, surpassing the WHO benchmark of achieving a score of less than 2 in 75% of participants. For the criteria “Redness” and “Visual scoring of skin”, observers recorded scores of less than 2 in 98% of participants during the initial visit, which increased to 100% in the subsequent two visits. The observer scores for “scaliness” were less than 2 for 98% of the participants at the first and second visits, and the scores for “fissures” remained less than 2 for 100% of the participants across all visits. Table 2 provides details on the outcomes of the objective evaluations performed throughout this study.
Subjective evaluations by participants further corroborated the high tolerability of the hand rub. During the second visit, 97% of participants rated their overall skin condition with scores of 4 or higher, which slightly increased to 98% at the third visit. Figure 1 provides a visual representation of the subjective evaluation scores regarding the state of the skin on hands by participants.
User acceptability of the WHO-formulated locally produced ABHR
Acceptability ratings exceeded 4 on a scale of 1 to 5 in more than 50% of participants during the second and third visits, with a notable increase in scores observed in subsequent visits. Specifically, the acceptability of the hand rub based on color improved, with positive responses increasing from 93% in the initial assessment to 95% at the follow-up visit. On the other hand, the criterion for smell witnessed a marginal enhancement, moving from an 87% approval rating to 88%. However, the drying effect of the hand rub presented a consistent challenge to user acceptability across both visits. Despite the high product satisfaction among health workers, the scores related to the drying effect of the hand rub did not meet the predetermined benchmark of acceptability, with only 52% of participants expressing satisfaction during the second visit and 58% during the third visit (Fig. 2; Table 3).
Hand hygiene practices and behaviours of health workers
At the second and third visits, 90% and 97%, respectively, of the participants reported using the tested product for at least five consecutive days. A significant proportion (88%, n = 53) of the participants acknowledged that the present study had changed their HH practice. Furthermore, 55% (n = 33) of the participants utilized the handrub for cleaning their hands during the last five opportunities for HH, and 63% (n = 38) confirmed adherence to HH practices in 90 to 100% of the recommended instances. Remarkably, 93% (n = 56) of the health workers expressed a preference for the tested product over the formulations routinely used in hospital settings. Despite this preference, 77% (n = 46) of the participants unequivocally expressed that the tested product could further improve their HH compliance. Nonetheless, barriers such as forgetfulness and time constraints were identified as obstacles to the consistent use of ABHR, which had median scores (IQRs) of 3 (2–5) and 4 (2-6.25), respectively (Table 4).
The mean daily consumption of alcohol-based hand rubs was 71.6 ml (SD ± 35), with a median usage of 65.1 ml per person per shift. Among the different professional cadres shown in Fig. 3, nurses and medical doctors were the highest consumers, with average daily usage values of 80.1 ml and 74.0 ml, respectively, while midwives and pharmacists reported the lowest consumption rates, averaging 36.4 ml and 33.1 ml, respectively.
Discussion
This study demonstrated that healthcare professionals exhibited a high level of tolerability and acceptance of ABHRs, in accordance with the WHO protocol for evaluating the tolerability and acceptability of ABHRs [15].
This study is important because it provides justification for the continuous utilization of an evidence-based, cost-effective intervention for a reliable supply of HH products at the point of patient care. This finding is in line with the WHO’s multimodal HH improvement strategy [11] and the recently published WHO research agenda for HH in health care [31]. The insights garnered from this study are particularly valuable for informing policy and strategic decisions to finance the production of local ABHR and inclusion of ABHR in the EML and EMLc countries to strengthen IPC programmes within healthcare settings in LMICs such as Sierra Leone. These settings often involve maintaining and optimizing IPC programs at both the national and facility levels with regard to the availability of essential consumables such as soap, ABHR, and personal protective equipment (PPE) [20, 32,33,34].
Our study showed that the locally produced ABHR met the criteria for user tolerability for all assessment items according to the WHO single-product evaluation protocol. The objective evaluation of the state of skin on hands by the trained observer showed increases in scores at each visit of up to 100% in proportion to a score > 2 (WHO benchmark), similar to the findings of a study by Wolsfensbergeret et al., who used the same WHO protocol [29]. Similarly, subjective evaluation of the state of the skin by health workers also underscored the product’s high tolerability, with “overall skin integrity” values of 97% and 98% at the second and third visits, respectively. These figures are in concordance with the findings of Tarka et al., where more than 95% of participants reported favorable tolerability outcomes [28]. Moreover, the self-assessment of skin condition by health workers consistently surpassed WHO benchmarks for each criterion for skin tolerability.
The test product met the acceptability threshold for all criteria except for the “drying effect”, which did not meet the requisite benchmark established by the WHO protocol. This benchmark requires more than 75% of the participants to rate the product with scores above 4. Contrary to our observations, Wolsfensbergeret et al. reported a satisfactory “drying effect” for their investigational product, albeit failing to meet WHO acceptability criteria for “texture” and “speed of drying” [29]. In our study, participants reported that the product had a strong “drying effect” on the skin of their hands, although this effect was not as pronounced as the drying effect associated with the use of soap and water [35]. In actuality, alcohol-based handrubs should not (if used correctly) dry their hands because they contain skin softeners (emollients). In the case of our test product, the locally produced ABHR (test product) was based on WHO formula 1 and contained 98% glycerol at a final concentration of 1.45% (v/v) to increase the ability of the product to be sent to hands. However, the production guidelines cautioned against strict adherence to concentration metrics to prevent less or more humectant effects that may result in dryness, dermatitis or stickiness of the hands, as reported in other previous studies [29, 36, 37]. This observation from our study possibly suggested the need for special consideration of the concentration of glycerol recommended in the WHO Formal 1. Findings in support of using lower glycerol concentrations were reported in another study that recommended 0.5% glycerol instead of 1.45% glycerol in tropical climate settings such as Sierra Leone [36]. Interestingly, our study showed that “color” was more favorably perceived than “smell”, although both met the WHO acceptability standards; these findings echo those of other similar studies [28, 29]. However, anecdotal feedback from participants indicated a preference for mitigating the wine-like odor associated with the product’s ethanol content, suggesting that the addition of a fragrance may enhance acceptability while maintaining its efficacy and quality. This underscores the notion that optimal acceptability may be achieved by providing healthcare workers with a selection of products, allowing for personal preference and thereby increasing adherence to hand hygiene protocols [27].
Addressing the challenge of suboptimal HH compliance necessitates a multifaceted strategy that extends beyond simply ensuring access to essential supplies such as ABHR. The incorporation of supplementary interventions targeting the underlying determinants of compliance, including social influences, attitudes, and behaviours, is needed. This comprehensive approach aligns with the WHO advocacy for a multimodal strategy to improve HH practices [38].
Our study described the participants’ HH behaviour based on the set of HH promotion and practice variables on the same WHO protocol and verbal feedback recorded by observers during the evaluation sessions. Although most participants believed that they could improve their HH compliance, forgetfulness and lack of time were the major limitations, as previously reported in other studies [28, 39, 40]. During the study period, 55% of participants reported having used hand rubs to clean their hands during the last five HH opportunities. Notably, more than half (63%) of the health workers adhered to HHs in more than 90% of the recommended cases. This performance was better than that documented by a study conducted between 2007 and 2015 in which 42% of nurses reported usage of ABHRs in more than 90% of the opportunities [41]. Most of the participants in our study felt very positive about the test product and preferred it to the usual product used by the hospitals. Even though a significant proportion of the participants perceived that the test product could improve their HH compliance and enjoyed wearing the 500 ml bottle containing the test product in their bags or pockets, this perception does not automatically translate into consistent HH practices. This observed behaviour might be partially influenced by the participants’ awareness of being part of a study and their conscious efforts to comply with the study’s expectations, potentially leading to a distortion commonly referred to as the Hawthorne effect [42, 43]. The Hawthorne effect suggests that individuals alter their behaviour in response to their awareness of being observed, which could skew genuine practice patterns. Therefore, while the introduction of intervention might initially seem to enhance practice, such improvements should be cautiously interpreted, acknowledging the possible influence of observational biases on reported HH compliance rates. This underscores the importance of considering both the psychological and behavioural dimensions when evaluating the impact of HH interventions to ensure a holistic understanding of compliance dynamics within healthcare settings.
The mean quantity of ABHR products used during daily shifts was 71.6 ml (SD ± 35), and the median was 65.1 ml. Our study recorded greater consumption of the test product than did other studies [37, 44]. A double-blind, randomized, crossover trial of 3 hand rub formulations by Pittet et al. reported that the mean amount of product used per daily shift was 54.9 ml (SD ± 23.5 ml) [37]. Nurses (mean = 80.1 ml) and medical doctors (mean = 74.0 ml) in our study recorded the highest daily consumption of the tested product among the various professional categories of health workers. A possible inference is that nurses have more HH opportunities because of their greater patient contact time (average of 6–8 h). The cadres with the least consumption were the pharmacists (mean = 33.1 ml); this is in tandem with their minimal patient contact time, as they are not routinely involved in direct patient care, such as nurses and doctors. Conversely, midwives (mean = 36.4 ml) preferred to use soap and water instead of ABHR, likely because of their work environment (delivery rooms) and greater propensity for visibly soiled hands. Despite the high daily usage of the tested ABHR product among nurses and doctors, we cannot correlate consumption with hand hygiene compliance. However, in a recent study, the authors used multivariable regression analysis to show that direct observation of hand hygiene practices was independently associated with an increase in ABHR consumption [45]. In contrast, another study showed no correlation between HH compliance rates and the quantity of ABHR consumed [46].
This study has several methodological strengths. First, adherence to the WHO standardized protocol and quality benchmark criteria facilitates comparability with the findings of other studies and enhances the robustness of our findings. Additionally, consistency in the objective evaluation achieved by employing the same trained observers across all three visits improved the internal validity by minimizing observer bias in the objective assessments of participants. Finally, the inclusion of secondary and tertiary hospitals and a wide array of professional categories provided a comprehensive overview of hygiene behaviours among health workers.
Despite these strengths, this study has several limitations. The exclusive use of a single test product, as prescribed by Method 1 of the WHO protocol, precluded comparison against multiple products. To address this gap, we advocate for subsequent research utilizing Method 2 of the WHO protocol for the evaluation of tolerability and acceptability of ABHR, which allows for such comparative analysis. Additionally, this study does not validate product consumption as an indirect measure of hand hygiene best practices and behaviour, indicating the need for further research in this area.
Conclusion
This was the first study to evaluate the tolerability and acceptability of a WHO-formulated locally produced ABHR and to evaluate health workers’ HH practices. The product was highly tolerable and acceptable by health workers and surpassed the WHO quality benchmark for all assessment criteria except for its drying effect on the skin of the hands. The test product and the assessment potentially influenced health workers’ behaviour and practices, including high handrub consumption, based on increased HH performance. These findings indicate that a continuous supply of evidence-based, cost-effective interventions for HH would be pivotal for sustaining IPC programmes in LMICs, including Sierra Leone. Further research is warranted to optimize the product formulation for the skin tolerability drying effect; moreover, a robust behaviour and practice study is required to illuminate the association between ABHR consumption and true HH compliance.
Data availability
The data used in this paper is available upon reasonable request to the corresponding author.
Abbreviations
- ABHR:
-
Alcohol-based hand rub
- IPC:
-
Infection prevention and control
- HH:
-
Practice hand hygiene
- HAI:
-
Healthcare-associated infection
- WHO:
-
World Health Organization
- BMS:
-
WHO Benchmark score
References
Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci IS. 2012;7:17.
Abou Mrad Z, Saliba N, Abou Merhi D, Rahi A, Nabulsi M. Sustaining compliance with hand hygiene when resources are low: A quality improvement report. Nkomazana O, editor. PLOS ONE. 2020;15(11):e0241706.
World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care [Internet]. Geneva: World Health Organization. 2009 [cited 2023 Dec 23]. (WHO Guidelines Approved by the Guidelines Review Committee). http://www.ncbi.nlm.nih.gov/books/NBK144013/
Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–41.
Burke JP. Infection control — a problem for Patient Safety. N Engl J Med. 2003;348(7):651–6.
World Health Organization. Report on the burden of endemic health care-associated infection worldwide [Internet]. Geneva: World Health Organization. 2011 [cited 2023 Jun 26]. 40 p. https://apps.who.int/iris/handle/10665/80135
Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305–15.
Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL, Donaldson L, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641–52.
Tusabe F, Kesande M, Amir A, Iannone O, Ayebare RR, Nanyondo J. Bacterial contamination of healthcare worker’s mobile phones: a case study at two referral hospitals in Uganda. Glob Secur Health Sci Policy. 2022;7(1):1–6.
World Health Organization. Global report on infection prevention and control [Internet]. Geneva: World Health Organization. 2022 [cited 2023 Jun 26]. 148 p. https://www.who.int/publications-detail-redirect/9789240051164
Allegranzi B, Gayet-Ageron A, Damani N, Bengaly L, McLaws ML, Moro ML, et al. Global implementation of WHO’s multimodal strategy for improvement of hand hygiene: a quasi-experimental study. Lancet Infect Dis. 2013;13(10):843–51.
World Health Organization. Guide to local production: WHO-recommended handrub formulations [Internet]. World Health Organization; 2010 [cited 2023 Jun 26]. https://www.who.int/publications-detail-redirect/WHO-IER-PSP-2010.5
World Health Organization. eEML - Electronic Essential Medicines List [Internet]. [cited 2023 Jun 26]. https://list.essentialmeds.org/recommendations/215
Siddharta A, Pfaender S, Vielle NJ, Dijkman R, Friesland M, Becker B, et al. Virucidal Activity of World Health Organization–recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215(6):902–6.
World Health Organization. Hand hygiene for all initiative: improving access and behaviour in health care facilities [Internet]. World Health Organization. 2020 [cited 2023 Jun 26]. https://www.who.int/publications-detail-redirect/9789240011618
Bauer-Savage J, Pittet D, Kim E, Allegranzi B. Local production of WHO-recommended alcohol-based handrubs: feasibility, advantages, barriers and costs. Bull World Health Organ. 2013;91(12):963–9.
Tusabe F, Lamorde M, Medley A, Kesande M, Lozier MJ, Yapswale S, et al. Establishment of district-led production of WHO-recommended alcohol-based Hand Rub (ABHR) during the COVID-19 pandemic: a model for improving access to ABHR during health emergencies. J Water Sanit Hyg Dev. 2023;13(10):847–56.
Gould DJ, Moralejo D, Drey N, Chudleigh JH, Taljaard M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2017;2017(9):CD005186.
Moiwo MM, Kamara GN, Kamara D, Kamara IF, Sevalie S, Koroma Z, et al. Have Hand Hygiene practices in two Tertiary Care hospitals, Freetown, Sierra Leone, Improved in 2023 following operational research in 2021? Trop Med Infect Dis. 2023;8(9):431.
Lakoh S, Maruta A, Kallon C, Deen GF, Russell JBW, Fofanah BD, et al. How Well Are Hand Hygiene Practices and Promotion Implemented in Sierra Leone? A cross-sectional study in 13 public hospitals. Int J Environ Res Public Health. 2022;19(7):3787.
Lakoh S, Firima E, Williams CEE, Conteh SK, Jalloh MB, Sheku MG, et al. An Intra-COVID-19 Assessment of Hand Hygiene Facility, Policy and Staff Compliance in two hospitals in Sierra Leone: is there a difference between Regional and Capital City hospitals? Trop Med Infect Dis. 2021;6(4):204.
World Health Organization. WHO protocol-for-evaluation-of-tolerability and acceptability of handrub-meth1 - Google Search [Internet]. 2020 [cited 2023 Jun 26]. https://www.google.com/search?hl=en&q=WHO+protocol-for-evaluation-of-tolerability+and+acceptability+of+handrub-meth1
Tusabe F, Nanyondo J, Lozier MJ, Kesande M, Tumuhairwe O, Watsisi M, et al. Improving Access to WHO formulations of Alcohol-based Hand Rub in Healthcare Facilities: a district-wide Approach. Am J Trop Med Hyg. 2023;109(1):191–200.
Ndegwa L, Hatfield KM, Sinkowitz-Cochran R, D’Iorio E, Gupta N, Kimotho J, et al. Evaluation of a program to improve hand hygiene in Kenyan hospitals through production and promotion of alcohol-based Handrub – 2012–2014. Antimicrob Resist Infect Control. 2019;8:2.
Selam MN, Bayisa R, Ababu A, Abdella M, Diriba E, Wale M, et al. Adequacy of alcohol-based handrub solution production practice in response to COVID-19 in public hospitals found in Addis Ababa, Ethiopia: a multicentered cross-sectional study. J Pharm Policy Pract. 2021;14(1):39.
Fofanah B, Tengbe S, Kamara I, Korjie MM. Local production of alcohol-based hand rub to optimize hand hygiene facility in healthcare settings during COVID-19. Antimicrob Steward Healthc Epidemiol. 2022;2(S1):s47–8.
Peters A, Cave C, Carry J, Sauser J, Pittet D. Tolerability and acceptability of three alcohol-based hand-rub gel formulations: a randomized crossover study. J Hosp Infect. 2022;123:112–8.
Tarka P, Gutkowska K, Nitsch-Osuch A. Assessment of tolerability and acceptability of an alcohol-based hand rub according to a WHO protocol and using apparatus tests. Antimicrob Resist Infect Control. 2019;8(1):191.
Wolfensberger A, Durisch N, Mertin J, Ajdler-Schaeffler E, Sax H. Evaluating the tolerability and acceptability of an alcohol-based hand rub–real-life experience with the WHO protocol. Antimicrob Resist Infect Control. 2015;4:1–7.
Hand hygiene tools. and resources [Internet]. [cited 2024 Jan 10]. https://www.who.int/teams/integrated-health-services/infection-prevention-control/hand-hygiene/monitoring-tools
WHO research for hand hygiene in. health care 2023–2030: summary [Internet]. [cited 2024 Jan 9]. https://www.who.int/publications-detail-redirect/9789240073715
Fofanah BD, Abrahamyan A, Maruta A, Kallon C, Thekkur P, Kamara IF, et al. Achieving Minimum standards for Infection Prevention and Control in Sierra Leone: Urgent need for a Quantum Leap in Progress in the COVID-19 era! Int J Environ Res Public Health. 2022;19(9):5642.
Squire JS, Conteh I, Abrahamya A, Maruta A, Grigoryan R, Tweya H, et al. Gaps in Infection Prevention and Control in Public Health Facilities of Sierra Leone after the 2014–2015 Ebola Outbreak. Trop Med Infect Dis. 2021;6(2):89.
Kamara IF, Tengbe SM, Fofanah BD, Bunn JE, Njuguna CK, Kallon C, et al. Infection Prevention and Control in Three Tertiary Healthcare Facilities in Freetown, Sierra Leone during the COVID-19 pandemic: more needs to be done! Int J Environ Res Public Health. 2022;19(9):5275.
Boyce JM, Kelliher S, Vallande N. Skin irritation and dryness associated with two hand-hygiene regimens: soap-and-water hand washing versus hand antisepsis with an alcoholic hand gel. Infect Control Hosp Epidemiol. 2000;21(7):442–8.
Menegueti MG, Laus AM, Ciol MA, Auxiliadora-Martins M, Basile-Filho A, Gir E, et al. Glycerol content within the WHO ethanol-based handrub formulation: balancing tolerability with antimicrobial efficacy. Antimicrob Resist Infect Control. 2019;8(1):109.
Pittet D, Allegranzi B, Sax H, Chraiti MN, Griffiths W, Richet H, et al. Double-Blind, randomized, crossover trial of 3 Hand Rub formulations: fast-track evaluation of tolerability and acceptability. Infect Control Hosp Epidemiol. 2007;28(12):1344–51.
Huis A, van Achterberg T, de Bruin M, Grol R, Schoonhoven L, Hulscher M. A systematic review of hand hygiene improvement strategies: a behavioural approach. Implement Sci. 2012;7(1):92.
Pittet D. Compliance with hand disinfection and its impact on hospital-acquired infections. J Hosp Infect. 2001;48:S40–6.
Awoke N, Geda B, Arba A, Tekalign T, Paulos K. Nurses Practice of Hand Hygiene in Hiwot Fana Specialized University Hospital, Harari Regional State, Eastern Ethiopia: Observational Study. Nurs Res Pract. 2018;2018:2654947.
Kingston LM, Slevin BL, O’Connell NH, Dunne CP. Hand hygiene: attitudes and practices of nurses, a comparison between 2007 and 2015. Am J Infect Control. 2017;45(12):1300–7.
Chen LF, Vander Weg MW, Hofmann DA, Reisinger HS. The Hawthorne Effect in infection Prevention and Epidemiology. Infect Control Hosp Epidemiol. 2015;36(12):1444–50.
Purssell E, Drey N, Chudleigh J, Creedon S, Gould DJ. The Hawthorne effect on adherence to hand hygiene in patient care. J Hosp Infect. 2020;106(2):311–7.
Vicentini C, Libero G, Bordino V, Zotti CM. Hand Hygiene practices during the COVID-19 pandemic in Northern Italy: Assessment of Compliance Rates measured by Direct Observation and Alcohol-based handrub usage. Antibiot Basel Switz. 2022;11(11):1510.
Jw RF, R A. Y, S H. A multi-centre study of the effects of direct observation of hand hygiene practices on alcohol-based handrub consumption. Infect Prev Pract [Internet]. 2022 Oct 20 [cited 2024 Jan 8];4(4). https://pubmed.ncbi.nlm.nih.gov/36387608/
Zahar JR, Masse V, Watier L, Lanternier F, Degand N, Postaire M, et al. Is hand-rub consumption correlated with hand hygiene and rate of extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE)-acquired infections? J Hosp Infect. 2012;80(4):348–50.
Acknowledgements
This research was conducted through the partnership of the WHO country office, the Ministry of Health in Sierra Leone, and the WHO Regional Office for Africa. Recognizing the contribution of the national IPC program, IPC focal points and pharmacists at Princess Christian Maternity Hospital, Kingarman Maternal and Child Hospital, Connaught Hospital, and 34 Military Hospital in Sierra Leone.
Funding
The Government of the United Kingdom of Great Britain, represented by its Foreign, Commonwealth & Development Office (FCDO), has contributed designated funding for the production and evaluation of the local alcohol-based handrub in Sierra Leone under the Saving Lives in Sierra Leone (SLiSL) II project, implemented by the WHO Sierra Leone under award number 68282.
Author information
Authors and Affiliations
Contributions
B.D.F, I.F.K, and L.K designed the study. B.D.F was responsible for writing the original draft. S.M.T, S.L, R.K, V.K, R.M, I.N, A.M, C.K, T.M, C.N were responsible for visualization. B.D.F, I.F.K, J.S.K, M.K, B.S, A.K, B.G.H, and L.K were responsible for validation. B.D.F, I.F.K, S.M.T, R.K, S.L, and L.K contributed to the methods; and B.D.F and L.K performed the data analysis. All authors contributed to reviewing and editing the manuscript until final approval. BDF is the guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was sought from the Sierra Leone Ethics and Scientific Review Board (SLREC—013/03/2023) in Freetown, Sierra Leone. Participation was voluntary, and informed consent to participate was obtained from all of the participants. This study is a recognized component of infection prevention and control program implementation, and as such, health workers who volunteered to participate were evaluated for tolerability and acceptability of the ABHR product as they performed their routine duties.
Consent for publication
Not applicable.
Disclaimer
According to the WHO’s open-access publication policy for all work funded by the WHO or authored/coauthored by WHO staff members, permits unrestricted use, distribution and reproduction in any medium provided that the original work was properly cited. There is no suggestion that the WHO endorses any specific organization, product, or service. The views expressed in this article are those of the authors and do not necessarily reflect those of their affiliated institutions.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fofanah, B.D., Kamara, I.F., Kallon, C. et al. Evaluating the tolerability and acceptability of a locally produced alcohol-based handrub and hand hygiene behaviour among health workers in Sierra Leone: a longitudinal hospital-based intervention study. BMC Health Serv Res 24, 940 (2024). https://doi.org/10.1186/s12913-024-11368-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-024-11368-3