Abstract
Background
Permanent left bundle branch area pacing (LBBAP) has been established as an effective means to correct left bundle branch block. Right bundle branch block (RBBB), emerge as a distinct form of cardiac conduction abnormality, can be seen in the context of LBBAP procedure. However, the correction potential of LBBAP in patients with RBBB remains largely unexplored.
Objective
The objective of this study was to evaluate the efficacy and safety of permanent LBBAP in patients with RBBB.
Methods
Ninety-two consecutive patients who underwent successful permanent LBBAP were recruited from May. 2019 to Dec. 2022 in Fuwai Central China Cardiovascular Hospital. Among them, 20 patients with RBBB were included in our analysis. These patients were followed up at 1, 3, 6 and 12 months post-LBBAP. The QRS duration (QRSd) on the V1 lead of the 12-lead elctrocardiogram was measured and compared before and after the LBBAP procedure. Additionally, mitral regurgitation, tricuspid regurgitation and cardiac function were assessed using transthoracic echocardiography, specifically focusing on left ventricular ejection fraction (LVEF) and mitral regurgitation severity. The acute pitfills and delayed complications associated with the LBBAP procedure were recorded to evaluate its safety. SPSS 23.0 was used to perform statistical analysis with Student’s t test or one way ANOVA or nonparametric tests (paired Wilcoxon test). A p value less than 0.05 was defined as significant.
Results
The demographic breakdown of the RBBB cohort revealed a mean age of 66.35 ± 11.55 years, 60% being male. Comorbidities were prevalent, including severe atrioventricular block (AVB) in 75%, sick sinus syndrome (SSS) in 20%, heart failure in 25%, atrial fibrillation in 30%, coronary heart diseases in 45%, hypertension in 35%, and diabetes mellitus in 15%. Regarding the LBBAP procedure, the average operation time was 106.53 ± 2.72 min, with 45% of patients (9 individuals) requiring temporary cardiac pacing during the surgery. Notably, the LBBAP procedure significantly narrow the QRS duration in RBBB patients, from 132.60 ± 31.49ms to 119.55 ± 18.58 ms (P = 0.046). Additionally, at the 12-month follow-up, we observed a marked improvement in LVEF, which increased significantly from 55.15 ± 10.84% to 58.5 ± 10.55% (P = 0.018). Furthermore, mitral regurgitation severity improved, with a median reduction from 4.46 (0.9, 7.3) to 2.29 (0, 3.49) cm2 (P = 0.033). Importantly, no cases of ventricular septum perforation or pericardial effusion were reported during the LBBAP procedure or during the follow-up period.
Conclusion
LBBAP provides an immediate reduction in QRS duration for patients suffering from RBBB, accompanied by improvements in mitral regurgitation and cardiac function as evident in the 12-month follow-up period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
In recent decades, His bundle pacing (HBP) has evolved from an experimental technique to a preferred pacing method for patients with cardiac conduction diseases. HBP offers the most physiological ventricular activation pattern [1,2,3], yet its clinical adoption has been hindered by several challenges. The high likelihood of encountering a high pacing threshold, low R wave amplitude, and the intricate surgical techniques required for successful implantation pose significant barriers. The success of HBP often hinges on the surgeon’s mastery of these complex skills, and a deficiency in them can result in procedural failure, thereby limiting its clinical applicability.
Permanent left bundle branch area pacing (LBBAP), initially reported by Huang et al. [4], is a novel pacing method. It consistently produce a QRS morphology resembling right bundle branch block (RBBB) by preferentially activating the left bundle branch (LBB). LBBAP boasts a more stable and lower pacing threshold, along with relatively simpler operation skills. Furthermore, it is particularly suited for patients with infra-His block, as the pacing lead is positioned distal to the block site. In patients with left bundle branch block (LBBB), LBBAP has been established as an effective means to narrow QRS duration, reduce left ventricular end-systolic diameter, and enhance the left ventricular ejection fraction (LVEF) [5, 6]. However, the efficacy of LBBAP in RBBB patients remains suboptimal, with limited research exploring this aspect [7, 8]. Existing studies indicate that LBBAP can also correct RBBB, improving both left and right ventricular synchrony [8]. While LBBAP has been shown to decrease mitral regurgitation rates in LBBB patients [9], its impact on mitral regurgitation in RBBB remains elusive.
The aim of our study was to elucidate the effect of permanent LBBAP on mitral regurgitation specifically in RBBB patients. Additionally, we investigated the clinical characteristics, pacing parameters, and potential complications associated with this procedure.
Methods
Patient population: This is a retrospective study in our single center. Ninety-two consecutive bradycardia patients were evaluated in Fuwai Central China Cardiovascular Hospital from May 2019 to December 2022. Among them, 20 patients with RBBB were recruited in our study.
The definition of RBBB encompasses the presence of an rsR’ or RR’ pattern in leads V1 and/or V2, along with a wide S wave in leads V6 and I [10]. It is crucial to exclude patients with other conduction diseases, such as left posterior fascicular block, left anterior fascicular block or septal fascicular block.
Severe atrioventricular block (AVB) is defined as third-degree AVB; type two second-degree AVB; high-degree AVB; or first-degree AVB accompanied by symptoms like chest tightness, dizziness, amaurosis, or syncope. Notably, patients who require cardiac resynchronization therapy (CRT) and have failed coronary sinus lead placement are also considered candidates for LBBAP.
All patients provided written informed consent prior to LBBAP surgery with the patient’s consent to publish identified data. The institutional review board of Fuwai Central China Cardiovascular Hospital approved the study protocol.
LBBAP procedures: For the execution of Left Bundle Branch Area Pacing (LBBAP), the Model 3830 pacing lead from Select Secure by Medtronic Inc. (Minneapolis, MN) was selected. Utilizing the C315His delivery sheath, also from Medtronic Inc., the 3830 pacing lead was guided to the left bundle branch area. Positioning of the C315 sheath occurred precisely at the distal His location, visible on a right anterior oblique (RAO) 30° fluoroscopy view, accessed via the left or right axillary vein, with the left being the primary route.
Subsequently, the 3830 lead was delicately maneuvered through the C315 sheath into the ventricular septum. Upon successful positioning, a characteristic unipolar paced morphology of QRS waves emerged in lead V1, featuring a distinct notch at its nadir. To secure the lead’s position, one or two rotations were applied. By advancing the sheath was towards the septum and rotating it counterclockwise (90–180 degrees), the 3830 lead was then swiftly rotated 4–5 times deeping into the septum. The left bundle branch potential might be recorded, and the unipolar pacing morphology of the QRS complex in V1 resembled the pattern observed in RBBB. LBBAP was obtained by deeply screwing the 3830 pacing lead into the left ventricular septum where the left bundle branch was located. The depth of the 3830 lead into the ventricular septum was evaluated by angiography of the C315 sheath. The detailed procedures of LBBAP were performed according to those reported previously [8]. Importantly, a temporary actuator is previously prepared to mitigate the risk of cardiac arrest that could potentially arise during the screwing process into the septum.
The LBBAP should be confirmed as previously reported [11]; otherwise, the lead will be relocated. LBBAP was confirmed by two electrocardiographic criteria and at least one intracardiac electrogram criterion. The electrocardiographic criteria include: (1) RBBB-like QRS morphology during pacing with a terminal R-wave appearing in lead V1; and (2) the left ventricular activation time (LVAT) remains unchanged according to the pacing voltage. The intracardiac electrogram criteria include: (1) the left bundle branch potential can be recorded on the 3830 lead (only in normal ventricular activation pattern); and (2) the local ventricular electrogram during pacing shows a fusion pattern of native ventricular activation and pacing potential; and (3) the paced QRS duration is shorter than that of native activation. Both selective and nonselective LBBAP were acceptable. The depth of lead fixation in the septum was verified via angiography. Lead fixation should be stopped when the values of unipolar pacing impedance approach 500Ω or when the impedance drops by 200Ω. The criteria proposed by Jastrzebski M et al. [12] were used to distinguish LBBP from left ventricular septal pacing.
Measurements: QRS duration (QRSd) was measured at baseline and postoperatively following LBBAP using standard 12-lead electrocardiography settings (25 mm/s, 10 mm/mV), respectively. The QRSd was defined consistently as the temporal span from the initial onset of the QRS complex to its termination, aligned with the isoelectric line. Comparative analysis was conducted on the changes in QRSd (△QRS) between the preoperative and postoperative states of LBBAP, with the V1 lead selected for measurement accuracy. Furthermore, the LVAT was utilized as a metric to assess the activation sequence from the pacing site to the LV. This was achieved by recording the interval from the pacing stimulus to the peak of the R wave on the V5 lead, thereby quantifying the time taken for ventricular activation. To ensure reliability, all measurements were independently verified by two investigators on two separate occasions.
The pacing morphology of the QRS wave was analyzed by unipolar pacing, as well as the pacing threshold and impedance. The R wave amplitude was measured through bipolar pacing. Mitral regurgitation, tricuspid regurgitation and cardiac function were evaluated through transthoracic ultrasound using a GE Vivid E95 ultrasound machine by two independent ultrasound doctors. The average value was chosen. We used two-dimensional transthoracic echocardiography to evaluate mitral regurgitation and tricuspid regurgitation, measuring the area of regurgitation in square centimeters through a parasternal long-axis view.
Procedure-related complications: The acute complications associated with the LBBAP procedure encompass pneumothorax, pericardial effusion, and the dire risk of acute cardiac arrest. Notably, ventricular septum perforation stands as a unique and critical complication that may manifest when there is an abrupt decrease in lead impedance exceeding 400 Ω. As for delayed complications, including a threshold increase, lead dislodgement, and device-related infections, were documented during follow-up evaluations.
Follow-up: Patients underwent comprehensive follow–up evaluations at 1, 3, 6 and 12 months post-discharge. At baseline and during each follow-up visit, a 12-lead ECGs was administered. During these sessions, encompassing the lead threshold, impedance and R wave amplitude, to ensure optimal performance and patient outcomes.
Statistical analysis: SPSS 23.0 was used to perform statistical analysis. Continuous data with a normal distribution are presented as the mean ± standard deviation and were assessed using Student’s t test or one way ANOVA. Continuous data with skewed distributions are described as medians with interquartile ranges (IQRs: 25th to 75th percentiles) and were analyzed using nonparametric tests (paired Wilcoxon test). Categorical data are expressed as numbers and percentages (%). A p value less than 0.05 was defined as significant.
Results
General characteristics of RBBB patients undergoing the LBBAP procedure
For our study, we recruited twenty patients with RBBB out of 92 consecutive bradycardia patients, all of whom were intending to undergo the LBBAP procedure. Of these twenty patients, cardiac resynchronization therapy (CRT) was attempted in 5. The baseline clinical characteristics of the study population are summarized in Table 1.
The mean age of the patients was 66.35 ± 11.55 years, with 60% being male. Among them, 75% had severe atrioventricular block (AVB), 20% had sick sinus syndrome (SSS), 25% had heart failure, 30% had atrial fibrillation, 45% had coronary heart diseases, 35% had hypertension, and 15% had diabetes mellitus. At baseline, the QRS duration (QRSd) averaged 132.6 ± 31.49 ms. Notably, nearly 75% of the patients with RBBB exhibited severe atrioventricular block. Furthermore, the average left ventricular end-diastolic diameter (LVEDD) at baseline was 54.20 ± 9.92 mm, accompanied by a left ventricular ejection fraction (LVEF) of 55.15 ± 10.84%, indicating slight cardiac dysfunction among the RBBB patients included in this study.
The detailed procedures and lead parameters of LBBAP
The total operative duration for the LBBAP procedure averaged 106.53 ± 2.72 min, with an X-ray exposure time of 5.11 ± 0.28 min. Among the patients, five patients (25%) underwent successful selective LBBAP. Additionally, nine patients (45%) accepted temporary cardiac pacing support during the LBBAP procedure. The mean time from stimulus to left ventricular activation (LVAT) in RBBB patients was 76.55 ± 1.03 ms.
The LBBAP lead parameters, comprising the lead threshold, R-wave amplitude, and impedance, remained stable and within acceptable ranges. A comprehensive summary of the detailed data is presented in Table 2. Moreover, the pacing parameters maintained stability throughout periods at 1, 3, 6, and 12 months post-LBBAP procedure, as outlined in Table 3.
Changes in QRS duration, mitral regurgitation, and cardiac function in RBBB post LBBAP
After LBBAP, the paced QRS duration (QRSd paced) for RBBB patients was 119.55 ± 18.58 ms, resulting in a significant QRSd reduction (△QRSd = QRSd paced - QRSd baseline) of -13.05 ± 27.29 ms (P = 0.046, Fig. 1A). Notably, LBBAP completely narrowed the wide QRS complex typically observed in RBBB patients, as evidenced by the reduction from 132.60 ± 31.49ms at baseline to 119.55 ± 18.58 ms post-pacing.
Changes in QRSd, cardiac structure and function in RBBB patients before and after LBBAP. A. The mean paced QRSd of LBBAP was associated with a significant reduction in RBBB patients (P < 0.05). B. The LVEF in RBBB patients increased significantly after 12 months of LBBAP follow-up (P < 0.05). C. Mitral regurgitation decreased significantly at the 12-month follow-up with LBBAP in RBBB patients (P < 0.05). D-F. The LA (D), LVEDD (E) and tricuspid regurgitation (F) of echocardiogram at baseline and at 12 months post LBBAP pacing showed no significant change in RBBB patients (P > 0.05). G-H. The mitral regurgitation (red dotted line) decreased from 14.2 cm2 (G) to 7.1 cm2 (H) in an RBBB patient at the 12-month follow-up of the LBBAP procedure. LBBAP = left bundle branch pacing; RBBB = right bundle branch block; QRSd = QRS duration; LVEF = left ventricular ejection fraction; LA = left atrium; LVEDD = left ventricular end-diastolic dimension; MR = mitral regurgitation; TR = tricuspid regurgitation. P < 0.05 indicates statistical significance. Student’s t test used in A, B D, E, nonparametric tests (paired Wilcoxon test) used in C, F
In RBBB patients, the baseline LVEF was 55.15 ± 10.84%, which significantly improved to 58.50 ± 10.55% after 12 months of LBBAP pacing (P = 0.018, Fig. 1B). Additionally, the median mitral regurgitation area decreased significantly from 4.46 (0.9, 7.3) cm2 at baseline to 2.29 (0, 3.49) cm2 at the 12-month follow-up (P = 0.033, Fig. 1C). However, no significant changes were observed in left atrial dimensions (LA), LVEDD, or tricuspid regurgitation (all P > 0.05, Fig. 1D-F).
The correction of RBBB depends on the recruitment of the distal right bundle branch
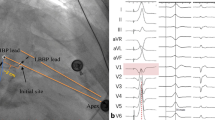
In our study, all 20 patients with RBBB underwent successful correction via LBBAP, achieving a 100% success rate. This correction was attributed to the distal recruitment of the RBB through transverse interconnection, which was manifested by typical electrocardiographic changes. Specifically, there was either disappearance or significant shortening of the R wave in lead V1 among RBBB patients post-LBBAP (Fig. 2).
A patient with second-degree type II AVB and RBBB was implanted with a dural-chamber pacemaker with a 3830 lead at the left bundle branch area. A. The basic ECG illustrated bradycardia and RBBB with a QRS duration of 130 ms. B. RBBB was corrected after LBBAP with disappearance or shortening of the R wave on V1. C-E. The LBBAP pacing lead (black arrow) is shown in three different orientations of chest X-ray: PA position (C), RAO position (D), and LAO position (E). AVB = Atrioventricular Block; LBBAP = Left Bundle Branch Area Pacing; RBBB = Right Bundle Branch Block; PA = Posteroanterior view; RAO = Right Anterior Oblique view; LAO = Left Anterior Oblique view
Analogous to the traditional His-Purkinje system, transverse interconnection can also be functionally blocked. The morphology of the paced QRS complex exhibits variability contingent upon the conduction patterns within the left bundle branch and the activation status of the distal RBB. Modulation of the atrio-ventricular (AV) pacing interval can dictate whether the distal RBB is activated via transverse interconnection. As illustrated in Fig. 3, with an AV pacing interval of 150 ms, complete correction of RBBB was achieved, manifesting as the disappearance or shortening of the R wave in lead V1 lead on the electrocardiogram. Conversely, prolonging the AV pacing interval to 250ms failed to correct RBBB, indicating the absence of transverse interconnection activation. Notably, at an AV pacing interval of 200 ms, a functional conduction block within transverse interconnection was revealed, as evidenced by the presence of a typical Qr wave in V1 lead, suggesting that a portion of the electrical impulse fell within the refractory period of the distal right bundle branch.
Paced QRS morphology varied depending on recruitment of the distal right bundle branch in an RBBB patient. A. This patient exhibited sinus bradycardia and RBBB at baseline. B. The QRS morphology of RBBB was fully corrected by LBBAP with a pacing AV interval of 150 ms, which exhibited the recruitment of distal RBB via 3830 leads with the disappearance or shortening of the R wave on the V1 lead. C. The pacing QRS morphology with RBBB was uncorrected when the pacing AV interval was prolonged to 250 ms, which demonstrated nonparticipation of distal RBB. D. The RBBB was partly corrected (a typical Qr wave on V1 lead) when the parameter was set with a pacing AV interval of 200 ms, which showed a functional block of transverse interconnection while part of the electrical impulse fell in the refractory period of the distal right bundle branch. RBBB = right bundle branch block; RBB = right bundle branch; LBBAP = left bundle branch area pacing
Procedural-related complications of LBBAP
One patient passed away one month post-LBBAP procedure due to unclear causes of heart failure. Another patient experienced lead dislodgement during the two-month follow-up period and subsequently underwent lead repositioning surgery. Notably, neither ventricular septum perforation nor pericardial effusion was encountered during the LBBAP procedure or during the subsequent follow-up assessments.
Discussion
LBBAP emerges as a promising therapeutic modality in the realm of physiological pacing. Its advantages lie in a more stable pacing threshold, a reduced procedural time, and a heightened success rate compared to HBP, achieved through the deep implantation of a pacing lead into the left intraventricular septum, thereby ensuring capture of the left bundle branch. Notably, HBP falls short for patients with bundle branch block whose block sites extend beyond the pacing site. In contrast, LBBAP presents a variable alternative for these patients, effectively traversing the block site to physiologically stimulate the left ventricle, thereby expanding treatment options.
LBBAP was initially documented by Huang et al. [4] in a case study involving a patient with dilated cardiomyopathy and LBBB, where conventional HBP had failed. By advancing the lead deeper into the interventricular septum, they unexpectedly activated the left bundle branch, resulting in the resolution of LBBB on electrocardiogram (ECG). Subsequent follow-up revealed marked enhancement in cardiac function. Furthermore, Chen et al. [13] pioneeringly discovered that the tip of the lead helix used for LBBAP was positioned in proximity to the LBB in an in vivo canine model. In their subsequent work [11], Chen et al. succinctly outlined the key features of LBBAP as follows: (1) an ECG pattern resembling RBBB; (2) clear demonstration of LBB potential; (3) Selective-LBBAP with distinct ECG morphology changes and a discernible component in the EGM; and (4) a consistent and shortest stimulus duration to achieve LVAT across varying pacing outputs.
LBBAP has proven to be both effective and safe in clinical applications. Notably, it has the ability to narrow the QRS complex in patients diagnosed with LBBB [5, 6], resulting in a shortened left ventricular activation time and significantly enhancing cardiac function among heart failure patients with LBBB [6]. Ravi et al. [14] conducted a comprehensive study on the success rate and complications associated with LBBAP, reporting a success rate of 97%. Following a mean follow-up period of 6.2 ± 5 months, only 7 lead-related complications (12.3%) were observed, with 3 patients (5.3%) requiring lead revision and a single case of interventricular septal perforation. In contrast, our study demonstrated an even lower incidence of LBBAP-related complications. Specifically, no instances of ventricular septum perforation or pericardial effusion were encountered during the LBBAP procedure or during follow-up. Furthermore, the lead parameters remained stable throughout the follow-up period, with only one patient requiring lead revision.
When discussing the intricacies of LBBAP, a more natural and academically sound way to express the phenomena and its underlying mechanisms would be as follows: LBBAP initiates early activation of the left bundle branch, leading to the manifestation of a RBBB morphology in lead V1, typically exhibiting patterns such as Qr, qR, rSR’, or QS. Since the RBBB block site is spatially distant from the LBBAP site, this conduction anomaly can potentially be clinically ameliorated through LBBAP. The inherent mechanism underlying this process is multifaceted and intricate. One hypothesis revolves around the phenomenon of longitudinal dissociation within the His bundle, which may offer some insight. It is conceivable that the simultaneous stimulation of a parallel conduction pathway, extending from the LBBAP site to the right bundle branch, serve as the fundamental mechanism. Furthermore, research has documented the existence of transverse interconnections within the His bundle and its branching system, which may further contribute to the complex electrophysiological interplay observed during LBBAP. Lazzara and colleagues [15] conducted observations on intracellular stimulation within the proximal conduction system, specifically focusing on its impact on distal activation. They discovered that stimulating various points within the His bundle, via intracellular means, could elicit activation of the right bundle branch in a manner that closely mimics the physiological atrioventricular conduction process. Furthermore, Chu and his team [16] reported a case where intrinsic RBBB was correction by strategically capturing either the intra-Hisian left bundle branch or the distal His bundle, depending on the pacing output settings. Notably, pacing at high output activated the distal right bundle branch, whereas pacing at a low output preferentially activated the high right ventricular septal branch. Mahmud et al. [17] conducted a study involving 39 patients with RBBB during HBP. The findings revealed that RBBB could be effectively corrected through nonselective HBP with higher pacing voltages, as opposed to selective HBP, indicating that the pacing signal was capable of traversing the RBBB site. This suggests that the conduction block may be bypassed or conducted via parallel pathway. Consequently, the right bundle branch can be activated by capturing the intra-Hisian LBB through functional transverse interconnections. The recruitment of the RBB via transverse interconnections by NS-LBBAP aids in achieving synchronized ventricular activation in the presence of RBBB.
In patients with RBBB, the QRS morphology can undergo narrowing via LBBAP. Zhu et al. [18] described a case of 63-year-old male with high-degree atrioventricular block and complete RBBB, where the intrinsic QRS duration markedly shortened following LBBAP and further decreased with an increase in pacing output. In a subsequent study by Zhu et al. [19], 32 patients with complete RBBB underwent the LBBAP, revealing a statistically significant reduction in the paced QRS duration compared to their intrinsic QRS duration (115.58[5.80] ms vs. 144.31[4.83] ms, P < 0.001), with a mean difference of 28.74(3.30) value. Our study aligns with these findings, demonstrating that the paced QRSd (119.55 ± 18.58 ms) was significantly shorter than the baseline QRSd in RBBB patients (P = 0.046).
In RBBB patients undergoing LBBAP, the QRS morphology assumes distinctive features. The most prevalent LBBAP-associated RBBB morphology in lead V1 exhibits Qr, qR, rSR’, or QS pattern, which reflects the activation of both the LV and RV. Specifically, the downslope of the QS pattern in lead V1 is indicative of high RV septal pacing, whereas an abruptly narrower QRS waveform suggests left bundle branch pacing, thereby signifying the correction of the RBBB status. Notably, the disappearance or shortening of the R wave in V1 is a hallmark of His bundle capture accompanied by recruitment of the RBB [16, 18]. Consistent with these observations, we noted similar alterations in the R wave of lead V1 among RBBB patients undergoing LBBAP.
The inclusion of LBBAP has been shown to be beneficial to heart function in individuals suffering from AVB [20] or LBBB complicated by heart failure [5, 6, 14]. Li et al. [21] conducted a study demonstrating the positive impact of LBBAP on clinical outcomes in patients who underwent cardiac resynchronization therapy (CRT). At the nine-month follow-up mark, among the 25 patients who received LBBAP therapy, significant improvements were observed Specifically, the New York Heart Association (NYHA) functional class improved dramatically, declining from a baseline of 2.6 ± 0.6 to 1.48 ± 0.6 (P < 0.001), indicating an overall enhancement in functional capacity. Furthermore, the LVEF increased substantially, rising from 35.2 ± 7.0% at baseline to 46.9 ± 10.2% (P < 0.001), signifying an improvement in cardiac contractility and ejection performance. In our research, we discerned a favorable impact of LBBAP on heart function specifically in patients with RBBB. Notably, at the 12-month follow-up subsequent to LBBAP implantation, we observed a statistically significant improvement in LVEF among RBBB patients (P = 0.018), underscoring the therapeutic benefits of this approach. Additionally, we found that LBBAP mitigated mitral regurgitation in these patients, with a notable reduction recorded (P = 0.033), further highlighting its potential to address multiple cardiac manifestions. Importantly, given that heart failure accompanied by RBBB is generally not amenable to cardiac resynchronization therapy (CRT), our findings suggest that LBBAP could emerge as a promising therapeutic option for such patients, owing to its demonstrated positive effects on heart function. The observed improvement in mitral regurgitation could potentially stem from the restoration of physiological ventricular conduction, thereby mitigating the abnormal motion pattern between the right and left ventricles. However, the long-term impact of LBBAP on LA and LVEDD remains to be rigorously assessed through extended follow-up studies. Moreover, there is a pressing need to delve deeper into the underlying mechanisms of mitral regurgitation and how they intertwine with LBBAP.
A recent study [22] has drawn attention to the association between exacerbated tricuspid regurgitation and a proximal positioning of the LBBAP lead, particularly when the distance from the lead to the tricuspid valve falls below 24.6 mm. In contrast, our study did not observe a worsening of tricuspid regurgitation post-LBBAP, which could plausibly be attributed to a more distal placement of the lead relative to the tricuspid valve. Nonetheless, this hypothesis necessitates rigorous validation through additional research endeavors.
Limitations
In our study, we presented the QRS complex modification of RBBB achieved via LBBAP. Nevertheless, our findings are subject to several limitations: Firstly, the sample size is limited due to the rarity of such patient cases, which may impact the generalizability of our results. Secondly, being a retrospective, single-center study, we were unable to comprehensively investigate the effects of LBBAP. To gain a more comprehensive understanding of the underlying mechanisms and to validate our findings, large-scale, multi-center studies are warranted.
Conclusion
LBBAP has demonstrated the potential to enhance mitral regurgitation and cardiac function in patients with RBBB, suggesting it as a promising and safe therapeutic option for this patient population.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct his-bundle pacing: a novel approach to cardiac pacing in patients with normal his-purkinje activation. Circulation. 2000;101(8):869–77. https://doi.org/10.1161/01.cir.101.8.869.
Vijayaraman P, Chung MK, Dandamudi G, et al. His bundle pacing. J Am Coll Cardiol. 2018;72(8):927–47. https://doi.org/10.1016/j.jacc.2018.06.017.
Vijayaraman P, Bordachar P, Ellenbogen KA. The continued search for physiological pacing: where are we now? J Am Coll Cardiol. 2017;69(25):3099–114. https://doi.org/10.1016/j.jacc.2017.05.005.
Huang W, Su L, Wu S, et al. A Novel Pacing Strategy with Low and stable output: pacing the Left Bundle Branch immediately beyond the Conduction Block. Can J Cardiol. 2017;33(12):1736. e1-1736.e3.
Zhang J, Wang Z, Cheng L, et al. Immediate clinical outcomes of left bundle branch area pacing vs conventional right ventricular pacing. Clin Cardiol. 2019;42(8):768–73. https://doi.org/10.1002/clc.23215.
Zhang W, Huang J, Qi Y, et al. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019;16(12):1783–90. https://doi.org/10.1016/j.hrthm.2019.09.006.
Huang W, Chen X, Su L, et al. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16(12):1791–6. https://doi.org/10.1016/j.hrthm.2019.06.016.
Li X, Fan X, Li H, et al. ECG patterns of successful permanent left bundle branch area pacing in bradycardia patients with typical bundle branch block. Pacing Clin Electrophysiol. 2020;43(8):781–90. https://doi.org/10.1111/pace.13982.
Peng X, Chen Y, Wang X, Hu A, Li X. Safety and efficacy of his-bundle pacing/left bundle branch area pacing versus right ventricular pacing: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2021;62(3):445–59. https://doi.org/10.1007/s10840-021-00998-w.
Surawicz B, Childers R, Deal BJ, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e235-e240. https://doi.org/10.1161/CIRCULATIONAHA.108.191095
Chen X, Wu S, Su L, et al. The characteristics of the electrocardiogram and the intracardiac electrogram in left bundle branch pacing. J Cardiovasc Electrophysiol. 2019;30(7):1096–101. https://doi.org/10.1111/jce.13956.
Jastrzębski M, Kiełbasa G, Curila K, et al. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm. 2021;18(6):935–43. https://doi.org/10.1016/j.hrthm.2021.02.021. indexed in Pubmed: 33677102.
Chen X, Jin Q, Li B, et al. Electrophysiological parameters and anatomical evaluation of left bundle branch pacing in an in vivo canine model. J Cardiovasc Electrophysiol. 2020;31(1):214–9. https://doi.org/10.1111/jce.14300.
Ravi V, Hanifin JL, Larsen T, et al. Pros and cons of Left Bundle Branch Pacing: a single-center experience. Circ Arrhythm Electrophysiol. 2020;13(12):e008874. https://doi.org/10.1161/CIRCEP.120.008874.
Lazzara R, Yeh BK, Samet P. Functional transverse interconnections within the his bundle and the bundle branches. Circ Res. 1973;32(4):509–15. https://doi.org/10.1161/01.res.32.4.509.
Chu SY, Sheng QH, Chen ED, et al. Is the left bundle branch pacing a choice to conquer the right bundle branch block?-A case report. Ann Noninvasive Electrocardiol. 2021;26(2):e12797. https://doi.org/10.1111/anec.12797.
Mahmud R, Jamal S. Effect of his bundle pacing on right bundle branch block located distal to site of pacing. J Electrocardiol. 2021;64:58–65. https://doi.org/10.1016/j.jelectrocard.2020.11.009.
Zhu K, Lin M, Li L, et al. Left bundle branch pacing shortened the QRS duration of a right bundle branch block. J Electrocardiol. 2021;68:153–6. https://doi.org/10.1016/j.jelectrocard.2021.08.018.
Zhu K, Sun Y, Cai B, et al. Left bundle branch pacing in patients with right bundle branch block. Kardiol Pol. 2021;79(10):1127–9. https://doi.org/10.33963/KP.a2021.0091.
Hu Y, Li H, Gu M, et al. Comparison between his-bundle pacing and left bundle branch pacing in patients with atrioventricular block. J Interv Card Electrophysiol. 2021;62(1):63–73. https://doi.org/10.1007/s10840-020-00869-w.
Li Y, Yan L, Dai Y, et al. Feasibility and efficacy of left bundle branch area pacing in patients indicated for cardiac resynchronization therapy. Europace. 2020;22(Suppl2):ii54–60. https://doi.org/10.1093/europace/euaa271.
Bednarek A, Kiełbasa G, Moskal P, et al. Left bundle branch area pacing improves right ventricular function and synchrony. Heart Rhythm Published Online May. 2024;13. https://doi.org/10.1016/j.hrthm.2024.05.019.
Funding
This study get a finical support from funding of The Medical Science and Technology Commission Foundation of Henan Province (No. LHGJ20231349).
Author information
Authors and Affiliations
Contributions
Ji-Fang Ma, Hai-Xia Fu contributed to conception and design of the study. Ji-Fang Ma, Juan Hu, Ke Chen, Xiao-Biao Zang organized the database. You Zhou, Wei-Feng Song performed the statistical analysis. Ji-Fang Ma wrote the first draft of the manuscript. Xian-Qing Wang, Yong-Hui Zhao wrote sections of the manuscript, Hai-Xia Fu complete the review of the article and revised it. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional review board of Fuwai Central China Cardiovascular Hospital approved the study protocol. Written informed consent for publication was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, J., Hu, J., Song, W. et al. Permanent left bundle branch area pacing improves mitral regurgitation and cardiac function in patients with right bundle branch block. BMC Cardiovasc Disord 24, 501 (2024). https://doi.org/10.1186/s12872-024-04175-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04175-5