Abstract
Background
Ferroptosis is a newly recognized form of regulatory cell death characterized by severe lipid peroxidation triggered by iron overload and the production of reactive oxygen species (ROS). However, the role of ferroptosis in severe acute pancreatitis(SAP) has not been fully elucidated.
Methods
We established four severe acute pancreatitis models of rats including the sham control group, the SAP group, the Fer -1-treated SAP (SAP + Fer-1) group, the 3-MA-treated SAP (SAP + 3-MA) group. The SAP group was induced by retrograde injection of sodium taurocholate into the pancreatic duct. The other two groups were intraperitoneally injected with ferroptosis inhibitor (Fer-1) and autophagy inhibitor (3-MA), respectively. The model of severe acute pancreatitis with amylase crest-related inflammatory factors was successfully established. Then we detected ferroptosis (GPX4, SLC7A1 etc.) and autophagy-related factors (LC3II, p62 ect.) to further clarify the relationship between ferroptosis and autophagy.
Results
Our study found that ferroptosis occurs during the development of SAP, such as iron and lipid peroxidation in pancreatic tissues, decreased levels of reduced glutathione peroxidase 4 (GPX 4) and glutathione (GSH), and increased malondialdehyde(MDA) and significant mitochondrial damage. In addition, ferroptosis related proteins such as GPX4, solute carrier family 7 member 11(SLC7A11) and ferritin heavy chain 1(FTH1) were significantly decreased. Next, the pathogenesis of ferroptosis in SAP was studied. First, treatment with the ferroptosis inhibitor ferrostatin-1(Fer-1) significantly alleviated ferroptosis in SAP. Interestingly, autophagy occurs during the pathogenesis of SAP, and autophagy promotes the occurrence of ferroptosis in SAP. Moreover, 3-methyladenine (3-MA) inhibition of autophagy can significantly reduce iron overload and ferroptosis in SAP.
Conclusions
Our results suggest that ferroptosis is a novel pathogenesis of SAP and is dependent on autophagy. This study provides a new theoretical basis for the study of SAP.
Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is an inflammatory process of the pancreas, which is characterized by local and systemic inflammatory responses, as well as multiple organ failure. Most patients present with mild acute pancreatitis, which is usually self-limited and resolves within 1 week. But about 20% of patients develop into severe acute pancreatitis (SAP) with necrosis of the pancreas or peripancreatic tissues or distal organs, and mortality can be 20 to 40% higher [1]. Gallstones (45%) and alcohol abuse (20%) are the most frequent causes of AP in most high-income countries. Less common causes include drugs, endoscopic retrograde cholangiopancreatography (ERCP), hypercalcemia, hypertriglyceridemia, infection, genetics, autoimmune diseases, and (surgical) trauma [2]. AP is characterized by damage to acinar cells (the functional unit of the outer membrane pancreas), resulting in the inappropriate release and activation of trypsinogen into trypsin within the acinar. This triggers the activation of other digestive enzymes, the kinin system, and the complement cascade, leading to the self-digestion of the pancreatic parenchyma [3]. Cell death plays a central role in all aspects of life and is involved in the development of multicellular organisms and tissue homeostasis. Regulatory cell deaths (RCDs) not only plays a role in keeping the body's internal environment stable, the danger-associated molecular patterns(DAMPs) released during RCDs also provide a powerful signal to stimulate local inflammation or systemic immune response [4]. The early death mode of acinar cells determines the severity and prognosis of AP. In recent years, various RCDs, such as apoptosis, pyroptosis, autophagy and necroptosis, have also been found to play important roles in the pathogenesis of AP. The progression of AP is closely related to the regulatory transitions between different RCDs. These death modes will eventually lead to cell rupture and release of cell contents [5]. However, in a recent study inhibition of pyroptosis, autophagy, or necroptosis only partially alleviates pancreatic damage, suggesting that other types of cell death may be involved in acinous damage in AP. The treatment of AP especially the SAP mainly includes fluid resuscitation and supportive treatment, and there is a lack of effective prevention and treatment methods [6]. Therefore, it is urgent to explore the pathogenic basis of AP.
Ferroptosis is a novel form of cell death triggered by lipid peroxidation in an iron-dependent manner. Ferroptosis is morphologically, biochemical, and genetically distinct from other types of cell death. Cells that undergo ferroptosis are morphologically characterized by increased membrane density, few or no cristae, and crumpled mitochondria with a ruptured outer membrane (Fig. 1) [7]. Ferroptosis is associated with pathological cell death associated with degenerative diseases (e.g., Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease), carcinogenesis, stroke, cerebral hemorrhage, traumatic brain injury, ischemia–reperfusion injury, and renal degeneration, and the progression of these diseases can be mitigated by activating or inhibiting the ferroptosis pathway [8]. Ferroptosis is defined by three indispensable markers, including impaired lipid peroxide repair caused by loss of glutathione peroxidase 4 (GPX4) activity, availability of reductively active iron, and oxidation of phospholipids containing polyunsaturated fatty acids (PUFAs). Currently, three biomarkers are available to identify the occurrence of ferroptosis: protein markers (GPX4, solute carrier family 7 member 11(SLC7A11), prostaglandin-endoperoxide synthase 2(PTGS2)), lipid peroxidation(acyl-CoA synthetase long-chain family member 4(ACSL4)) and lipid reactive oxygen species (ROS) [4, 9]. Moreover, this form of cell death, which is regulated by a distinct set of genes including ACSL4, GPX4, and SLC7A11, can be distinguished morphologically by the existence of shrunken mitochondria and can be inhibited specifically by ferrostatin-1(Fer-1) (Fig. 1) [10]. Studies have shown that ferroptosis plays an important role in SAP-induced acute kidney, intestinal, and lung injury, and that the use of ferroptosis inhibitors such as Fer-1 can effectively alleviate inflammation, oxidative stress, and excessive ferroptosis in these tissues during SAP [5]. Autophagy performs a housekeeping function for the degradation and recycling of cytoplasmic proteins and organelles, which plays a crucial role in maintaining homeostasis. Both excessive and insufficient autophagy can trigger cell death. In recent years, more and more evidence supports that ferroptosis is autophagy dependent cell death.Ferroptosis is strictly regulated by iron metabolism. Ferritin is the main intracellular iron-storage protein complex, comprising FTL (ferritin light chain) and FTHl (ferritin heavy chain 1) [6]. Autophagy activation degrades ferritin to increase intracellular iron levels and subsequently leads to oxidative damage through the Fenton reaction [7]. Wei et al. reported that mitochondrial ROS autophagy lysosome pathway is involved in ferroptosis induced by inorganic arsenic, and chronic arsenic poisoning can cause ferroptosis in mouse cortical neurons [10]. Yu’s research has confirmed that ferroptosis mediated by ferritin is involved in vascular endothelial damage caused by zinc oxide nanoparticles (ZnONP) [11]. Studies have shown that autophagy is involved in SAP in rats through oxidative stress-mediated AKT/AMPK/mTOR pathway [2].
To explore the role of ferroptosis and autophagy in SAP, a model of SAP induced by sodium taurocholate was established. In the present study, we determined that ferroptosis occurs in SAP by iron accumulation and accumulation of lipid peroxidation products, and by examining mitochondrial ultrastructural changes. In addition, we found that autophagy also plays a key role in the ferroptosis in SAP, and 3-methyladenine(3-MA), an autophagy inhibitor, can alleviate ferroptosis in SAP, which deeps our understanding of autophagy and ferroptosis in the pathogenesis of SAP and may provide a new treatment for SAP.
Materials and methods
Chemicals and antibodies
Antibodies were purchased for immunoblot analysis, including anti-rabbit SLC7A11 (#26864–1-AP, Proteintech, China), anti-Mouse glutathione peroxidase 4 (GPX4) (#67763–1-Ig, Proteintech, China), anti-rabbit Ferritin Heavy Chain 1(FTH)1 (#10727–1-AP,Proteintech, China), anti-rabbit microtubule-associated protein1 light chain 3B (LC3B) (#14600–1-AP, Proteintech, China), anti-rabbit microtubule-associated P62/SQSTM1 Polyclonal antibody (#18420–1-AP, Proteintech, China) and GAPDH (#10494–1-AP, Proteintech,China). Autophagy inhibitor 3-Methyladenine (3-MA) (#S2767, Selleck,USA). Ferroptosis specific inhibitor Fer-1 (#S6243,Selleck,USA).
Animals and animal model
The animal procedures used in this study were performed according to the National Institutes of Health guidelines for laboratory animals and were approved by the Animal Care and Experiment Committee of Lanzhou University (Lanzhou, China). All animals received humane care and all efforts were made to minimize suffering .28 adult male Sprague–Dawley rats (age, 8–10 weeks; weight, 250-290 g) were obtained from the animal center of Lanzhou University (Lanzhou, China). The rats were randomly assigned to four groups: the sham control group, the SAP group, the Fer -1-treated SAP (SAP + Fer-1) group, the 3-MA-treated SAP (SAP + 3-MA) group. (each subgroup contained 7 rats).
Before the experiment, the rats fasted for 12 h. After anesthesia with 3% pentobarbital sodium (1 ml/kg body weight), the SAP model was induced by standard pressure-controlled infusion of freshly prepared 5% sodium taurocholate (Sigma) solution (0.1 ml/kg body weight) into the biliary pancreatic duct [12]. In the sham control group, the duct was infused with an equal quantity of sterile saline. In the SAP + Fer-1 group, Fer-1 (Selleck), a ferroptosis inhibitor, was administered i.p. at a concentration of 10 mg/kg body weight 1 h before the establishment of the SAP model, following previous study protocols [13, 14]. In the SAP + 3-MA group, 3-MA (Selleck), a autophagy inhibitor, was administered i.p. at a concentration of 20 mg/kg body weight 1 h before the establishment of the SAP model, following previous study protocols. Mouse in each group were fed standard granular food, free drinking water, and a sterile environment (25 ± 2° C; Light–dark cycle 12/12 h). All rats were killed 24 h later and samples were collected for follow-up experiments.
Sample collection
After SAP was induced, the rats were anesthetized again 24 h later. Blood samples were collected by puncture from the right ventricle. The blood samples were centrifuged at 3000 rpm at 4 °C for 15 min after standing for 30 min. The supernatant was subpacked into a group labeled, dried and sterilized EP centrifuge tube (200 µl/ tube) and frozen in a -80℃ refrigerator for the detection of amylase(AMY), lipase activity(LIPA), interleukin-6(IL-6), and tumor necrosis factor α (TNF-α) levels. The pancreatic head, ileum, lung, and kidney tissues near the cecum were removed and fixed with 4% paraformaldehyde for sectionalization. The remaining tissues were stored at -80 °C for subsequent analysis.
Serum assays
The activity of AMY and LIPA in blood samples was measured by the amylase kit. Serum TNF-α and IL-6 levels were detected using the standard diagnostic kit (Elabscience,China) according to the manufacturer’s instructions.
Iron measurements
Pancreatic tissue was prepared into 10% tissue homogenate. Tissue iron determination reagent (#E-BC-K773-M,Elabscience,China) to determine the relative iron content in the kidney. After treatment according to the manufacturer's instructions, the absorbance is measured at 520 nm and the iron content is calculated by using the corresponding formula.
Assessment of MDA, GPX4 and GSH content
Fresh pancreatic tissue was analyzed for malondialdehyde (MDA) and glutathione (GSH) levels and GPX4 activity using a commercially available kit (Jiancheng Biotech).
Transmission Electron Microscopy (TEM)
Pancreatic tissue was cleaned with pre-cooled PBS (pH 7.4) and then fixed in phosphate buffered glutaraldehyde (2.5%) and osmium tetroxide (1%). The pancreas sample was then cut, stained whole with 2% uranyl acetate (UA), dehydrated in a graded ethanol series, and embedded in epoxy resin. The slices (70–90 nm) are then stained with UA and lead citrate. Ultrastructure images were captured using transmission electron microscopy (Hitachi HT 7700, Tokyo, Japan).
Tissue histology and immunofluorescence
For histological analysis, the rat pancreas was immobilized in 4% formalin followed by paraffin embedding. The specimen was cut into 4 μm thick slices and stained with hematoxylin and eosin (H&E). The slices are then examined with an optical microscope. The pathological score was evaluated blind by two independent pathologists based on previously established scoring criteria [15, 16].
The expressions of ACSL4, FTH1 and LC3 were determined by immunofluorescence analysis. The low-temperature sections (4 μm thick) were fixed with 4% paraformaldehyde fixative at room temperature for 20 min, and then washed in PBS 3 times for 3 min. The slices were then incubated with a blocking solution (normal goat serum) at room temperature for 20 min. Next, the slices were incubated overnight with rabbit anti-ACSL 4 (Proteintech), rabbit anti-FTH1 (Proteintech), and rabbit anti-LC3 (Proteintech) primary antibodies at 4 °C. The slices were washed three times with PBS for 3 min each time, and incubated with secondary antibodies at room temperature for another 1 h. Then the slices were washed again with PBS, covered with a cover glass, and observed and photographed under a fluorescence microscope (Olympus, Tokyo, Japan).
Western bolt analysis
The expressions of SLC7A11, GPX 4, LC3, p62 and FTH1 in pancreatic tissues were detected by Western blot. Total protein was extracted from rat pancreas and quantified by BCA protein assay kit (Solarbio, Beijing, China). The extracted proteins were separated on a 10 or 15% sodium dodecyl sulphate–polyacrylamide gel and then transferred to a polyvinylidene fluoride (PVDF) membrane. Incubate the membrane overnight at 4 °C with the following primary antibodies: SLC7A11 (Proteintech), GPX 4 (Proteintech), LC3 (Proteintech), p62 (Proteintech), FTH 1 (Proteintech), GAPDH (Proteintech). Finally, the membrane was incubated with the corresponding secondary antibody at room temperature for 60 min, and the density of the blotted bands was measured using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
The data were presented as means ± standard deviation (S.D.), analyzed and visualized using ggplot2 in R version 3.6.3. Kaplan–Meier analysis was used to assess the survival rate, and the survival curves were plotted by GraphPad Prism 6.0 (San Diego, CA, USA). Two groups were compared by Student’s t-test and multiple groups were compared by one-way analysis (ANOVA) of variance followed by Tukey’s post hoctest. P values < 0.05 were considered statistically significant.
Results
Ferroptosis was activated in Rats with Taur-induced SAP
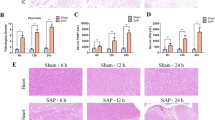
To assess sensitivity to ferroptosis in SAP, we measured Iron, MDA, and GSH levels in pancreatic tissue, as shown in Fig. 2A-C. Iron was an important factor in the execution of ferroptosis, and iron accumulated significantly in the SAP group compared to the sham group. In addition, MDA content in pancreatic tissue was significantly increased, while GSH level was decreased. Some core factors, such as SLC7A11, GPX4, and FTH1, are considered key ferroptosis regulatory proteins [17]. Therefore, we determined the expression levels of these proteins, as shown in Fig. 2D-G, the expression of these proteins decreased significantly in injured pancreatic tissues compared with normal tissues. Immunofluorescence microscopy shows changes in the expression of ACSL4 (red) and FTH1 (green) in the pancreas (Fig. 2H), the change is consistent with that of Western bolting. Next, TEM was used to study the morphological features of ferroptosis (Fig. 2I), and significant mitochondrial contraction was observed in the SAP group. These findings suggest that ferroptosis is activated in SAP.
Ferroptosis was activated in Rats with Taur-induced SAP. A Iron content in pancreatic tissue of rats. B GSH content in rats. C MDA content in pancreatic tissue of rats. D-G Protein levels of GPX4, SLC7A11 and FTH1 in rat pancreatitis. H Levels of ACSL4 (red) and FTH1 (green) proteins in the pancreas of rats. H Representative transmission electron microscopy image of mitochondria in rat pancreatic tissue. The data are expressed as the mean ± SD. **p < 0.01 versus the sham group
Ferroptosis inhibition improved survival and pancreas function in Rats with Taur-induced SAP
We further investigated the effects of inhibiting ferroptosis on survival and inflammation in SAP rats (Fig. 3A). As shown in Fig. 3B-E, inhibiting ferroptosis can significantly inhibit the rise of pancreatic amylase and lipase, as well as the inflammatory factors interleukin-6 and tumor necrosis factor in blood. Representative HE stained (Fig. 3F-G) sections of pancreas showed that the histological scores of pancreas in SAP rats were significantly higher than those in Sham group, and could be significantly improved by Fer-1. Overall, these data suggest that inhibiting ferroptosis improves survival and inflammation levels in SAP rats.
The role of autophagy in Taur-induced SAP
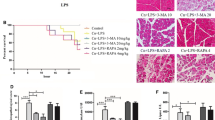
Autophagy is a process in which eukaryotic cells use lysosomes to degrade cytoplasmic proteins and destroy organelles under the regulation of autophagy related genes (Atg). p62 and LC3 are used as biomarkers of autophagy [18]. The role of autophagy in ferroptosis induced by sodium taurine cholate was studied with 3-MA. 3-MA is an inhibitor of PI3K. It is widely used as an autophagy inhibitor by inhibiting Class III PI 3 K (Fig. 4A) [19]. Compared with SAP group, 3-MA + SAP group showed lower Iron content (Fig. 4B), MDA levels (Fig. 4D), and higher GSH (Fig. 4C) and GPX4 (Fig. 4E) content levels. In Fig. 4F-J shows that the 3-MA + SAP group decreased the levels of LC3II/I and p62, and increased the expression levels of GPX 4 and FTH1. In addition, fluorescent double staining also showed a decrease in LC3II/I (green fluorescence) expression and an increase in FTH1 (red fluorescence) expression in the 3-MA + SAP group compared with the SAP group (Fig. 4K).
The role of autophagy in Taur-induced SAP. A Molding method and time. B Iron content in rats. C GSH content in pancreatic tissue of rats. D MDA content in pancreatic tissue of rats. E Pancreatic GPX4 content in rats. Protein levels of GPX4, FTH1, LC3 and p62 in F-J rats with pancreatitis. K Levels of LC3 (green) and FTH1 (red) proteins in the pancreas of rats. H Representative transmission electron microscopy image of mitochondria in rat pancreatic tissue. The data are expressed as the mean ± SD. **p < 0.01 versus the sham group
Discussion
Acute pancreatitis (AP) is a common and potentially threatening inflammation of the pancreas. Although usually self-limiting, up to 20% of patients may develop severe acute pancreatitis (SAP), which leads to systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction (MOF), affecting the lungs, liver, kidneys, and heart, among others. The main injury mechanisms are oxidative damage, apoptosis, autophagy, cell cycle inhibition and endoplasmic reticulum stress [20]. Ferroptosis is a novel form of cell death triggered by lipid peroxidation in an iron-dependent manner. Recently, the Committee on Cell Death Nomenclature (NCDD) classified ferroptosis as one of the regulators of cell death (RCD). When ferroptosis occurs in cells, the cells exhibit necro-like changes. These features include loss of plasma membrane integrity, organelle swelling, and moderate coagulation of chromatin [21]. Until now, it has been unclear whether ferroptosis is involved in the pathological damage of severe acute pancreatitis. In this study, we investigated the mechanism of ferroptosis in severe acute pancreatitis in rats. Ferroptosis is caused by oxidative perturbations in the intracellular microenvironment controlled by GPX 4. The accumulation of ferrous iron (Fe 2+) and the excessive oxidation of lipids are the key events that induce ferroptosis [22]. GPX 4 is a key negative regulator of ferroptosis that converts hydrogen lipid peroxide (L-OOH) to lipids (L-OH), thereby preventing lipid peroxide-induced cell death. System Xc− (a heterodimer composed of SLC 7A11 and SLC 3A2) can transport cystine into cells to produce GSH, and it can work with GPX 4 to eliminate excess lipid peroxides [23]. Our study found that the expression of SLC 7A11 and GPX 4 proteins, biomarkers of ferroptosis, was reduced, while the electron microscope showed mitochondrial atrophy in the pancreatic tissue of rats. The contents of GSH and MDA were dose-dependent. These results confirmed the occurrence of ferroptosis in severe acute pancreatitis in rats. Immunofluorescence showed that ACSL4 and FTH1 were mainly expressed in pancreatic acinar cells, suggesting that ferroptosis mainly occurred in the pancreatic head. This study also found that the pathological injury of pancreatitis in rats was significantly reduced after Fer-1 treatment, indicating that Fer-1 treatment reduced the damage of pancreatic tissue caused by ferroptosis, confirming that ferroptosis was an important factor in severe acute pancreatic injury in rats. Our previous studies have confirmed the occurrence of autophagy in rats with severe acute pancreatitis. Autophagy is a process used to degrade and recycle biological macromolecules and damaged or damaged cells within cells. In recent years, more and more research results support that ferroptosis is a kind of autophagy dependent cell death and a kind of selective autophagy [4, 24]. In order to verify the relationship between autophagy and ferroptosis in SAP, the autophagy inhibitor 3-MA was used in this study. The experimental results confirmed that compared with SAP group, 3-MA + SAP group could effectively inhibit the formation of ferroptosis. The autophagy mechanism of ferroptosis in SAP was also discussed. In summary, the results suggest that ferroptosis occurs in SAP in rats, and autophagy plays an important role in the occurrence of ferroptosis.
Conclusion
Autophagy mediated ferroptosis is one of the important mechanisms of severe acute pancreatitis. However, this study does not further investigate how ferroptosis and autophagy affect each other, nor does it further study at the cellular level.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–34. https://doi.org/10.1016/S0140-6736(20)31310-6.
Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–96. https://doi.org/10.1038/s41575-019-0158-2.
Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–90. https://doi.org/10.1001/jama.2020.20317.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64.
FFan R, Sui J, Dong X, Jing B, Gao Z. Wedelolactone alleviates acute pancreatitis and associated lung injury via gpx4 mediated suppression of pyroptosis and ferroptosis. Free Radic Biol Med. 2021;173:9–40.
Li HY, Lin YJ, Zhang L, Zhao J, Xiao DY, Huang ZZ, et al. Progress of pyroptosis in acute pancreatitis. Chin Med J (Engl). 2021;134(18):2160–2. https://doi.org/10.1097/CM9.0000000000001589.
Li H, Lin Y, Zhang L, Zhao J, Li P. Ferroptosis and its emerging roles in acute pancreatitis. Chin Med J (Engl). 2022;135(17):2026–34. https://doi.org/10.1097/cm9.0000000000002096.
Yuan H, Pratte J, Giardina C. Ferroptosis and its potential as a therapeutic target. Biochem Pharmacol. 2021;186:114486. https://doi.org/10.1016/j.bcp.2021.114486.
Liu J, Zhang Z, Yang Y, Di T, Wu Y, Bian T. NCOA4-mediated ferroptosis in bronchial epithelial cells promotes macrophage M2 polarization in COPD emphysema. Int J Chron Obstruct Pulmon Dis. 2022;17:667–81. https://doi.org/10.2147/COPD.S354896.
Wei S, Qiu T, Yao X, Wang N, Jiang L, Jia X, et al. Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. J Hazard Mater. 2020;384:121390. https://doi.org/10.1016/j.jhazmat.2019.121390.
Qin X, Zhang J, Wang B, Xu G, Yang X, Zou Z, et al. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17(12):4266–85. https://doi.org/10.1080/15548627.2021.1911016.
Ma D, Li C, Jiang P, Jiang Y, Wang J, Zhang D. Inhibition of ferroptosis attenuates acute kidney injury in rats with severe acute pancreatitis. Dig Dis Sci. 2021;66(2):483–92. https://doi.org/10.1007/s10620-020-06225-2.
Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivationof the ferroptosis regul- ator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91.
Li Y, Feng D, Wang Z, et al. Ischemia-induced ACSL4 activation contributes to ferropt- osis mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26(22):84–2299.
Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scori- ng in research. Vet Pathol. 2013;50:1007–15.
Zhang YM, Ren HY, Zhao XL, et al. Pharmacokinetics andpharmacodynamics of Da- Cheng-Qi decoction in the liver of rats with severe acute pancreatitis. World J Gastroenterol. 2017;23:1367–74.
Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023:1–15. https://doi.org/10.1080/15548627.2023.2165323.
Garg PK, Singh VP. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology. 2019;156(7):2008–23. https://doi.org/10.1053/j.gastro.2018.12.041.
Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent fer- roptosis: machinery and regulation. Cell Chem Biol. 2020;27(4):420–35. https://doi.org/10.1016/j.chembiol.2020.02.005.
Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Toth M. Genetics, cell biology, and pathophysiology of pancreatitis. Gastroenterology. 2019;156(7):1951-1968 e1951. https://doi.org/10.1053/j.gastro.2018.11.081.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis : a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85. https://doi.org/10.1016/j.cell.2017.09.021.
Yuan H, Pratte J, Giardina C. Ferroptosis and its potential as a therapeutic target. Biochem Pharmacol. 2021;186:114486. https://doi.org/10.1016/j.bcp.2021.114486.
Anandhan A, Dodson M, Shakya A, Chen J, Liu P, Wei Y, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023;9(5):eade9585. https://doi.org/10.1126/sciadv.ade9585.
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, et al. Autophagy promotes ferrop- tosis by degradation of ferritin. Autophagy. 2016;12(8):1425–8. https://doi.org/10.1080/15548627.2016.1187366.
Funding
This work was supported by the Cuiying Science and Technology Innovation program of the Second Hospital of Lanzhou University(No.CY2020-MS03).
Author information
Authors and Affiliations
Contributions
Hongyao Li and Ding Wu designed the studies. Haidan Zhang and Shixian Liu assisted with animal experiments. Jiahui Zhen and Yufen Yan participated in cell experiments.Hongyao Li wrote the manuscript. Hongyao Li revised the manuscript. All authors reviewed the manuscript. Peiwu Li is the guarantor of the article. Hongyao Li and Ding Wu Contributed equally.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal procedures used in this study were performed according to the National Institutes of Health guidelines for laboratory animals and were approved by the Animal Care and Experiment Committee of Lanzhou University (Lanzhou, China,Number:D2022-108).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Wu, D., Zhang, H. et al. Autophagy-mediated ferroptosis is involved in development of severe acute pancreatitis. BMC Gastroenterol 24, 245 (2024). https://doi.org/10.1186/s12876-024-03345-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03345-1