Abstract
Background
A persisting high-risk human papillomavirus (HR-HPV) infection is causal for cervical cancer; however, there is limited population-based data on the prevalence of HPV infections in Germany. We assessed the age and type-specific HPV prevalence, and associated risk factors in HPV unvaccinated women aged 30 and above.
Methods
The MARZY prospective population-based cohort study was conducted between 2005 and 2012 in Mainz and Mainz-Bingen, Germany. Eligible women were randomly recruited from population registries and invited for cervical cancer screening (n = 5,275). A study swab (liquid-based cytology) was taken and HPV testing was performed with GP5+/6 + polymerase chain reaction (PCR) followed by genotyping. We assessed HPV types as HR-HPV, ‘moderate’ risk and low-risk (LR-HPV). Logistic regression was performed to identify factors associated with HPV infection, stratified by HPV types.
Results
2,520 women were screened with a valid PCR result. Overall HPV prevalence was 10.6% (n = 266), with 6.5% HR-HPV positive (n = 165), 1.5% ’moderate’ risk type (n = 38) and 3.3% LR-HPV type (n = 84) positive. 8.9% had a single infection (n = 225) and 1.6% had multiple types (n = 41). The most common HR-HPV types were 16, 56, 52 and 31 and LR-HPV 90 and 42. Of 187 HR-HPV infections detected (among 165 women), 55.1% (n = 103) were with HPV types not covered by available bivalent or quadrivalent HPV vaccines. About 23% (n = 43) were of types not covered by the nonavalent vaccine (HPV 35, 39, 51, 56, 59). The HR and LR-HPV prevalence were highest in the age group 30–34 years (HR 9.8%, ‘moderate’ risk 3.0% and LR 5.6%), decreasing with increasing age. HR-HPV prevalence in women with normal cytology was 5.5%. In women with a high-grade squamous intraepithelial lesion (HSIL), prevalence was 66.7%. Women currently not living with a partner and current smokers had increased chances of an HR-HPV infection.
Conclusion
The overall population-based HPV prevalence was relatively high. An important share of prevalent HR-HPV infections constituted types not covered by current HPV vaccines. With the advent of HPV screening and younger vaccinated cohorts joining screening, HPV types should be monitored closely, also in older women who were not eligible for HPV vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
An infection with high-risk (HR) human papillomavirus (HPV) is a necessary cause for cervical cancer and contributes to almost all cervical cancer cases [1]. Currently, HR-HPV comprises types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 2, with HPV 16 and HPV 18 accounting for about 70% of all cervical cancers worldwide [3], while low-risk (LR) HPV types 6 and HPV 11 cause around 90% of genital warts. The persistence of specific HR-HPV types is associated with the development of high-grade cervical cancer lesions and cervical cancer [4,5,6]. Additional risk factors such as age, early sexual debut, parity, oral contraceptive use and tobacco smoking have also been observed to influence the onset of cervical cancer [6,7,8,9].
Given the role of HPV infection in cervical cancer development, effective primary and secondary prevention methods have been developed and continuously improved. Prophylactic HPV vaccination was first recommended by the World Health Organization (WHO) for girls aged 12 to 17 years in 2006 [10]. The WHO later revised the age group to target younger girls aged 9 to 14 years to ensure HPV vaccination prior to sexual debut. The first two HPV vaccines approved were Cervarix® (bivalent) and Gardasil® (quadrivalent), which cover the two most relevant HR-HPV types 16 and 18. The nonavalent vaccine Gardasil®9 covers additional HR-HPV types 31, 33, 45, 52 and 58. Both quadrivalent and nonavalent vaccines also protect against LR-HPV types 6 and 11 [11].
Prior to prophylactic vaccination against HPV and since the 1960s, cytology-based cervical cancer screening has been routinely offered in high income countries. In Europe, screening involved Pap smears obtained regularly between every one to five years and relied upon proper smear sampling, adequate assessment and quality assurance [12]. Despite its successes in reducing cervical cancer incidence and mortality, cytological screening is hampered by issues of poor sensitivity and reproducibility [13]. Recently, owing to the superior detection abilities and high negative predictive value of HPV testing compared to cytology [13], HPV testing has been implemented as a primary screening tool in several countries, such as the Netherlands and Australia [14]. In Germany, opportunistic screenings have been offered since 1971 and were based on annual cytological assessments with the Pap smear for women aged 20 years and above [15]. In 2020, an organised programme was implemented including HPV testing as a co-test alongside cytology at triennial intervals for women aged 35 and older. HPV testing includes target amplification methods such as GP5+/6 + polymerase chain reaction (PCR), capable of identifying HPV DNA and distinct genotypes. These methods can determine whether a screened woman has an HPV infection and whether this warrants further follow-up based on the oncogenicity of the HPV type [2].
The worldwide prevalence of overall HPV (HR and LR-HPV combined) in women with normal cytology is estimated at 11.7%, with major regional differences ranging from 9% in western Europe to 21.4% in eastern Europe [16]. Globally, Latin America (16.1%) and Sub-Sahara Africa (24.0%) have even higher HPV prevalence. These meta-analysis estimates are based on studies with women of all ages eligible for screening, including young women below 30 years of age in whom HPV infections are frequent [17]. Identifying the prevalence of HR-HPV in older women is important due to their ineligibility for HPV vaccination and higher risk of cervical cancer [18]. Previous studies reported HPV prevalences among women already attending routine screening [19] and few studies have focussed on women above the age of 29 years [20,21,22,23,24,25]. Among these older age groups, the HR-HPV prevalence in Germany ranged between 5 and 6% [20, 21, 25], and in women with normal cytology results, the prevalence ranged from 4 to 6% [20, 21, 25]. These studies, however, estimated prevalence from women attending screening opportunistically rather than from population-based samples. In opportunistic screening systems, uptake is known to be sub-optimal [12] and HPV prevalence in the general population appears to be substantially higher than in a screening population [6]. Results from other populations above 29 years including the United States, United Kingdom and Denmark demonstrated large variation in HR-HPV prevalence from less than 10% to up to 15–20% 22–24. Additionally, previous investigations of factors associated with HR-HPV infection were mostly conducted for all age groups, not specifically for women from age 30 years [23, 26,27,28].
This analysis aimed to estimate the population-based age and type-specific HR, moderate risk and LR-HPV prevalence in HPV unvaccinated women aged 30 years and above. In addition, the association of the different HPV types with socio-demographic characteristics and cytology results were investigated. These baseline estimates in unvaccinated women are relevant to determine and understand the mid- and long-term impact of HPV vaccination and screening efforts in the near future, especially as screening shifts towards primary HPV testing.
Methods
Study population
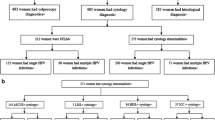
The MARZY study is a prospective population-based cohort study conducted between 2005 and 2012, investigating cervical cancer screening and HPV testing. The study design regarding invitation, screening and test accuracy is described in detail elsewhere [29, 30]. In brief, the study was conducted in two neighbouring regions in western Germany: Mainz, the capital of the state of Rhineland-Palatinate and the surrounding rural district of Mainz-Bingen. The study population (n = 5,275) aged between 30 and 65 years was randomly selected via population registries and invited via postal letter to participate in cervical cancer screening at a gynaecologist of their preference. The exclusion criteria were hysterectomy, pregnancy, childbirth in the past six months, temporary residence in the study area, history of cervical cancer, intellectual disability, transsexuality or employment at the study centre. HPV vaccinations in Germany were only approved in 2007 and for young girls, therefore, none of the study participants were eligible for vaccination.
Study design
The present analyses were based on baseline data (2005–2007) of the cohort study. Participating women gave informed consent and completed a questionnaire prior to screening, documenting socio-demographic characteristics, history of participation in cervical cancer screening, and risk factors for cervical cancer, including smoking, oral contraceptive use, and hormone replacement therapy [29]. As described previously, the study swab was taken using an Ayres spatula and endocervical broom or a cytobrush if the transformation zone was not visible [30]. The swab was processed in PreservCyt® solution for liquid-based cytology (ThinPrep®, Cytyc/Hologic®, Bedford, MA, USA) and was used for both cytological and hc2 HPV testing and processed centrally at a routine laboratory (CytoMol, Frankfurt, Germany).
HPV testing and genotyping
The study swab was additionally used for HPV genotyping, and the testing procedures have been described in detail previously [30]. For these analyses, additional post-hoc HPV DNA testing was performed in a reference laboratory in The Netherlands (Department of Pathology, Amsterdam UMC, location Vrije Universiteit Amsterdam, Amsterdam) [31, 32]. For GP5+/6 + PCR HPV testing and genotyping, an aliquot of each PreservCyt® sample was used for DNA extraction and 1/10 of the resulting DNA eluate was subjected to a human β-globin PCR reaction to verify the presence of sufficient amplifiable DNA. GP5+/6 + PCR was subsequently performed, followed by an enzyme immunoassay (EIA) using two cocktail probes: one for HR-HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 and another for HPV types 6, 11, 26, 30, 32, 34, 40, 42, 43, 44, 53, 54, 55, 57, 61, 64, 67, 69, 70, 71, 72, 73, 81, 82 (variants mm4 and is39), 83, 84, 85, 86, 89 (formerly cp6108), 90 (formerly jc9710). HPV genotyping of GP5+/6 + PCR-EIA positive samples was performed by reverse line blot (RLB) hybridisation of GP5+/6 + PCR products. Samples that were EIA positive but in which no genotypes could be detected by RLB were considered to contain uncharacterised types, referred to as HPV X.
Classification of cytological results
The cytological results were originally classified according to the Munich Nomenclature II [33] and later, for analyses purposes, converted to the International Bethesda Classification for cytology [34] as: negative for intraepithelial lesion malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade intraepithelial lesion (LSIL) or high-grade intraepithelial lesion (HSIL) [33].
Statistical analysis
In our analyses, HPV types were classified according to the International Agency for Research on Cancer (IARC) as HR with sufficient evidence (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59) and limited evidence group 2 (26, 53, 66, 67, 68, 70, 73, 82), which was categorised as ‘moderate’ risk due to their potential for carcinogenicity [2]. All other HPV types were considered LR. Additionally, we classified HR-HPV types as being covered by the different nonavalent (16, 18, 31, 33, 45, 52 and 58), quadrivalent (16, 18) and bivalent (16, 18) vaccines. For the bivalent vaccine, we also considered HPV types 31, 33 and 45 for which cross-protection lasting at least seven years [35] or longer [36, 37] has been reported.
Age-specific overall HPV prevalence based on GP5+/6 + PCR test results with 95% confidence intervals (95% CI) using the Clopper-Pearson method were calculated. Additionally, the prevalence of individual HPV genotypes were determined. The prevalence of HR, ‘moderate’ risk and LR-HPV type groups were also reported stratified by cytology result. Associations between socio-demographic factors, other characteristics and HPV infection were assessed using univariable and multivariable logistic regression. Women who had infections with both HR and LR types or moderate risk types were categorised as HR. Analyses were stratified by HR-HPV, ‘moderate’ risk HPV, LR-HPV and HPV positive overall. A Cochran-Armitage trend test was performed in order to assess age trends.
Since the variables currently living with a partner and marital status were highly correlated, only the variable currently living with a partner was used in multivariable analyses. Since net household income and employment status were also highly correlated, only employment was included in the analysis. Smoking exposure as total cigarette pack-years was assessed. This was determined by the number of daily cigarettes reportedly smoked divided by 20 (the amount in a standard cigarette pack), multiplied by the duration of reported years of smoking [38]. The following variables were considered in multivariable regression due to their clinical relevance: age, living with a partner and smoking. Multiple imputation was applied for any missing data in both univariable and multivariable analyses, and results were pooled based on Rubin’s rule. All analyses were carried out using R (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria) and the R packages survival and mice were employed to carry out multiple imputed regression.
Ethical considerations
The MARZY study was approved by the ethics committee of the state of Rhineland-Palatinate [Landesärztekammer Rheinland-Pfalz: 837.438.03 (4100)] and by the state government data protection office. The study was conducted following the guidelines of Good Epidemiological Practice (GEP).
Results
In total, 5,275 women were eligible for inclusion in the study at baseline. Of those, 2,627 women (49.8%) participated in cervical cancer screening and received a study swab. 2,520 had a valid PCR test result and were included in the present analyses. The median age of the study population when receiving the baseline study swab was 46 years (95% CI 45, 46; range: 30–68 years). Few women aged above 65 years participated only after receiving a reminder but within the baseline study period.
Table 1 provides detailed information on the socio-demographic characteristics of the study population. Half of the screened women had reported ever smoking (49.0%), with 19.1% currently smoking. Among ever smokers 42.2% had moderate to heavy exposure to cigarette smoking (10+ total pack-years). Abnormal cytological findings (ASC-US+) were diagnosed in 3.7% of women. Overall, increasing age compared to the youngest age group (30–39) highlighted a lower likelihood of an HPV infection of any type and this likelihood decreased as age increased (Table 2). After adjustment of confounders, not living with a partner led to a 1.8-fold increase in HPV infection (adjusted odds ratio: aOR 1.78, 95% CI 1.28, 2.46) and high exposure to tobacco smoking (more than 20 total pack-years) led to a 1.6-fold increase (aOR 1.55, 95% CI 1.01, 2.39). Older age (60+ years) was also associated with a 63% reduced odds of HR-HPV infection (aOR 0.37, 95% CI 0.20, 0.68) compared to younger women. Women currently not living with a partner (aOR 1.72 95% CI 1.15, 2.56) and women who smoked 10 or more total pack-years (10–19 pack-years: aOR 1.94 95% CI 1.25, 3.03; 20+ pack-years: aOR 1.91, 95% CI 1.13, 3.24) had increased odds of HR-HPV infection.
HPV prevalence
The overall prevalence of any HPV infection was 10.6% (n = 266, 95% CI 9.4, 11.8). Of the 266 HPV positive women, the majority (84.6%) had a single infection (n = 225, 95% CI 79.7, 88.7). After counting all HPV infections separately (Table 3), regardless if they occurred in women as a single or multiple infections, HR-HPV prevalence was 6.5% (n = 165, 95% CI 5.6, 7.6), IARC classified types with limited evidence or ‘moderate’ risk was 1.5% (n = 38, 95% CI 1.1, 2.1) and LR-HPV prevalence was 3.3% (n = 84, 95% CI 2.7, 4.1).
Type-specific HPV prevalence
Among all 2,520 women with a PCR result, HPV 16 was the most frequent type (2.8%) (Table 3). We observed 187 infections with HR-HPV types among 165 women. Among HR-HPV types, HPV 16 contributed to almost half the HR types detected (43.0%, 95% CI 35.4, 51.0), followed by HPV 56 (13.9%, 95% CI 9.0, 20.2), HPV 52 (9.7%, 95% CI 5.6, 15.3) and HPV 31 (9.1%, 95% CI 5.2, 14.6). About half (55.1%, n = 103) of all the 187 HR-HPV infections were with types not covered by the quadrivalent vaccine or the bivalent vaccine, already taking into account cross protection of HPV types 31, 33, and 45 [35,36,37]. A quarter (23%, n = 43) of detected infections in our population were not covered by the nonavalent vaccine (Supplements Table S1).
As for the HPV types of ‘moderate risk’, HPV 66 ranked first among its subgroup and accounted for half of these infections (50.0%, 95% CI 33.4, 66.6), corresponding to 0.8% (95% CI 0.5, 1.2%) of all women included and 7.1% (95% CI 4.4, 10.9) of all HPV types detected (Table 3). Among the women positive for LR-HPV, HPV 90 and HPV 42 were the most common types (33.3%, 96% CI 23.4, 44.5 and 28.6%, 95% CI 19.2, 39.5 respectively). Both types each represented approximately 1% of all HPV types detected in all HPV-tested women. Only five women were positive for HPV 6 (0.2%, 95% CI 0.1, 0.5%) and 2 for HPV 11 (0.1%).
Age-specific HPV prevalence
Age-specific HR-HPV, ‘moderate’ risk and LR-HPV prevalence are shown in Fig. 1. HR-HPV prevalence decreased from 9.8% (95% CI 6.3, 14.4%) in the youngest age group (30–34 years) to 3.6% (95% CI 2.0, 5.9%) in the oldest age group (60+ years), including a slight increase of prevalence between ages 55–59 years (Fig. 1A). Similar decreasing patterns of prevalence with increasing age were observed for ‘moderate’ types (Fig. 1B) and LR-HPV (Fig. 1C). A statistically significant inverse linear trend was found between age and the prevalence of HR-HPV infection (p = 0.003; Table 2).
Prevalence of HPV by cytological findings
The large majority (96.3%) of women in the study population had normal cytological findings (NILM; Table 1). Among these women with normal cytology, 9.2% were positive for any HPV type, 5.5% for a HR type, 1.3% were positive for a ‘moderate’ risk type and 3.1% were positive for any LR-HPV (Table 4). In women with any borderline to low-grade cytological abnormality (ASC-US/LSIL), HR-HPV was detected in 31.4% (n = 27) and LR-HPV in 11.6% (n = 10). Two-thirds of the women with HSIL (66.7%; n = 4) had an HR-HPV infection. Single HPV infections were observed in 7.7% of NILM results (n = 188), 38.4% of ASC-US or LSIL results (n = 33) and 66.7% in HSIL results (n = 4). 7.0% of abnormal and low-grade results had multiple HPV types (n = 6).
Discussion
Our study investigated age and type-specific HPV prevalence as well as socio-demographic and risk factors in a population-based sample of HPV unvaccinated women aged 30 and above in Germany. In the total population, overall HPV positivity was 10.6% and HR-HPV prevalence was 6.5%, highest among younger women and consistently decreasing with increasing age. HR-HPV prevalence in women with normal cytological results was 5.5%. HPV prevalence of ‘moderate’ types was 1.5% and 3.3% had LR-HPV infections. The most common HR-HPV types detected were 16, 18, 31, 45, 52 and 56. Interestingly, of the 187 HR-HPV infections observed, 55% comprised HPV types not covered by bivalent or quadrivalent vaccines, and 23% were not covered by nonavalent vaccines. Our findings highlight the need to monitor the prevalence of these non-vaccine covered but HR-HPV genotypes over time to assess potential consequences for screening and vaccination strategies.
A global meta-analysis estimated an overall HPV prevalence in women with normal cytology of 9.0% (95% CI 8.8, 9.2) for western Europe [16], comparable to our results (9.5%). These western European estimates included two previous German studies that were not population-based by design, as well as data from Belgium, France and the Netherlands [16], which are countries with fairly similar cervical cancer incidence [39]. We report a HR-HPV prevalence of 6.5% using GP5+/6 + PCR in our sample of women aged 30 years and older with a median age of 46 years (95% CI 45, 46), which included previously unscreened women [29]. Other studies reported much higher HR-HPV prevalence in women above 30 years, ranging from approximately 15% in Denmark [24] and up to 28% in the United States [23]. It is possible that selection of study sample, different HPV assays, categorisation of more HPV types as HR and use of self-collected vaginal smears contribute to these differences [23, 24]. Among women attending opportunistic screening in Germany, HR-HPV prevalence (6.4% overall and 3.7% among women with normal cytology) were comparable to our findings [20].
Type-specific prevalence
In our study, HPV 16 was by far the most frequent HR-HPV type, affecting 2.8% of all women in the study sample, with a proportion of 43% of HR-HPV positive women. This is in agreement with previous studies in women aged over 30 years [20,21,22]. In contrast, HPV 18, considered to be the second most common HR-HPV type worldwide and third most common in Europe after HPV 31 [16], only ranked fifth in our study with a prevalence of 0.5% (7.9% of all HR-HPV infections), following HPV types 16, 56, 52 and 31. Studies from Germany [21] and other European countries, including Denmark and Netherlands, also identified genotypes other than HPV 18, such as HPV 52, 51, 31, 33, as the most frequently observed types [24, 40, 41].
Impact of HPV vaccination status on screening
Interestingly, we observed that 23% of all HR-HPV infections detected were of types not covered by any of the available vaccines. This information is important, firstly because our results provide baseline evidence of the HR-HPV types prevalent in the HPV non-vaccinated population, which impacts current and future screening and disease management policies [18]. It highlights the necessity to continue screening efforts, particularly since women who are immunised against HPV, are not fully protected against all the HR-HPV types associated with cervical cancer, even considering cross-protection effects [35,36,37]. Second, although younger cohorts are eligible for HPV vaccination, HPV vaccination coverage in Germany and in other countries worldwide is lagging. In 2020, only 54.1% of 18 year old women in Germany were fully immunised [42]. This underscores the importance of maximising both primary and secondary prevention measures by improving vaccination rates and screening uptake, also in high income countries such as Germany.
Benefits of HPV type monitoring
We also report a strong association between abnormal cytological diagnosis and HPV infection consistent with the literature [1, 16]. This association was observed in a large meta-analysis of HPV-positive women worldwide, showing particular HR-HPV types such as HPV 16 to be common contributors to HPV infections among women with invasive cervical cancers [27]. Assessing both HPV and cytological status as triage is an important step for narrowing risk and guiding management [18].
As for ‘moderate’ risk and LR-HPV types, HPV 90 (1.1%), HPV 42 (1.0%) and HPV 66 (0.8%) were the three most commonly found HPV types in this study. However, HPV prevalence and distribution varies by population and region. In a population-based Danish study [24], HPV 6 (1.6%) and HPV 74 (1.4%) were most prevalent among the LR-HPV types, while in a Polish study, these were HPV 42 (2.3%), HPV 66 (1.0%) and HPV 83 (0.9%) [43]. In a representative sample of a United States screening population, HPV 62 and HPV 84 (3.3%), as well as HPV 89 and HPV 61 (2.4%), were the most common types [23]. These findings may be of relevance for HPV type monitoring purposes in the population. Additionally, genotyping of HPV may be beneficial as a form of risk stratification in follow-up monitoring i.e. as a triage method for abnormal cytological findings (ASC-US+). However, it is worthy to note that the risk of developing precancer and cancer from ‘moderate’ risk and LR HPV types is low, representing less than 5% of invasive cervical cancer cases [2, 44]. Therefore, genotyping of non-HR types may be excessive and costly, although they may reduce follow-up monitoring in discordant HPV negative but ASC-US + cases.
Age-specific prevalence
As consistently described in previous research [16, 40, 45, 46], the prevalence of both HR and LR-HPV decreased with increasing age. This pattern is likely a result of behavioural and biological aspects. In terms of behaviour, sexual activity and the number of sexual partners tends to decrease with age [47]. Our cross-sectional comparison includes several birth cohorts, with more recent birth cohorts having, on average, a higher number of lifetime sexual partners than older ones, as observed in other populations [48]. We observed a slight increase in HPV prevalence in the age group 50–59 years. This second peak of HPV positivity may be explained by hormonal and immune system changes, particularly in the cervix, which might affect HPV detection rather than an actual change in sexual activity during this life period [49]. However, based on natural history studies, others argue that immune response and the number of lifetime sexual partners play a role in the second HPV prevalence peak among older women [50,51,52]. Since cervical cancer rates in older women are higher than previously estimated after hysterectomy rates were considered [53], these unvaccinated age groups need to be considered in future screening efforts.
Strengths and limitations
The findings from the MARZY study regarding HPV prevalence provide the first population-based data among HPV non-vaccinated women aged 30 years and above in Germany. Our study reported results by HPV type, age group and cytology including prevalence among women previously unscreened and in an older population, contrasting to the majority of previous studies reporting primarily HPV prevalence of younger age groups and based on routinely screened populations. Obtaining these estimates provide a necessary baseline for understanding the mid- and long-term impact of HPV vaccination status on screening, particularly since screening shifts towards primary HPV testing, there will be a co-existence of HPV vaccinated and unvaccinated cohorts.
There are limitations to our results. We rely on cross-sectional reporting of HPV prevalence where it was not possible to determine whether the infection was newly acquired, persistent or reactivated. Women who participated in the study may not reflect the entire eligible population comprehensively, with older women and women with a migration background less likely to participate [54]. Additionally, we did not adjust our analyses for other known confounding factors as these items were not included in the baseline questionnaire, including lifetime number of sexual partners, age at first sexual intercourse, and sexually transmitted infections. Finally, in contrast to women with lower-grade lesions (ASC-US/LSIL) or normal cytology, our findings relating to high-grade lesions (HSIL+) may be limited due to the low number of cases (n = 6) detected, two of which were negative for any HPV type. Our results nonetheless capture HPV prevalence and type distribution on an unvaccinated and population-based sample of older women in Germany and shed valuable light on HPV types not covered by available vaccines and their impact on screening efforts.
Conclusion
As cohorts of vaccinated women become eligible for screening, and changes in screening recommendations shift towards HPV-based screening, knowing the prevalence and distribution of HPV types in non-vaccinated women is necessary for studying effects of vaccination and screening. A considerable share of HR-HPV infections circulating in the population are due to HPV genotypes not covered by the available vaccines, even when taking cross-protection into account. Monitoring vaccinated and unvaccinated women for these non-vaccine but high-risk HPV genotypes is important for understanding the impact of HPV vaccination and screening efforts and identify optimal prevention strategies. These analyses provide important evidence from a population-based sample of women and can be utilised for screening, monitoring and modelling purposes.
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- aOR:
-
Adjusted odds ratio
- ASC-US:
-
Atypical squamous cells of undetermined significance
- CI:
-
Confidence interval
- EIA:
-
Enzyme immunoassay
- GEP:
-
Good Epidemiological Practice
- HSIL:
-
High-grade intraepithelial lesion
- HR:
-
High-risk
- HPV:
-
Human papillomavirus
- IARC:
-
International Agency for Research on Cancer
- LSIL:
-
Low-grade intraepithelial lesion
- LR:
-
Low-risk
- NILM:
-
Negative for intraepithelial lesion malignancy
- PCR:
-
Polymerase chain reaction
- RLB:
-
Reverse line blot
- WHO:
-
World Health Organization
References
Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents: human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:255–313.
Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:K1–16.
Bonde J, Bottari F, Iacobone AD, Cocuzza CE, Sandri MT, Bogliatto F, et al. Human papillomavirus same genotype persistence and risk: a systematic review. J Low Genit Tract Dis. 2021;25(1):27–37.
Chen HC, Schiffman M, Lin CY, Pan MH, You SL, Chuang LC, et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst. 2011;103(18):1387–96.
Schulte-Frohlinde R, Georges D, Clifford GM, Baussano I. Predicting Cohort-Specific Cervical Cancer Incidence from Population-based surveys of human papilloma virus prevalence: a Worldwide Study. Am J Epidemiol. 2022;191(3):402–12.
Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–24.
Vaccarella S, Herrero R, Dai M, Snijders PJF, Meijer CJLM, Thomas JO, et al. Reproductive factors, oral contraceptive use, and human papillomavirus infection: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2148–53.
Plummer M, Herrero R, Franceschi S, Meijer CJ, Snijders P, Bosch FX, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case–control study. Cancer Causes Control. 2003;14(9):805–14.
Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399.
Osmani V, Klug SJ. [HPV vaccination and the prevention of genital warts and precancerous lesions-current evidence and evaluation]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2021;64(5):590–9.
Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, et al. European guidelines for quality assurance in cervical cancer screening. Second Edition—Summary Doc Ann Oncol. 2010;21(3):448–58.
Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587.
Maver PJ, Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2020;26(5):579–83.
Bujan Rivera J, Klug SJ. [Cervical cancer screening in Germany]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(12):1528–35.
Bruni L, Diaz M, Castellsagué M, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: Meta-Analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–99.
Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–26.
Lehtinen M, Pimenoff VN, Nedjai B, Louvanto K, Verhoef L, Heideman DAM, et al. Assessing the risk of cervical neoplasia in the post-HPV vaccination era. Int J Cancer. 2023;152(6):1060–8.
Schneider A, Hoyer H, Lotz B, Leistritz S, Kühne-Heid R, Nindl I, et al. Screening for high grade cervical intraepithelial neoplasia and cancer by testing for high risk HPV, routine cytology or colposcopy. Int J Cancer. 2000;89(6):529–34.
Klug SJ, Hukelmann M, Hollwitz B, Duzenli N, Schopp B, Petry KU, et al. Prevalence of human papillomavirus types in women screened by cytology in Germany. J Med Virol. 2007;79(5):616–25.
Luyten A, Scherbring S, Reinecke-Lüthge A, Braun BE, Pietralla M, Theiler K, et al. Risk-adapted primary HPV cervical cancer screening project in Wolfsburg, Germany–experience over 3 years. J Clin Virol. 2009;46(Suppl 3):S5–10.
Cuzick J, Beverley E, Ho L, Terry G, Sapper H, Mielzynska I, et al. HPV testing in primary screening of older women. Br J Cancer. 1999;81(3):554–8.
Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–9.
Kjaer SK, Breugelmans G, Munk C, Junge J, Watson M, Iftner T. Population-based prevalence, type- and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer. 2008;123(8):1864–70.
Petry KU, Menton S, Menton M, van Loenen-Frosch F, Gomes HD, Holz B, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer. 2003;88(10):1570–7.
De Sanjose S, Almirall R, Lloveras B, Font R, Diaz M, Munoz N, et al. Cervical human papillomavirus infection in the female population in Barcelona, Spain. Sex Transm Dis. 2003;30(10):788–93.
Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–59.
Roura E, Iftner T, Vidart JA, Kjaer SK, Bosch FX, Munoz N, et al. Predictors of human papillomavirus infection in women undergoing routine cervical cancer screening in Spain: the CLEOPATRE study. BMC Infect Dis. 2012;12:145.
Radde K, Gottschalk A, Bussas U, Schulein S, Schriefer D, Seifert U, et al. Invitation to cervical cancer screening does increase participation in Germany: results from the MARZY study. Int J Cancer. 2016;139(5):1018–30.
Liang LA, Einzmann T, Franzen A, Schwarzer K, Schauberger G, Schriefer D, et al. Cervical Cancer screening: comparison of Conventional Pap Smear Test, Liquid-based cytology, and human papillomavirus testing as stand-alone or cotesting strategies. Cancer Epidemiol Biomarkers Prev. 2021;30(3):474–84.
Husman AMDR, Walboomers JMM, Vandenbrule AJC, Meijer CJLM, Snijders PJF. The Use of General primers GP5 and GP6 elongated at their 3’ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–62.
van den Brule AJC, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJLM, Snijders PJF. GP5+/6 + PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40(3):779–87.
Cirkel C, Barop C, Beyer DA. Method comparison between Munich II and III nomenclature for pap smear samples. J Turkish German Gynecol Association. 2015;16(4):203–7.
Herbert A, Bergeron C, Wiener H, Schenck U, Klinkhamer P, Bulten J, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for cervical cytology terminology. Cytopathology. 2007;18(4):213–9.
Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infec Dis. 2017;17(12):1293–302.
Tsang SH, Sampson JN, Schussler J, Porras C, Wagner S, Boland J, et al. Durability of Cross-protection by different schedules of the Bivalent HPV Vaccine: the CVT Trial. J Natl Cancer Inst. 2020;112(10):1030–7.
Mariz FC, Gray P, Bender N, Eriksson T, Kann H, Apter D, et al. Sustainability of neutralising antibodies induced by bivalent or quadrivalent HPV vaccines and correlation with efficacy: a combined follow-up analysis of data from two randomised, double-blind, multicentre, phase 3 trials. Lancet Infect Dis. 2021;21(10):1458–68.
Roura E, Castellsagué X, Pawlita M, Travier N, Waterboer T, Margall N, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer. 2014;135(2):453–66.
European Cancer Information System. Estimates of cervical cancer incidence and mortality in 2020, for all countries 2022 [cited. https://ecis.jrc.ec.europa.eu
Coupe VMH, Berkhof J, Bulkmans NWJ, Snijders PJF, Meijer CJLM. Age-dependent prevalence of 14 high-risk HPV types in the Netherlands: implications for prophylactic vaccination and screening. Br J Cancer. 2008;98(3):646–51.
Forslund O, Antonsson A, Edlund K, van den Brule AJC, Hansson BG, Meijer CJLM, et al. Population-based type-specific prevalence of high-risk human papillomavirus infection in middle-aged Swedish women. J Med Virol. 2002;66:535–41.
Rieck T, Feig M, Siedler A. Impfquoten Von Kinderschutzimpfungen in Deutschland – Aktuelle Ergebnisse Aus Der RKI-Impfsurveillance. Epid Bull. 2022;48:3–25.
Bardin A, Vaccarella S, Cliffoyd GM, Lissowska J, Rekosz M, Bobkiewicz P, et al. Human papillomavirus infection in women with and without cervical cancer in Warsaw, Poland. Eur J Cancer. 2008;44(4):557–64.
Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73.
De Vuyst H, Clifford G, Li N, Franceschi S. HPV infection in Europe. Eur J Cancer. 2009;45(15):2632–9.
Jacobs MV, Walboomers JMM, Snijders PJF, Voorhorst FJ, Verheijen RHM, Fransen-Daalmeijer N, et al. Distribution of 37 mucoscopic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer. 2000;87(2):221–27.
Haversath J, Gärttner KM, Kliem S, Vasterling I, Strauss B, Kröger C. Sexual behavior in Germany. Dtsch Arztebl Int. 2017;114(33–34):545–50.
Hansen BT, Kjær SK, Arnheim-Dahlström L, Liaw K-L, Juul KE, Thomsen LT, et al. Age at first intercourse, number of partners and sexually transmitted infection prevalence among Danish, Norwegian and Swedish women: estimates and trends from nationally representative cross-sectional surveys of more than 100 000 women. Acta Obstet Gynecol Scand. 2020;99(2):175–85.
Castle PE, Jeronimo J, Schiffman M, Herrero R, Rodríguez AC, Bratti MC, et al. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006;66(2):1218–24.
Gravitt PE, Winer RL. Natural history of HPV infection across the Lifespan: role of viral latency. Viruses. 2017;9(10):267.
Muñoz N, Méndez F, Posso H, Molano M, van den Brule AJ, Ronderos M, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–87.
Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on Incident Human Papillomavirus detection: Acquisition and Reactivation in Older Women. Cancer Res. 2012;72(23):6183–90.
Neumeyer S, Tanaka LF, Liang LA, Klug SJ. Epidemiology of cervical cancer in elderly women: analysis of incidence, treatment, and survival using German registry data. Cancer Med. 2023;12(16):17284–95.
Brzoska P, Aksakal T, Yilmaz-Aslan Y. Utilization of cervical cancer screening among migrants and non-migrants in Germany: results from a large-scale population survey. BMC Public Health. 2020;20:5.
Acknowledgements
The authors would like to thank all women, gynaecologists and regional cytology laboratories and their staff for participating in the study. We would like to thank the study team at the Institute of Medical Biostatistics, Epidemiology and Informatics at the University Medical Center in Mainz. We would also like to thank all colleagues who supported the study, in particular Prof. Peter J. F. Snijders† (Dept. Pathology, Amsterdam UMC, location Vrije Universiteit Amsterdam). We are indebted to the technicians of the Molecular Pathology Unit of Dept. Pathology, Amsterdam UMC, location Vrije Universiteit Amsterdam, for excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. The MARZY Study was funded by grants from the German Cancer Aid (Deutsche Krebshilfe; No. 105827, 106619, 107159, 107247, 108047). The funding agency did not interfere with the study results.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study conception and design: SJK, MB. Data collection and sample processing: KR, UB, HI, DAMH, CJLMM, SJK. Study co-ordination: KR, UB, SJK. Data analysis and interpretation: LAL, LFT, SJK. Drafting of the manuscript: LAL, LFT, SJK. Supervision of the findings: SJK. Funding: SJK. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent to participate in the MARZY prospective cohort study was provided by all study participants prior to screening at study baseline. The MARZY study was approved by the ethical committee of the state of Rhineland-Palatinate (Landesärztekammer Rheinland-Pfalz: 837.438.03 (4100)) and the state government data protection office. All recruitment, data collection and analyses were performed in accordance to Good Epidemiological Practice guidelines and the Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
LAL, LFT, KR, UB, MB, and SJK declare no conflict of interest. DAMH and CJLMM are minority shareholders of Self-screen B.V., a spin-off company of Amsterdam UMC, location VUmc, which develops, manufactures, and licenses high-risk HPV and methylation marker assays for cervical cancer screening and holds patents on these tests. CJLMM is part-time CEO of Self-screen B.V., and has a very small number of shares of MDXHealth and previously QIAGEN, has received speaker’s fees from GSK, QIAGEN and SPMSD/Merck, and served occasionally on the scientific advisory boards of these companies. HI has received contributions for speaking engagements and travels as well as support for research projects from B&D, Hologic, Otto Bock, Qiagen and Roche Diagnostics during the last three years. HI is a shareholder in a laboratory that carries out conventional cytology, liquid-based cytology and molecular diagnostics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, L.A., Tanaka, L.F., Radde, K. et al. Population-based age- and type-specific prevalence of human papillomavirus among non-vaccinated women aged 30 years and above in Germany. BMC Infect Dis 24, 1008 (2024). https://doi.org/10.1186/s12879-024-09827-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09827-7