Abstract
Background
Agitation/delirium is commonly seen in children after anesthesia, and a proper dose of dexmedetomidine can prevent this complication. This study aimed to investigate the effects of different doses of Dexmedetomidine (DEX) on agitation/delirium and other complications in anesthetized children, providing clinical evidence for dose recommendations of DEX.
Methods
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A systematic search was conducted in the Cochrane Library, PubMed, Web of Science, and EMBASE. Two independent researchers performed literature screening, data extraction, and assessed the methodological quality. Data analysis was conducted using R and STATA 16.0.
Results
In the final analysis, 20 randomized controlled trials (RCTs) involving 2521 children were included. The results showed that in comparison to normal saline, 1 µg/kg, 1.5 µg/kg, and 2 µg/kg intranasal DEX significantly reduced the incidence of post-anesthetic emergence agitation in children with the most effective dose being 2 µg/kg (SUCRA = 0.91). Compared with normal saline, 1 µg/kg, 1.5 µg/kg, and 2 µg/kg intranasal DEX reduced patient’s need for postoperative analgesia, with the most effective dose being 1.5 µg/kg (SUCRA = 0.78). However, 1 µg/kg DEX performed the best in reducing Pediatric Anaesthesia Emergence Delirium (PAED) Scale score (SUCRA = 0.88).
Conclusion
Compared with normal saline, intranasal administration of 2 µg/kg DEX and 1.5 µg/kg DEX are the optimal doses to reduce the incidence of agitation and the need for postoperative pain relief in children under general anesthesia. Given effectiveness and safety, intranasal use of 1 µg/kg DEX appears to be the most effective dosage for anesthetized children.
Similar content being viewed by others
Background
It is common for pediatric patients to feel nervous and anxious during anesthesia and operation, with the incidence of severe preoperative anxiety reaching up to 60% [1]. This may be due to the unfamiliar operating room environment and the fear of being separated from their parents before operation. In this case, the pediatric patients tend to experience emotional outbursts and refuse to cooperate, which not only increases the incidence of anesthesia risks and adverse events, but also increases the negative emotions of the patients and their family members [2]. Research indicates that preoperative anxiety can result in postoperative agitation in children, as well as have adverse psychological and behavioral effects [3]. Emergence agitation (EA) is a postoperative complication that occurs during the emergence period of general anesthesia. It refers to a state in which pediatric patients experience dissociation between their consciousness and behavior. EA can trigger severe complications such as vomiting, aspiration, and even laryngospasm [4]. Emergence delirium (ED) represents a behavioral disorder in children characterized by crying, fear, instability, and disorientation in the early stages of anesthesia recovery. Improper anesthesia techniques and postoperative airway obstruction and pain significantly contribute to the occurrence of these complications [5, 6]. Therefore, the use of effective interventions to promote cooperation, ensure clinical safety, enhance overall comfort, and reduce postoperative adverse reactions has become a focal point in anesthesia research and efforts.
Currently, numerous interventions have been studied for managing EA and emergence delirium, including pharmacological and behavioral approaches [7]. Among these interventions, Dexmedetomidine (DEX)is a highly selective α2-adrenergic receptor agonist. It possesses sedative, hypnotic, anxiolytic, and analgesic properties while maintaining a short half-life, providing easily reversible sedation without respiratory depression [8]. It achieves maximum average blood drug concentration within approximately 37 min after administration via the intranasal route, which is well-tolerated without causing pain or irritation. In 1999, it was first approved by the FDA for sedation in critically ill patients. DEX provides sedation and anxiolysis by acting on α2 receptors located in the pontine locus coeruleus, and it exerts dose-dependent analgesic effects through binding to α2 receptors in the dorsal horn and upper spinal cord. In recent years, DEX has been widely used for preventing EA and ED in pediatric patients, although dose-related side effects such as hypotension and bradycardia may increase [9].
DEX holds significant potential in various clinical scenarios. To ensure the safe use of DEX, it is necessary to carefully determine appropriate dosages. Therefore, this systematic review aimed to observe the effects of intranasal DEX at different doses on the occurrence of emergence agitation or delirium in children undergoing general anesthesia.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10], and this study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (https://www.crd.york.ac.uk/PROSPERO), with the ID of CRD42023441872.
Inclusion and exclusion criteria
Based on the definitions of participants, interventions, comparators, outcomes, and study design (PICOS) in the Cochrane Handbook for Systematic Reviews of Interventions, the following inclusion and exclusion criteria were formulated.
Inclusion criteria
(1) Participants (P): Children assessed as American Society of Anesthesiologists (ASA) physical status I-III, < 12 years old, scheduled for examination or surgery under general anesthesia, and without known allergies to DEX.
(2) Intervention (I): Experimental group receiving intranasal administration of DEX.
(3) Comparator (C): Control group receiving different doses of intranasal DEX or normal saline compared to the experimental group.
(4) Outcomes (O): Incidence of emergence delirium, Pediatric Anesthesia Emergence Delirium (PAED) score, Postanesthesia care unit (PACU) stay time, postoperative length of hospital stay, postoperative gastrointestinal adverse reactions, parent’s satisfaction, analgesics, and postoperative pain scores.
(5) Study Types (S): Randomized controlled trials.
Exclusion criteria
(1) DEX combined with other narcotics.
(2) Reviews, expert consensus, in vitro studies, animal experiments, case reports, letters, and replies.
(3) Data that contain significant errors or missing information, and it is not possible to contact the corresponding author of the literature.
Search strategy
We conducted a systematic search in PubMed, Embase, Cochrane Library, and Web of Science databases for relevant studies published from the inception of databases up to June 25, 2023. The keywords used were (“Dexmedetomidine”) and (“Child” or “Adolescent” or “Pediatrics” or “Youth” or “Teen”), without restrictions on region or language. Table S1 describes the detailed search strategies for each database.
Literature screening and data extraction
Two researchers (Hu Wei and Wang Ming) strictly followed the inclusion criteria to screen articles and extract data and cross-checked their findings. In cases of disagreement, a third researcher (Sun Fei) joined the discussion for consensus.
The retrieved articles were imported into Endnote X9 for management and screened by two researchers (Hu Wei and Wang Ming). Duplicate articles were firstly removed manually and by machine marking. Then, the two researchers screened the remaining articles independently by reading the title, abstracts and full texts according to the pre-designed inclusion and exclusion criteria. After obtaining the full texts of the articles meeting the inclusion criteria, two researchers independently extracted the following data: (1) basic study characteristics such as authors, publication year, country, patient source, age, gender, and sample size; (2) key elements for assessing bias risk; and (3) outcome measures.
Risk of bias in included studies
The quality assessment of included studies was independently conducted by two researchers (Hu Wei and Wang Ming) using Version 2 of the Cochrane tool (RoB2) [11]. Any discrepancies were resolved through consultation with a third reviewer (Sun Fei). The assessment comprised five domains: bias in the randomization process, bias due to deviations from intended interventions, bias caused by missing outcome data, bias in outcome measurement, and bias in the selection of reported results. Each included study was evaluated according to the above criteria. If the five domains of a study were assessed as low risk of bias (RoB), the overall RoB was evaluated as low risk. If all five domains were not assessed as high RoB, but one of the domains was rated as having a possibility of RoB, the overall RoB was classified as unclear risk. If one of the five domains was assessed as high RoB, or multiple domains were graded as unclear RoBs and have a great impact on the credibility of the study results, the overall RoB was high [12].
Statistical analysis
All the data analyses in this study were performed by Stata 15.1 and R software (VER. 4.0.3) and Rstudio. For dichotomous variables, we calculated the risk ratio (RR) with corresponding 95% confidence intervals (CI). For continuous variables with the same scale and unit, we used the mean difference (MD) with corresponding 95%CI. In cases where the units were not uniform or different scales were used, we employed the standardized mean difference (SMD) with corresponding 95%CI. Given the heterogeneity among different experiments, a Bayesian random-effects model was employed for multiple comparisons of various treatment regimens of intranasal DEX on postoperative agitation or delirium and other complications in pediatric patients under general anesthesia. We employed a Markov Chain Monte Carlo method for modeling, with four Markov chains running simultaneously and setting the number of annealing iterations to 20,000. The modeling process was completed after 50,000 simulation iterations. The deviance information criterion (DIC) was used to compare whether the overall consistency and inconsistency of model fitting are unified. If the DIC value is less than 5, the overall consistency and inconsistency are unified, and vice versa. If there were closed loops, we further analyzed local consistency using node-splitting. Additionally, we ranked the interventions based on Surface Under the Cumulative Ranking curve (SUCRA), which shows the likelihood of each treatment being the best invention and generated a league table for comparison of treatment efficacy among various interventions [13, 14]. The closer the SUCRA value is to 100%, the higher the ranking of a therapy, and the better the intervention effect. The funnel plot was used to visualize the publication bias of the study.
Results
Systemic Retrieval results
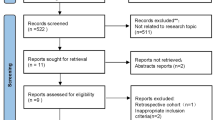
A total of 10,232 articles were obtained from the databases. After removing duplicates (n = 3408), 6824 studies remained. By excluding irrelevant articles (n = 6752) based on titles and abstracts, 72 articles were retained. Among these, full text could not be found for one article, and after reading the remaining 71 articles, 51 articles that did not meet the inclusion criteria were excluded. Finally, 20 RCTs were included in this network meta-analysis [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The flowchart is shown in Fig. 1.
Basic Information of included studies
A total of 2521 children younger than 18 years old from 20 RCTs were included in this network meta-analysis, with a sex ratio (Man/Female) of 1607: 914. Among the included studies, 10 articles were from China [20, 24,25,26, 28, 30,31,32,33,34], 4 from India [19, 22, 23, 29], 3 from Egypt [17, 18, 21], 1 from United States of America [27], 1 from Australia [15] and 1 from Saudi Arabia [16]. Table 1 presents the baseline characteristics of each trial, and Fig. 2 displays the network plot of different interventions on the outcomes.
Risk of bias assessment results
All the 20 trials described the process of sequence generation. No reports were considered to have a high or unclear RoB in randomization process, deviations from intended interventions or missing outcome data. In the measurement of the outcome, 6 studies [16, 21, 22, 29, 32, 33] (28.6%) had an unclear RoB. Regarding the selective reporting, 8 studies [21,22,23,24, 26, 27, 31, 33] (47.6%) had a moderate RoB.
In terms of the overall bias, 42.9%, 57.1%, and 0% of studies had a low RoB, moderate RoB, and high RoB, respectively. The risks were mainly caused by the measurement methods of the outcome in 6 articles [16, 21, 22, 29, 32, 33] and selective reporting in 8 articles [21,22,23,24, 26, 27, 31, 33].
According to the quality assessment tool, all studies were of good quality. The RoB of the included studies is shown in Fig. 3.
Network Meta-analysis
The network plot is shown in Fig. 2: emergence agitation (A), PAED score (B), PACU stay time (C), postoperative length of hospital stay (D), postoperative gastrointestinal adverse reactions (E), parent’s satisfaction score (F), use of analgesics (G) and postoperative pain score (H).
Each dot represents different interventions, and the size of the dot represents the number of articles involved in the relevant intervention. The larger the dot, the more studies involved in the relevant intervention. The line indicates the comparison between interventions, and the thickness of the line indicates the number of related articles. The thicker the line, the more articles involved in the two related interventions.
Emergence agitation
Nine studies [16, 18, 20, 21, 24, 26, 27, 32, 34] reported the incidence of emergence agitation. The network meta-analysis revealed that after compared to normal saline, intranasal DEX intervention at 1 µg/kg [RR = 0.305, 95%CI= (0.154, 0.579)], 1.5 µg/kg [RR = 0.276, 95%CI= (0.085, 0.905)], and 2 µg/kg [RR = 0.163, 95%CI= (0.059, 0.373)] significantly decreased the incidence of emergence agitation. However, 0.5 µg/kg of DEX had a higher incidence of emergence agitation compared to 2 µg/kg [RR = 4.49, 95%CI= (1.124, 24.37)]. There was no significant difference between other pairwise interventions (Table S2).
PAEDs
Six studies reported the PAEDs [16, 18, 25, 28, 31, 33]. The meta-analysis revealed that compared to normal saline, intranasal DEX at 1 µg/kg [MD=-3.20, 95%CI= (-5.39, -1.00)] significantly reduced the PAEDs, and the difference was statistically significant. However, there was no statistically significant difference between DEX at 2 µg/kg [MD=-1.83, 95%CI= (-3.92,0.26)] or 0.3 µg/kg [MD=-0.14, 95%CI= (-3.80,3.52)] and normal saline. No statistically significant differences were observed in the pairwise comparison of different doses (Table S3).
PACU stay time
Six studies [16, 24, 26, 28, 32, 33] reported the PACU stay time. The meta-analysis revealed that compared to normal saline, intranasal DEX at 1.5 µg/kg [SMD = 0.83, 95%CI= (0.17, 1.5)] and 2 µg/kg [SMD = 0.59, 95%CI= (0.15, 1.02)] significantly prolonged the PACU stay time. Pairwise comparisons were conducted between different doses of DEX, and only the differences between intranasal DEX at 0.5 µg/kg and DEX at 1.5 µg/kg [SMD=-1.03, 95%CI= (-1.78, -0.28)] and between DEX at 0.5 µg/kg and 2 µg/kg [SMD=-0.79, 95%CI= (-1.46, -0.12)] were statistically significant. However, there was no statistically significant difference between DEX at 0.5 µg/kg [SMD=-0.2, 95%CI= (-0.86, 0.46)] or 1 µg/kg [SMD = 0.19, 95%CI= (-0.27, 0.65)] and normal saline (Table S4).
Postoperative length of hospital stay
Four studies [21, 24, 28, 34] reported postoperative length of hospital stay. The meta-analysis revealed no statistically significant differences (Table S5).
Postoperative gastrointestinal adverse reactions
Seven studies [16, 21, 25, 26, 28, 33, 34] reported the incidence of postoperative gastrointestinal adverse reactions. Among them, five studies [16, 25, 26, 28, 34] reported vomiting and three studies [21, 33, 34] reported vomiting and nausea. The meta-analysis revealed no statistically significant differences (Table S6).
Parental satisfaction
Three studies [31,32,33] reported parent’s satisfaction. The overall meta-analysis demonstrated that at doses of 1 µg/kg [SMD = 1.46, 95%CI= (0.57, 2.36)] and 2 µg/kg [SMD = 2.21, 95%CI= (1.3, 3.1)], intranasal DEX showed an increase in parent’s satisfaction scores compared to normal saline. No statistically significant differences were observed in the pairwise comparison of different doses (Table S7).
Analgesics
Six studies [16, 20, 21, 24, 27, 28] reported the use of analgesics, of which two studies [14, 25] focused on the use of acetaminophen, one study [20] on the use of remifentanil, one study [21] on 1% lidocaine, one [24] on nalbuphine, and one [28] on fentanyl. The meta-analysis demonstrated that at doses of 1 µg/kg [RR = 0.22, 95%CI= (0.082, 0.569)], 1.5 µg/kg [RR = 0.128, 95%CI= (0.011, 0.978)], and 2 µg/kg [RR = 0.241, 95%CI= (0.061, 0.656)], intranasal DEX improved the use of analgesics compared to normal saline. However, no statistically significant difference was found between 0.5 µg/kg DEX [RR = 0.283, 95%CI= (0.042, 1.791)] and normal saline. No statistically significant differences were observed in the pairwise comparison of four doses (Table S8).
Postoperative pain scores
Six studies [16, 21, 25, 26, 28, 33] reported on postoperative pain. Two of these studies [14, 19] used the Face, Legs, Activity, Cry and Consolability (FLACC) scale to evaluate pain, three [25, 26, 33] adopted the Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS), and one [28] employed the Wong-Baker Faces Pain Rating Scale. No statistically significant differences were found (Table S9).
SUCRA Ranking results
The ranking results of the SUCRA showed that the top three regimens for emergence agitation were 2 µg/kg DEX, 1.5 µg/kg DEX, and 1 µg/kg DEX, then 0.3 µg/kg DEX, 0.5 µg/kg DEX, and NS successively; the top three regimens for PAEDs were 1 µg/kg DEX, 2 µg/kg DEX, and 0.3 µg/kg DEX, with NS ranking the last; the top three regimens for PACU stay time were 0.5 µg/kg DEX, NS, and 1 µg/kg DEX, then 2 µg/kg DEX, and 1.5 µg/kg DEX successively; the top three regimens for postoperative hospital stay were 0.5 µg/kg DEX, 1 µg/kg DEX, and 1.5 µg/kg DEX, then NS, and 2 µg/kg DEX; The top three regimens for reducing postoperative gastrointestinal adverse reactions were 1.5 µg/kg DEX, 2 µg/kg DEX, and 1 µg/kg DEX, with NS ranking the last; the top three regimens for parental satisfaction were 2 µg/kg DEX, 1 µg/kg DEX, and NS; the top three regimens for requiring analgesics were 1.5 µg/kg DEX, 1 µg/kg DEX and 2 µg/kg DEX, then 0.5 µg/kg DEX, and NS; the top three in the postoperative pain score were 2 µg/kg DEX, 1 µg/kg DEX, and NS. Details are shown in Fig. 4 and Table S10. The cumulative probability suggests that there may be shorter PACU stay and shorter postoperative hospital stay with 0.5 µg/kg DEX; lower PAED scores with 1 µg/kg DEX; fewer analgesics and lower incidence of postoperative gastrointestinal adverse reactions with 1.5 µg/kg DEX; less emergence agitation, higher parent’s satisfaction, and less postoperative pain with 2 µg/kg DEX.
Publication bias and inconsistency
To assess publication bias, we created funnel plots using STATA software for outcome measures that were included in at least five studies. Different colors were used to indicate the comparisons between interventions. The funnel plot showed that there was no potential publication bias in the outcomes. The Deviance Information Criterion (DIC) and node-splitting analysis indicated no statistical inconsistency. Details are provided in Figure S1.
Discussions
To the best of our knowledge, this is the first Bayesian network meta-analysis focusing on the effects of different doses of DEX on postoperative agitation or delirium and other outcomes in anesthetized pediatric patients. In this systematic review and network meta-analysis, we incorporated direct and indirect evidence from 20 randomized controlled trials to compare the effects of intranasal DEX at doses of 0.3 µg/kg, 0.5 µg/kg, 1 µg/kg, 1.5 µg/kg, and 2 µg/kg on postoperative agitation or delirium and other outcomes in pediatric patients undergoing anesthesia. Our results indicate that the application of DEX in pediatric anesthesia is effective in reducing emergence agitation, PAED scores, PACU stay time and the use of additional anesthetics, and improving parent’s satisfaction, compared with the control group. In the comparison between different doses, we found that 0.5 µg/kg can significantly shorten the PACU stay time and lower the incidence of emergence agitation. The cumulative probability results show that in comparison of different DEX doses, 0.5 µg/kg DEX is associated with the shortest PACU stay, 1 µg/kg DEX with the lowest PAED scores; 1.5 µg/kg DEX with the least use of analgesics, and 2 µg/kg DEX with the lowest incidence of emergence agitation.
Zhang et al.‘s research revealed that nasal administration of DEX can reduce the incidence of agitation after inhalation anesthesia in pediatric patients, but the optimal dose is still uncertain [35]. Our research has shown that compared with the control group, 1 µg/kg DEX, 1.5 µg/kg DEX and 2 µg/kg DEX can significantly reduce the incidence of emergence agitation, with 2 µg/kg DEX showing the best performance. Aerosolized intranasal dexmedetomidine is an anesthetic strategy that can be used for sedation in pediatric patients without the need for vascular access during diagnostic procedures. Miller’s study showed that increasing the dose from 1 µg/kg to 2 µg/kg halved the time to mean arterial plasma concentration and almost doubled the mean peak plasma concentration. Higher doses of DEX may provide better sedation [36]. Besides, compared with 2 µg/kg DEX, 0.5 µg/kg DEX increased the incidence of agitation. Therefore, to reduce the incidence of emergence agitation in clinical practice, relatively higher doses of DEX are required under safe conditions.
We also found that 1 µg/kg DEX significantly reduced PAED scores, which may contribute to its neuroprotective properties as shown in a previous study [37]. DEX is an α2-adrenoceptor agonist [38], which exerts neuroprotective effects by α2-adrenoceptor-dependent or independent modulation of neuroinflammation, apoptosis, oxidative stress, and synaptic plasticity [37].
A meta-analysis by Zhang et al. demonstrated that DEX can reduce the use of postoperative analgesics in adult patients undergoing general anesthesia [39]. Similarly, we observed that all three doses of DEX (1 µg/kg, 1.5 µg/kg, and 2 µg/kg) reduced the use of analgesics in the pediatric population compared with the control group, Furthermore, we found that both 1 µg/kg and 2 µg/kg DEX exhibited similarly favorable results in terms of parent’s satisfaction. The cumulative probability results suggest that 1.5 µg/kg DEX may be the most ideal for reducing analgesic use, while 2 µg/kg DEX may provide the highest level of parent’s satisfaction. Infants and children experience pain in a manner similar to adults, with emotional factors particularly increasing their perception of pain, which is exactly where sedatives play a role [40, 41]. Although we couldn’t exactly explain the dose differentiation in pain due to the inclusion of patients of different age or disease background, and further research is needed to determine the effects of different doses on patients’ pain, it cannot be denied that 1 µg/kg, 1.5 µg/kg and 2 µg/kg DEX have an effect on pain relief in pediatric patients.
We also found that both 1.5 µg/kg and 2 µg/kg DEX prolonged PACU stay compared with normal saline. In addition, when comparing different doses, both 1.5 µg/kg and 2 µg/kg significantly prolonged PACU stay compared with 0.5 µg/kg. In contrast to our results, the study by Xu et al. showed that DEX did not reduce PACU stay compared with the control group [42]. This may be due to the relatively short duration of intranasal dexmedetomidine administration and outpatient surgery in some of the included studies.
The most common adverse events induced by DEX are hypotension and bradycardia, and cardiac arrest may occur when high-dose DEX is used [43]. Li et al. reported that 3 female patients suffered from bradycardia during intravenous (IV) dexmedetomidine injection under general anesthesia [44]. The use of dexmedetomidine can reduce the ventilatory responses to hypoxia and hypercapnia, and interact with the peripheral and central control of respiration, resulting in respiratory inhibition [45]. Although our results showed no significant difference in the incidence of postoperative gastrointestinal adverse reactions, the length of hospital stay, and postoperative pain score between the DEX group and the control group, the potential side effects of DEX should not be neglected.
This study has some limitations that currently cannot be resolved. Although we have carried out a comprehensive and systematic search, the relevant literature retrieved is still limited, so the age of the included population, the source of the drug, types of surgery and anesthesia, and the different administration times of DEX were not fully discussed. In addition, given the limitation of quantity, most of the treatment modalities included in this study were not directly compared, thus indirect comparisons played a crucial role in our study. Furthermore, although we carefully included the retrieved studies according to randomized controlled trials in the admission stage, the RCTs eventually included were small samples, single-center studies, which may lead to overestimating the efficacy and underestimating the risk of adverse reactions. In addition, there were few doses involved in the included intervention, so it was unfeasible to conduct multiple comparisons, and the effect of anesthesia was mostly affected by the patient’s own physical condition, so the results should be interpreted with caution.
Conclusions
In summary, our results have proved that intranasal DEX exhibits satisfactory sedative and analgesic effects, which can improve the quality of postoperative recovery in children under general anesthesia without increasing gastrointestinal adverse reactions. Therefore, given drug effectiveness and safety, a dose of 1 µg/kg intranasal DEX appears to be most effective for anesthetized children. More large-sample, multicenter clinical studies are warranted to further supplement the conclusions of this study.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- CI:
-
Confidence intervals
- DIC:
-
Deviance information criterion
- DEX:
-
Dexmedetomidine
- EA:
-
Emergence agitation
- ED:
-
Emergence delirium
- MD:
-
Mean difference
- PAED:
-
Pediatric Anesthesia Emergence Delirium
- PAEDs:
-
Pediatric anesthesia emergence delirium scale score
- PACU:
-
Postanesthesia care unit
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
Prospective Register of Systematic Reviews
- RoB:
-
Risk of bias
- SMD:
-
Standardized mean difference
- SUCRA:
-
Surface Under the Cumulative Ranking curve
- RoB2:
-
Version 2 of the Cochrane tool for assessing risk of bias in randomized trials
References
Sola C, Lefauconnier A, Bringuier S, Raux O, Capdevila X, Dadure C. Childhood preoperative anxiolysis: is sedation and distraction better than either alone? A prospective randomized study. Paediatr Anaesth. 2017;27(8):827–34.
Bevan JC, Johnston C, Haig MJ, Tousignant G, Lucy S, Kirnon V, Assimes IK, Carranza R. Preoperative parental anxiety predicts behavioural and emotional responses to induction of anaesthesia in children. Can J Anaesth = J Canadien D’anesthesie. 1990;37(2):177–82.
Ramayanti O, Juwana H, Verkuijlen SA, Adham M, Pegtel MD, Greijer AE, Middeldorp JM. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int J Cancer. 2017;140(1):149–62.
Kawai M, Kurata S, Sanuki T, Mishima G, Kiriishi K, Watanabe T, Ozaki-Honda Y, Yoshida M, Okayasu I, Ayuse T, et al. The effect of midazolam administration for the prevention of emergence agitation in pediatric patients with extreme fear and non-cooperation undergoing dental treatment under sevoflurane anesthesia, a double-blind, randomized study. Drug Des Devel Ther. 2019;13:1729–37.
Xiao Y, Jin X, Zhang Y, Huang T, Zhou L, Gao J. Efficacy of propofol for the prevention of emergence agitation after sevoflurane anaesthesia in children: a meta-analysis. Front Surg. 2022;9:1031010.
Tan Y, Shi Y, Ding H, Kong X, Zhou H, Tian J. µ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2016;26(2):139–50.
Mason KP. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. 2017;118(3):335–43.
Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59(2):263–8. discussion 269–270.
Gómez-Vázquez ME, Hernández-Salazar E, Hernández-Jiménez A, Pérez-Sánchez A, Zepeda-López VA, Salazar-Páramo M. Clinical analgesic efficacy and side effects of dexmedetomidine in the early postoperative period after arthroscopic knee surgery. J Clin Anesth. 2007;19(8):576–82.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Version 2 of the. Cochrane tool for assessing risk of bias in randomised trial (RoB2) [https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2/].
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58.
Trinquart L, Attiche N, Bafeta A, Porcher R, Ravaud P. Uncertainty in treatment rankings: reanalysis of Network Meta-analyses of Randomized trials. Ann Intern Med. 2016;164(10):666–73.
Lee-Archer PF, von Ungern-Sternberg BS, Reade M, Betts M, Haenke D, Keys A, Rance T, Gibbons K, Long D. Correction to: The effect of dexmedetomidine on postoperative behaviour change in children: a randomised controlled trial (Anaesthesia, (2020), 75, (1461–8), https://doi.org/10.1111/anae.15117). Anaesthesia 2021, 76(4):568.
Abdelaziz HMM, Bakr RH, Kasem AA. Effect of intranasal dexmedetomidine or intranasal midazolam on prevention of emergence agitation in pediatric strabismus surgery: a randomized controlled study. Egypt J Anaesth. 2016;32(3):285–91.
Ali RM, Mahmoud NMY. The effect of nebulized dexmedetomidine as sedative premedication in pediatrics undergoing cochlear implantation. Egypt J Anaesth. 2022;38(1):317–23.
Ali WA, Mohammed AK, Elshorbagy HM. Dexmedetomidine versus Ketofol effect on the incidence of emergence agitation associated with sevoflurane-based anesthesia in children undergoing orthopedic surgery. Egypt J Anaesth. 2016;32(3):277–84.
Anupriya J, Kurhekar P. Randomised comparison between the efficacy of two doses of Nebulised Dexmedetomidine for Premedication in Paediatric patients. Turkish J Anaesthesiol Reanimation. 2020;48(4):314–20.
Bi Y, Ma Y, Ni J, Wu L. Efficacy of premedication with intranasal dexmedetomidine for removal of inhaled foreign bodies in children by flexible fiberoptic bronchoscopy: a randomized, double-blind, placebo-controlled clinical trial. BMC Anesthesiol. 2019;19(1):219.
El-Hamid AMA, Yassin HM. Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy and/or adenoidectomy. Saudi J Anaesth. 2017;11(2):137–43.
Gupta A, Gupta H, Bhanotra K, Jamwal A, Gulati S. Comparative evaluation of efficacy of midazolam and dexmedetomidine as premedicants in children undergoing elective surgery. JK Sci. 2021;23(3):155–61.
Gyanesh P, Haldar R, Srivastava D, Agrawal PM, Tiwari AK, Singh PK. Comparison between intranasal dexmedetomidine and intranasal ketamine as premedication for procedural sedation in children undergoing MRI: a double-blind, randomized, placebo-controlled trial. J Anesth. 2014;28(1):12–8.
Lei DX, Wu CJ, Wu ZY, Wang LY, Zhao Q, She YJ. Efficacy of different doses of intranasal dexmedetomidine in preventing emergence agitation in children with inhalational anaesthesia: a prospective randomised trial. Eur J Anaesthesiol. 2022;39(11):858–67.
Li LQ, Wang C, Xu HY, Lu HL, Zhang HZ. Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Medicine. 2018;97(39):e12140.
Lin Y, Chen Y, Huang J, Chen H, Shen W, Guo W, Chen Q, Ling H, Gan X. Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J Clin Anesth. 2016;33:289–95.
Pestieau SR, Quezado ZM, Johnson YJ, Anderson JL, Cheng YI, McCarter RJ, Pena MT, Finkel JC. The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatr Anaesth. 2011;21(11):1128–35.
Shen F, Zhang Q, Xu Y, Wang X, Xia J, Chen C, Liu H, Zhang Y. Effect of Intranasal Dexmedetomidine or Midazolam for Premedication on the occurrence of respiratory adverse events in children undergoing Tonsillectomy and Adenoidectomy: a Randomized Clinical Trial. JAMA Netw open. 2022;5(8):e2225473.
Sidhu GK, Jindal S, Kaur G, Singh G, Gupta KK, Aggarwal S. Comparison of Intranasal Dexmedetomidine with Intranasal Clonidine as a premedication in surgery. Indian J Pediatr. 2016;83(11):1253–8.
Wang SS, Zhang MZ, Sun Y, Wu C, Xu WY, Bai J, Cai MH, Lin L. The sedative effects and the attenuation of cardiovascular and arousal responses during anesthesia induction and intubation in pediatric patients: a randomized comparison between two different doses of preoperative intranasal dexmedetomidine. Paediatr Anaesth. 2014;24(3):275–81.
Yao J, Gong H, Zhao X, Peng Q, Zhao H, Yu S. Parental presence and intranasal dexmedetomidine for the prevention of anxiety during anesthesia induction in children undergoing tonsillectomy and/or adenoidectomy surgery: a randomized controlled trial. Front Pharmacol. 2022;13:1015357.
Yao Y, Qian B, Lin Y, Wu W, Ye H, Chen Y. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr Anaesth. 2015;25(5):492–8.
Yao Y, Sun Y, Lin J, Chen W, Lin Y, Zheng X. Intranasal dexmedetomidine versus oral midazolam premedication to prevent emergence delirium in children undergoing strabismus surgery: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(12):1143–9.
Zhang S, Zhang R, Cai M, Zhang K, Zhang M, Zheng J. Intranasal dexmedetomidine premedication in children with recent upper respiratory tract infection undergoing interventional cardiac catheterisation: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(2):85–90.
Zhang X, Bai Y, Shi M, Ming S, Jin X, Xie Y. Effect of different administration and dosage of dexmedetomidine in the reduction of emergence agitation in children: a meta-analysis of randomized controlled trials with sequential trial analysis. Translational Pediatr. 2021;10(4):929–57.
Miller JW, Balyan R, Dong M, Mahmoud M, Lam JE, Pratap JN, Paquin JR, Li BL, Spaeth JP, Vinks A, et al. Does intranasal dexmedetomidine provide adequate plasma concentrations for sedation in children: a pharmacokinetic study. Br J Anaesth. 2018;120(5):1056–65.
Lv H, Li Y, Cheng Q, Chen J, Chen W. Neuroprotective effects against cerebral ischemic Injury exerted by Dexmedetomidine via the HDAC5/NPAS4/MDM2/PSD-95 Axis. Mol Neurobiol. 2021;58(5):1990–2004.
Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913.
Zhang J, Yu Y, Miao S, Liu L, Gan S, Kang X, Zhu S. Effects of peri-operative intravenous administration of dexmedetomidine on emergence agitation after general anesthesia in adults: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2019;13:2853–64.
Fantacci C, Fabrizio GC, Ferrara P, Franceschi F, Chiaretti A. Intranasal drug administration for procedural sedation in children admitted to pediatric emergency room. Eur Rev Med Pharmacol Sci. 2018;22(1):217–22.
Snyers D, Tribolet S, Rigo V. Intranasal Analgosedation for infants in the neonatal intensive care unit: a systematic review. Neonatology. 2022;119(3):273–84.
Xu C, Zhang Y, Zhang T, Wu D, Zhang K. Efficacy and safety of Intranasal Dexmedetomidine during Recovery from Sevoflurane Anesthesia in children: a systematic review and Meta-analysis. Clin Neuropharmacol. 2021;44(5):157–68.
Bharati S, Pal A, Biswas C, Biswas R. Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: a case series and review of published case reports. Acta Anaesthesiologica Taiwanica: Official J Taiwan Soc Anesthesiologists. 2011;49(4):165–7.
Li Y, Gao J, Jiang L, Sun C, Hong H, Yu D. Severe hypertensive response to Atropine Therapy for Bradycardia Associated with Dexmedetomidine: Case Report and Literature Review. Clin Pharmacol. 2024;16:27–31.
Lodenius Å, Ebberyd A, Hårdemark Cedborg A, Hagel E, Mkrtchian S, Christensson E, Ullman J, Scheinin M, Eriksson LI, Jonsson Fagerlund M. Sedation with dexmedetomidine or Propofol Impairs Hypoxic Control of Breathing in healthy male volunteers: a nonblinded, randomized crossover study. Anesthesiology. 2016;125(4):700–15.
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Wei Hu; Methodology: Wei Hu, Ming Wang; Formal analysis and investigation: Wei Hu, Ming Wang; Writing - original draft preparation: Wei Hu, Ming Wang; Writing - review and editing: Wei Hu, Ming Wang, Fei Sun; Resources: Fei Sun; Supervision: Fei Sun, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, W., Wang, M. & Sun, F. Effects of different doses of intranasal dexmedetomidine on related complications and parents’ satisfaction in anesthetized children: a systematic review. BMC Pediatr 24, 377 (2024). https://doi.org/10.1186/s12887-024-04832-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04832-w