Abstract
Background
The benefit of adding Zolbetuximab to the treatment in patients with Claudin-18 isoform 2 (CLDN18.2)-positive, human epidermal growth factor receptor 2-negative, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GC/GEJ) is not yet fully elucidated.
Methods
We searched PubMed, Embase and Cochrane databases for randomized controlled trials (RCTs) that investigated Zolbetuximab plus chemotherapy versus chemotherapy alone for GC or GEJ adenocarcinoma. We computed hazard-ratios (HRs) or odds-ratios (ORs) for binary endpoints, with 95% confidence intervals (CIs).
Results
Three studies and 1,233 patients were included. Comparing with Zolbetuximab plus chemotherapy versus chemotherapy alone, progression-free survival (PFS) rate (HR 0.64; 95% CI 0.49–0.84; p < 0.01) and overall survival (OS) rate (HR 0.72; 95% CI 0.62–0.83; p < 0.01) were significant in favor of the Zolbetuximab group. Regarding effectiveness, the Objective Response Rate (ORR) was (OR 1.15; 95% CI 0.87–1.53; p = 0.34).
Conclusions
In this comprehensive systematic review and meta-analysis of RCTs, the incorporation of Zolbetuximab alongside chemotherapy offers a promising prospect for reshaping the established treatment paradigms for patients diagnosed with advanced CLDN18.2-positive GC/GEJ cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric adenocarcinoma (GC) is the fifth most diagnosed type of cancer in the world and, together with adenocarcinomas of the gastro-oesophageal junction (GEJ) and oesophageal adenocarcinomas (EC), has a high mortality rate [1, 2]. Because of their aggressiveness and non-specific symptoms, these tumors are usually diagnosed at a late stage and the prognosis is poor, representing some of the greatest unmet medical needs [2,3,4]. The standard first-line therapy for human epidermal growth factor receptor 2-negative (HER2-), advanced, unresectable or metastatic GC/GEJ/EC is platinum-fluoropyrimidine-based chemotherapy [5,6,7,8,9]. Platinum-fluoropyrimidine-based chemotherapy resulting in a median overall survival (OS) duration of about 12 months [2, 10,11,12,13,14].
The addition of targeted therapies to chemotherapy improves survival for GC/GEJ, but there are currently few validated molecular targets for this scenario. Treatment with programmed death-ligand 1 (PD-L1) such as nivolumab is approved first-line in combination with chemotherapy in more than 50 countries, but its efficacy is linked to a combined positive score (CPS) of 5 or more [15,16,17]. The addition of pembrolizumab (PD-L1) to trastuzumab and first-line chemotherapy in the phase III KEYNOTE-811 [18] clinical trial showed an increase in objective response rate (ORR) of 74.4% versus 51.9% for trastuzumab and chemotherapy alone for GC. Currently an unmet need is the development of targeted therapies for patients with HER2-negative advanced, unresectable or metastatic GC/GEJ/EC.
Claudin-18 isoform 2 (CLDN18.2) is a tight junction protein that regulates the polarity and permeability of epithelial layers, which is frequently expressed in normal gastric mucosa cells and is maintained in the majority of GC/GEJ/EC adenocarcinomas [19,20,21,22]. During malignant transformation, cell polarity is lost, causing CLDN18.2 to become exposed on the cell surface and thus remain available for monoclonal antibody binding, making it a promising emerging therapeutic target [19, 23,24,25].
Zolbetuximab (IMAB363) is a first-in-class chimeric monoclonal immunoglobulin G1 antibody that targets CLDN18.2 and mediates tumour cell death by antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) in GC/GEJ/EC CLDN18.2-positive adenocarcinoma cells [23, 26]. Pre-clinical studies have shown significant therapeutic synergism when associated with cytotoxic agents [26]. The phase IIa MONO study showed anti-tumour action and good tolerability when Zolbetuximab was administered as a single agent in heavily pre-treated advanced GC/GEJ CLDN18.2-positive patients [24].
Therefore, in this systematic review and meta-analysis of randomized clinical trials (RCTs), we aim to clarify the benefits for progression-free survival (PFS), OS, response and safety using Zolbetuximab plus chemotherapy verus chemotherapy alone in advanced CLDN18.2-positive GC/GEJ/EC.
Methods
Protocol and registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [27]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42023455827.
Eligibility criteria
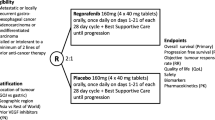
Studies that met the following eligibility criteria were included: (1) RCTs; (2) comparison of Zolbetuximab plus chemotherapy versus chemoterapy alone; (3) adult patients with histologically confirmed adenocarcinoma of the stomach, esophagus or GEJ; (4) locally advanced unresectable or metastatic disease; (5) CLDN18.2 expression confirmed by immunohistochemistry; (6) HER2 negative, HER2 unknown or HER2 positive status but not eligible to trastuzumab therapy; (7) Eastern Cooperative Oncology Group (ECOG) performance-status score of 0–1 (on a 5-point scale in which higher scores reflect greater disability; and (8) life expectancy ≥ 12 weeks. We excluded studies (1) with overlapping populations; (2) without any outcomes of interest; and (3) with unpublished complete results. Inclusion and exclusion criteria for the RCTs included in this systematic review and meta-analysis are detailed in Supplementary Table 1.
Thus, we sought to answer the following question: How effective is the addition of Zolbetuximab to chemotherapy versus chemotherapy alone for treatment of CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic GC or GEJ adenocarcinoma?
Search strategy and data extraction
Pubmed, Cochrane Library and Embase were systematically searched on August 20, 2023. The search strategy is detailed in Supplementary Table 1. In addition, backwards snowballing was performed aiming the inclusion of additional studies. Duplicate articles were removed, using both automated and manual methods. Subsequently, two reviewers (E.P. and M.P.C.) independently analyzed the titles and abstracts of the identified articles. Disagreements were resolved by consensus between the two authors and senior author (E.P., M.P.C. and R.M.R.B).
The following baseline characteristics were extracted: (1) ClinicalTrials.gov Identifier and study design; (2) number of patients allocated for each arm; (3) regimen details in experimental and control arm; and (4) main patient’s characteristics; (1) PFS, defined as the time from patient randomization to disease progression or death from any cause; (2) OS, defined as the period of time, from the randomization, that patients are still alive; (3) Radiographic response, according to the Response Evaluation Criteria for Solid Tumors (RECIST), version 1.1 [28] and (4) adverse events, defined as an unwanted effect of a treatment, which were evaluated by the Common Terminology Criteria for Adverse Events, version 5.0, in the included RCTs [29]. Two authors (E.P. and R.O.M.F.) collected pre-specified baseline characteristics and outcome data.
Endpoints
Outcomes of interest were: (1) PFS; (2) OS; (3) complete response (CR); (4) partial response (PR); (5) stable disease (SD); (6) progressive disease (PD); (7) objective response rate (ORR); (8) disease control rate (DCR); (9) any grade or grade ≥ 3 of all treatment-emergent events; patients with any grade or grade ≥ 3 of (10) nausea; (11) vomiting; (12) decreased appetite; (13) diarrhea; (14) neutropenia; (15) anaemia; (16) fatigue; (17) asthenia; (18) abdominal pain; (9 weight decreased; (20) pyrexia; (21) oedema peripheral; (22) aspartate aminotransferase increased; (23) alanine aminotransferase increased; and (24) thrombocytopenia.
Risk of bias assessment
The quality assessment of individual RCTs was carried out using the Cochrane Collaboration tool for assessing risk of bias in randomized trials (RoB 2) [30]. Two authors (E.P. and R.M.O.F.) independently conducted the assessment, and disagreements were resolved by consensus. For each trial, a risk of bias score was assigned, indicating whether it was at a high, low, or unclear risk of bias across five domains: randomization process, deviations from intended interventions, missing outcomes, measurement of outcomes, and selection of reported results [31].
Assessment of publication bias
Potential publication bias was evaluated through visual inspection of funnel plots and analysis of the control lines. No quantitative assessment of small studies or publication bias was performed due to the small number of included studies.
Sensitivity analyses
Leave-one-out procedures were used to identify influential studies and their effect on the pooled estimates, evaluating the heterogeneity. This procedure was carried out removing data from one study and reanalyzing the remaining data, confirming that the pooled effect-sizes did not result from a single-study dominance.
Statistical analysis
Binary endpoints were evaluated with Hazard-ratios (HRs) or odds-ratios (ORs), with 95% confidence intervals (CIs). The Cochrane Q-test and I2 statistics were used to assess heterogeneity; P values > 0.10 and I2 values > 25% were considered to indicate significance for heterogeneity [32]. The Sidik-Jonkman estimator was used to calculate the tau2 variance between studies [33]. We used DerSimonian and Laird random-effect models for all endpoints [34]. Statistical analyses were performed using R statistical software, version 4.2.3 (R Foundation for Statistical Computing).
Results
Study selection and baseline characteristics
The initial search yielded 241 results, as detailed in Fig. 1. After the removal of duplicate records, and the assessment of the studies based on title and abstract, 17 full-text remained for full review according to prespecified criteria. Of these, three RCTs were included [35,36,37], comprising 1,233 patients. A total of 614 patients with advanced CLDN18.2-positive GC/GEJ/EC were randomized to Zolbetuximab plus chemotherapy, while 619 received chemotherapy alone. The median age ranged from 57.0 to 62.0 years. 769 (62.4%) patients were men and 464 (37.6%) were female. 459 patients had diffuse classification and 255 had Lauren’s intestinal classification. The primary site of 987 patients was the stomach. 504 patients had an ECOG performance-status score of 0 and 716 patients had a score of 1. Study and participant characteristics are detailed in Table 1 and Supplementary Table S3.
Progression-free survival
PFS was significantly prolonged in patients randomized to receive Zolbetuximab plus chemotherapy versus chemotherapy alone (HR 0.64; 95% CI 0.49–0.84; p < 0.01; I²=59%; Fig. 2).
Overall survival
OS was significantly prolonged in patients randomized to receive Zolbetuximab plus chemotherapy versus chemotherapy alone (HR 0.72; 95% CI 0.62–0.83; p < 0.01; I²=31%; Fig. 3).
Radiographic response
Zolbetuximab plus chemotherapy significantly increased CR (OR 2.09; 95% CI 1.17–3.73; p = 0.01; I²=0%; Fig. 4C) and reduced PD (OR 0.52; 95% CI 0.28–0.96; p = 0.04; I²=40%; Fig. 4F). There was no significant different between groups for ORR (OR 1.15; 95% CI 0.87–1.53; p = 0.34; I²=30%; Fig. 4A), PR (OR 0.99; 95% CI 0.79–1.25; p = 0.95; I²=0%; Fig. 4D), SD (OR 0.79; 95% CI 0.60–1.04; p = 0.09; I²=0%; Fig. 4E), and DCR (OR 0.95; 95% CI 0.75–1.21; p = 0.68; I²=0%; Fig. 4B).
Radiographic response of patients with advanced CLDN18.2-positive gastric or gastro-oesophageal adenocarcinoma treated with Zolbetuximab plus chemotherapy versus chemotherapy alone. (A) Objective response rate (ORR). (B) Disease control rate (DCR). (C) Complete response (CR). (D) Partial response (PR). (E) Stable disease (SD). (F) Progressive disease (PD)
Safety
Zolbetuximab plus chemotherapy significantly increased any grade of nausea (OR 2.30; 95% CI 1.61–3.29; p < 0.01; I²=44%; Fig. 5A), vomiting (OR 3.27; 95% CI 2.10–5.09; p < 0.01; I²=68%; Fig. 5B), decrease appetite (OR 1.42; 95% CI 1.02–1.97; p = 0.04; I²=40%; Fig. 5C), and oedema peripheral (OR 2.49; 95% CI 1.52–4.06; p < 0.01; I²=20%; Fig. 5D). There was no significant difference between groups for any grade of all treatment-emergent events (OR 1.05; 95% CI 0.17–6.70; p = 0.96; I²=50%; Supplementary Figure S1), diarrhea (OR 0.73; 95% CI 0.51–1.06; p = 0.10; I²=53%; Supplementary Figure S2), neutropenia (OR 1.28; 95% CI 0.99–1.66; p = 0.06; I²=0%; Supplementary Figure S3), anaemia (OR 1.00; 95% CI 0.80–1.27; p = 0.97; I²=0%; Supplementary Figure S4), fatigue (OR 0.94; 95% CI 0.61–1.45; p = 0.78; I²=54%; Supplementary Figure S5), asthenia (OR 1.13; 95% CI 0.85–1.51; p = 0.39; I²=0%; Supplementary Figure S6), abdominal pain (OR 0.81; 95% CI 0.56–1.18; p = 0.28; I²=37%; Supplementary Figure S7), weight decrease (OR 1.34; 95% CI 0.81–2.23; p = 0.26; I²=64%; Supplementary Figure S8), pyrexia (OR 1.07; 95% CI 0.66–1.75; p = 0.78; I²=51%; Supplementary Figure S9), aspartate aminotransferase increased (OR 0.91; 95% CI 0.69–1.21; p = 0.53; I²=0%; Supplementary Figure S10), alanine aminotransferase increased (OR 0.78; 95% CI 0.57–1.06; p = 0.11; I²=0%; Supplementary Figure S11), or thrombocytopenia (OR 0.82; 95% CI 0.51–1.33; p = 0.43; I²=44%; Supplementary Figure S12).
In addition, Zolbetuximab plus chemotherapy significantly increased grade ≥ 3 of all treatment-emergent events (OR 1.44; 95% CI 1.09–1.90; p = 0.01; I²=8%; Supplementary Figure S13), nausea (OR 2.78; 95% CI 1.76–4.40; p < 0.01; I²=0%; Supplementary Figure S14), vomiting (OR 3.33; 95% CI 2.13–5.19; p < 0.01; I²=0%; Supplementary Figure S15), neutropenia (OR 1.55; 95% CI 1.08–2.22; p = 0.02; I²=14%; Supplementary Figure S16), asthenia (OR 2.48; 95% CI 1.24–4.95; p = 0.01; I²=0%; Supplementary Figure S17), and weight decrease (OR 2.69; 95% CI 1.02–7.15; p = 0.05; I²=0%; Supplementary Figure S18). There was no significant difference between groups for grade ≥ 3 of decrese appetite (OR 2.07; 95% CI 0.71–6.04; p = 0.18; I²=49%; Supplementary Figure S19), diarrhea (OR 1.00; 95% CI 0.59–1.68; p = 0.99; I²=0%; Supplementary Figure S20), anaemia (OR 1.00; 95% CI 0.68–1.46; p = 0.99; I²=0%; Supplementary Figure S21), fatigue (OR 1.13; 95% CI 0.65–1.94; p = 0.67; I²=0%; Supplementary Figure S22), abdominal pain (OR 0.88; 95% CI 0.22–3.48; p = 0.86; I²=44%; Supplementary Figure S23), pyrexia (OR 1.59; 95% CI 0.19–12.95; p = 0.67; I²=0%; Supplementary Figure S24), oedema peripheral (OR 3.90; 95% CI 0.43–35.44; p = 0.23; I²=0%; Supplementary Figure S25), aspartate aminotransferase increased (OR 0.79; 95% CI 0.36–1.73; p = 0.56; I²=0%; Supplementary Figure S26), alanine aminotransferase increased (OR 0.41; 95% CI 0.14–1.25; p = 0.12; I²=14%; Supplementary Figure S27), and thrombocytopenia (OR 0.86; 95% CI 0.38–1.93; p = 0.71; I²=0%; Supplementary Figure S28).
Sensitivity analyses and risk of bias
We performed leave-one-out sensitivity analyses for all outcomes. There was no significant difference in emergency events grade ≥ 3 omitting FAST 2021 or SPOTLIGHT 2023. There was a significant difference favoring the control group in decreasing appetite in both the general and the subgroup populations omitting FAST 2021. There was a significant difference favoring the control group in neutropenia in both the general and subgroup populations omitting SPOTLIGHT 2023. There was no significant difference in asthenia grade ≥ 3 omitting SPOTLIGHT 2023. There was a significant difference in favor of the intervention group for abdominal pain omitting FAST 2021. There was a significant difference in favor of the control group for weight decreased grade ≥ 3 omitting GLOW 2023. There was a significant difference in favor of the intervention group for ALT levels grade ≥ 3 FAST 2021. Leave-one-out sensitivity analysis of the main outcomes is detailed in Supplementary Figure S29.
Risk of individual within-study bias is represented in the Rob 2 traffic-light diagram (Supplementary Figure S30). All studies were considered low risk of bias.
Discussion
In this systematic review and meta-analysis involving 3 studies and 1,233 patients, we compared Zolbetuximab plus chermotherapy against chermotherapy only for patients with primary advanced or recurrent GC/GEJ/EC. The main findings from the pooled analyses were as follows: (1) PFS was improved in patients receiving Zolbetuximab; (2) OS showed a significant difference favoring Zolbetuximab; and (3) adverse events, particularly nausea and vomiting of any grade and grade 3 or more, increased in the Zolbetuzimab group.
Zolbetuximab (IMAB363) is a chimeric IgG1 monoclonal antibody with a high affinity for CLDN 18.2 present on the cell surface, which induces cell death due to antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity [23, 24, 38,39,40,41]. It was granted a priority review by the Food and Drug Administration (FDA) in 2023 for patients with locally advanced, inoperable or metastatic GC/GEJ with moderate to strong expression of CLDN18.2, HER2 negative GC or GEJ adenocarcinoma [42, 43].
A phase III study, CheckMate-649, showed that nivolumab improved PFS (HR 0.68; 98% CI 0.56–0.81; p < 0.0001) compared to chemotherapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (XELOX) for GC/GEJ cancer [16]. Additionally, the phase III ToGA study showed that trastuzumab plus chemotherapy improved PFS (HR 0.71; 95% CI 0.59–0.85; p < 0.0002) compared to chemotherapy alone in patients with HER2-positive, GC or GEJ cancer [44]. Therefore, similar to these studies, our meta-analysis shows a significant benefit with the addition of Zolbetuximab to chemotherapy (HR 0.64; 95% CI 0.49–0.84; p < 0.01) in patients with GC/GEJ cancer.
Regarding OS, our results show statistical significance in favor of the Zolbetuximab group for patients with CLDN18.2-positive, HER2-negative GC/GEJ cancer (HR 0.72; 95% CI 0.62–0.83; p < 0.01). Similarly, the addition of other targeted therapies to cytotoxic chemotherapy for GC/GEJ cancer also represents superior benefit over chemotherapy alone, such as anti-HER2 (HR 0.74; 95% CI 0.60–0.91; p < 0.0046) [44] and anti-PD1 (HR 0.71; 98.4% CI 0.59–0.86; p < 0.0001) [16] for advanced or metastatic GC/GEJ cancer.
In the analysis of the studies, the median PFS was higher than the chemotherapy-only group in all studies, with 7.5 months in Zolbetuximab plus EOX (Epirubicin, Oxaliplatin and Capecitabine) and 5.3 months with EOX in the FAST trial [35]; 8.21 versus 6.80 months with placebo plus CAPOX in the GLOW trial [36] and 10.61 in the Zolbetuximab plus mFOLFOX6 group versus 8.67 months in placebo plus mFOLFOX6 group in SPOTLIGHT trial [37]. Likewise, a benefit of increased OS was observed for all studies, with 13.0 versus 8.3 months in the NCT01630083 [35]; 14.39 versus 12.16 months in the NCT03653507 [36] and, NCT03504397 [37], 18.23 versus 15.54 months.
Although our meta-analysis shows no benefit for ORR (p = 0.34), DCR (p = 0.68), PR (p = 0.95) and SD (p = 0.09) for patients in the Zolbetuximab group, we found a positive association for CR (p = 0.01) and PD (p = 0.04). These data are similar to those found in the phase III JACOB study (NCT 01774786), which showed that the addition of Pertuzumab to Trastuzumab and chemotherapy resulted in CR for 5.7% (20) versus 2%(7) of the placebo group and a decrease in the incidence of PR, with 4.8% (17) versus 8.2% (29), for HER2-positive GC/GEJ cancer patients [45].
Adverse events on overall well-being associated with the chosen pharmacotherapy generally have a detrimental influence on the patient’s daily life, compromising their routine activities and emotional state. Although the frequency of adverse events is usually higher in combined chemotherapy, only nausea, vomiting, decreased appetite, peripheral edema, neutropenia, asthenia and decreased weight showed a statistically significant difference between the groups, both of which tended to favor chemotherapy alone [23, 24]. However, considering the overall benefit achieved with the addition of Zolbetuximab, its clinical use should be considered. Additionally, it is important to note that the included studies did not use steroids as prophylaxis for nausea and vomiting, due to the sponsor concern that they would abrogate the effect of Zolbetuximab.
This study has some limitations. Firstly, the analysis was based on a restricted (limited) number of RCTs. However, the pooled analysis of most of the results suggests that our meta-analysis conveys the best available evidence for the use of Zolbetuximab plus chemotherapy as a treatment for CLD18.2 positive, GC/GEJ adenocarcinoma.
Conclusion
This is the first meta-analysis of RCTs to evaluate first-line with Zolbetuximab for advanced CLDN18.2-positive gastric or gastro-oesophageal adenocarcinoma. Our results support that the addition of Zolbetuximab to chemotherapy is associated with significant improvement in PFS and OS. The combination is not associated with increased toxicities to the treatment.
Data availability
All data generated and/or analysed during this study are included in this published article [and its supplementary information files]. Requests for materials should be addressed to F.C.A.M.; francisco.cezar2205@gmail.com.
Abbreviations
- Cls:
-
Confidence intervals
- GC:
-
Gastric Adenocarcinoma
- CLDN18.2:
-
Claudin-18 isoform 2
- CR:
-
Complete Response
- GEJ:
-
Adenocarcinomas of the gastro-oesophageal junction
- HER2:
-
Human epidermal growth factor recptor 2
- HRs:
-
Hazard ratio
- OR:
-
Odds ratio
- ORR:
-
Objective Response Rate
- OS:
-
Overall survival
- RCTs:
-
Randomized controlled trials
- SD:
-
Stable Disease
- PD:
-
Progressive Disease
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- PR:
-
Partial Response
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–48.
Iwatsuki M, Matsumoto C, Mimori K, Baba H. The comprehensive review of gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) from diagnosis and treatment. Ann Gastroenterol Surg. 2023;7:725–32.
Al-Haddad MA, Elhanafi SE, Forbes N, Thosani NC, Draganov PV, Othman MO, et al. American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: methodology and review of evidence. Gastrointest Endosc. 2023;98:285–305e38.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai B-C, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064.
Liu C, Li S, Tang Y. Mechanism of cisplatin resistance in gastric cancer and associated microRNAs. Cancer Chemother Pharmacol. 2023;92:329–40.
Okines AFC, Norman AR, McCloud P, Kang Y-K, Cunningham D. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529–34.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1–21.
Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–20.
Shah MA, Bang Y-J, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin with or without Onartuzumab in HER2-Negative, MET-Positive gastroesophageal adenocarcinoma: the METGastric Randomized Clinical Trial. JAMA Oncol. 2017;3:620–7.
Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18:473–87.
Gupta J, Ahmed AT, Tayyib NA, Zabibah RS, Shomurodov Q, Kadheim MN, et al. A state-of-art of underlying molecular mechanisms and pharmacological interventions/nanotherapeutics for cisplatin resistance in gastric cancer. Biomed Pharmacother. 2023;166:115337.
Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, et al. Immunotherapy and targeted therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J Clin Oncol. 2023;41:1470–91.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40.
Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942–8.
Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–30.
Sawada N. Tight junction-related human diseases. Pathol Int. 2013;63:1–12.
Ding L, Lu Z, Lu Q, Chen Y-H. The claudin family of proteins in human malignancy: a clinical perspective. Cancer Manag Res. 2013;5:367–75.
Pellino A, Brignola S, Riello E, Niero M, Murgioni S, Guido M, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J Pers Med. 2021;11:1095.
Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, Popescu NC, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 2001;21:7380–90.
Sahin U, Schuler M, Richly H, Bauer S, Krilova A, Dechow T, et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur J Cancer. 2018;100:17–26.
Türeci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30:1487–95.
Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49:870–6.
Türeci Ӧzlem, Mitnacht-Kraus R, Wöll S, Yamada T, Sahin U. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology. 2019;8:e1523096.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Common Terminology. Criteria for Adverse Events (CTCAE). 2017.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Chapter 13.: Assessing risk of bias due to missing results in a synthesis. https://training.cochrane.org/handbook/current/chapter-13. Accessed 15 Sep 2023.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609–19.
Shah MA, Shitara K, Ajani JA, Bang Y-J, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29:2133–41.
Shitara K, Lordick F, Bang Y-J, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655–68.
Cao W, Xing H, Li Y, Tian W, Song Y, Jiang Z, et al. Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy. Biomark Res. 2022;10:38.
Ooki A, Yamaguchi K. The dawn of precision medicine in diffuse-type gastric cancer. Ther Adv Med Oncol. 2022;14:17588359221083049.
Kyuno D, Takasawa A, Takasawa K, Ono Y, Aoyama T, Magara K, et al. Claudin-18.2 as a therapeutic target in cancers: cumulative findings from basic research and clinical trials. Tissue Barriers. 2022;10:1967080.
Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10:105.
FDA Accepts Application for Zolbetuximab in Gastric, Cancers GEJ. Curetoday. 2023. https://www.curetoday.com/view/fda-accepts-application-for-zolbetuximab-in-gastric-gej-cancers. Accessed 15 Sep 2023.
News| Astellas Pharma Inc. 2023. https://www.astellas.com/en/news/27946. Accessed 15 Sep 2023.
Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Siddiqui A, et al. Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gastroesophageal junction cancer: end-of-study analysis of the JACOB phase III randomized clinical trial. Gastric Cancer. 2023;26:123–31.
Acknowledgements
We thank the Federal University of Pará (UFPA); the Center for Research in Oncology (NPO/UFPA). The design of the study, sample collection, data analysis, interpretation, and manuscript writing were conducted independently of any influence or involvement from the funding agencies.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Pró-Reitoria de Pesquisa e Pós-Graduação da UFPA (PROPESP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. [F.C.A.M.] conceived the project, material preparation, data collection and analysis were performed by [F.C.A.M., E.P., R.O.M.F., and M.P.C.]. The figures and tables were created by [E.P., F.C.A.M., R.O.M.F.].The first draft of the manuscript was written by [F.C.A.M., E.P., T.B.C., and R.M.R.B] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Moraes, F.C.A., Pasqualotto, E., Chavez, M.P. et al. Efficacy and safety of Zolbetuximab plus chemotherapy for advanced CLDN18.2-positive gastric or gastro-oesophageal adenocarcinoma: a meta-analysis of randomized clinical trials. BMC Cancer 24, 240 (2024). https://doi.org/10.1186/s12885-024-11980-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-11980-w