Abstract
Introduction
Total knee arthroplasty (TKA) is accompanied by severe postoperative pain, which is reported to be an important cause of chronic pain. Ultrasound-guided adductor canal block (ACB) combined with infiltration between the popliteal artery and posterior capsular of the knee (IPACK) has been proven to have a better effect on relieving acute pain after TKA. However, whether it has a significant effect on the incidence of chronic pain after TKA has not been reported. This trial was designed to investigate the effect of ultrasound-guided ACB combined with IPACK on the incidence and intensity of chronic pain after TKA.
Methods
In this prospective, randomized, double-blind, placebo-controlled study, 100 subjects scheduled for TKA were randomly (1:1) divided into two groups: the ropivacaine group and the placebo group. Patients in each group received ultrasound-guided ACB + IPACK procedures with 0.25% ropivacaine or equal volume normal saline. All patients received multimodal analgesia. Pain intensity was assessed using the Numerical Rating Scale (NRS). The primary outcome was the incidence of chronic pain at 3 months after TKA by telephone follow-up. In addition, pain intensity in early resting and mobilized states, chronic pain intensity, the time to first rescue analgesia; opioid consumption; CRP and IL-6 after the operation; length of postoperative hospital stay; and cost of hospitalization and postoperative complications; as well as the function of the knee in the early stage after the operation, were recorded.
Results
Ninety-one participants were included in the final analysis. At 3 months, the incidence of chronic pain was 30.4% in the ropivacaine group, significantly lower than 51.1% in the placebo group. Compared with the placebo group, the ACB + IPACK with ropivacaine group had significantly lower pain scores at 4 hours, 8 hours, 16 hours, and 24 hours after the operation; increased the knee range of motion at 8 hours and 24 hours after the operation; and a significantly decreased incidence of chronic pain at 3 months after the operation. During the follow-up period, there were no nerve block-related complications in either group.
Conclusion
In the context of multimodal analgesia protocols, ACB combined with IPACK before surgery decreases the incidence and intensity of chronic pain 3 months after TKA compared with placebo injection. In addition, it reduces the NRS scores, whether at rest or during mobilization, and improves knee function within 24 hours after TKA.
Trial registration
This trial was registered in the China Clinical Trial Center (registration number ChiCTR2200065300) on November 1, 2022.
Similar content being viewed by others
Introduction
Knee osteoarthritis is a pathological condition based on joint degenerative changes and is characterized by joint pain and dysfunction caused by the degeneration and destruction of articular cartilage [1]. Nearly 40% of the population older than 65 years is thought to have knee osteoarthritis, and the incidence is steadily increasing [2]. Total knee arthroplasty (TKA) is the most effective treatment for end-stage knee osteoarthritis [3]. However, TKA is often accompanied by severe pain. Postoperative pain not only limits early postoperative knee joint functional exercise but also affects the long-term knee joint function of patients, which may result in a decrease in patient quality of life and satisfaction [4, 5]. With the progress of surgical techniques and the implementation of multimodal analgesia strategies in recent years, postoperative pain is expected to be well controlled in most patients [6, 7]. Despite the good results of knee radiography after TKA, after excluding infection, prosthesis loosening and other causes, approximately 49% of patients still experience knee joint pain that lasts for more than 3 months [8]. 15% of patients still have extreme knee pain 3 to 4 years after surgery; however, the pathogenesis of pain is difficult to determine [8]. The International Association for the Study of Pain (IASP) defines postoperative chronic pain (chronic post-surgical pain, CPSP) as pain that lasts for more than 3 months [9].
The causes of CPSP after TKA are complex. Current studies have shown that it is related to preoperative knee pain, anxiety and depression, pain catastrophizing, preoperative medication, sex, age, BMI, intraoperative factors, and postoperative acute pain [10, 11]. When the known other risk factors are controlled, severe postoperative pain in the acute phase is an important risk factor for the occurrence of postoperative chronic pain [12, 13]. With the concept of accelerated rehabilitation surgery (enhanced recovery after surgery, ERAS), peripheral nerve block began to play an important role in multimodal analgesia after TKA. In the past, ultrasound-guided femoral nerve block combined with sciatic nerve block was the most effective nerve block protocol after TKA [14]. Considering its effect on lower limb muscle strength [15], adductor canal block (ACB) combined with infiltration between the popliteal artery and posterior capsular of the knee (IPACK) has been proven to be effective postoperative analgesic regimen following TKA procedures [16, 17]. Due to its analgesic effect and minimal effect on muscle strength, it has been favored by the majority of anesthesia providers, surgeons and patients.
Therefore, we designed this trial to investigate the effect of ultrasound-guided adductor canal block combined with local anesthetic infiltration between the popliteal artery and posterior capsular of the knee on the incidence of chronic pain after TKA. We hypothesized that ACB combined with IPACK could effectively relieve postoperative pain, thus reducing the incidence of chronic pain after TKA.
Materials and methods
This single-center, prospective, double-blind, randomized, placebo-controlled clinical trial was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (number: 2022ER399-1) and registered in the China Clinical Trial Center (registration number: ChiCTR2200065300 https://www.chictr.org.cn/showproj.html?proj=183866) on November 1, 2022, before patient enrollment. All patients provided written informed consent to participate.
Recruitment
After admission, the subjects were evaluated and recruited by a co-investigator before surgery. At the same time, the basic characteristics (including gender, age, BMI, and ASA status, etc.), preoperative pain score, Hospital Anxiety and Depression Scale (HADS) [18], Pain Catastrophizing Scale (PCS) [18], knee range of motion (ROM), and medication use were recorded. After providing consent, the patients were instructed on how to score their pain (NRS: 0–10; 0 for no pain, 10 for the most severe pain) and were randomly divided (1:1) into two groups (the ropivacaine group and saline group) according to computer-generated randomized numbers (Excel, Microsoft Corporation, Redmond, WA).
We enrolled patients aged 18–80 years between November 1, 2022, and July 1, 2023, with American Society of Anesthesiologists physical status I-III who underwent elective unilateral TKA. The exclusion criteria included preoperative complications of severe heart, brain, lung, liver, and kidney diseases; a history of rheumatism and rheumatoid arthritis; an allergy to drugs involved in the study; the use of chronic opioids (i.e., continuous use of opioids for more than 3 months); a history of other chronic pain diseases; skin rupture and infection at the puncture site; a preoperative NRS score greater than or equal to 4; preoperative anxiety and depression; and a preoperative pain catastrophizing state. All subjects had the right to terminate and drop out of the trial at any time.
Nerve block regimen
All patients received 200 mg of celecoxib orally on the day of the operation. The allocation information was contained in opaque envelopes and opened according to the order before the beginning of the nerve block by an anesthetic nurse who was not involved in the trial. Anesthesia nurses prepared the nerve block solution according to the allocation. For the ropivacaine group, 25 ml of ropivacaine (for the IPACK block) and 15 ml of ropivacaine (for the ACB block) were diluted to 0.25% with normal saline and prepared into two syringes. The saline group was prepared with the same volume of normal saline. The attending anesthesiologists, surgeons, patients, nurses, and data collectors were blinded to the group assignments.
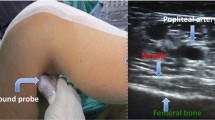
After entering the preoperative unit before anesthesia, patients received venous cannulation, standardized monitoring, and nasal cannula placement for oxygen inhalation. Midazolam (1 mg) and fentanyl (20 µg) were administered intravenously before puncture. All nerve block procedures were performed by the same experienced anesthesiologist. For IPACK, the patient was placed in a prone position, the popliteal vessel was scanned at the popliteal striation with a low-frequency (2–5 MHz) convex array probe (TE7, Mindray, Shenzhen, China), and then the probe was moved slowly toward the side of the head until the internal and external condyle of the femur and the popliteal artery were exposed. After local infiltration with 1% lidocaine, nerve block was performed with an 80 mm 22-gauge nerve stimulation needle (B. Braun, Hessen, Germany). The puncture needle was inserted into the space between the popliteal artery and the posterior capsule of the knee from medial to lateral through an in-plane approach, and local anesthetic (25 ml) was injected after aspiration without blood. Then, the patient was placed in a supine position and scanned at the middle and anteromedial parts of the thigh with a high-frequency (10 MHz) linear probe. Then, the probe was moved slowly toward the distal end until the sartorius muscle, adductor longus muscle, adductor major muscle, medial femoris muscle and superficial femoral artery were visualized, and the solution (15 ml) was injected into the adductor canal. Twenty minutes after injection, a researcher who was blinded to the group allocation tested the sensory distribution through acupuncture the medial knee and the anterior medial skin of the leg, which were divided into 0, 1, and 2 grades according to no sensation, only touch sensation, and sharp sensation, respectively. Blocking success was defined as the level of 0 or 1 of acupuncture sensation detected within 20 min of completion of the injection. Thirty minutes after the injection, the patients were sent to the operating room.
Intraoperative regimen
After monitoring the ECG, SPO2, and noninvasive blood pressure in the operating room, standardized anesthesia induction began. Tracheal intubation was performed after administration of 1–2 mg/kg propofol, 3 µg/kg fentanyl, or 0.15 mg/kg cisatracurium. After induction, a tourniquet was applied, and the position where the tourniquet was applied and the pressure at which it was inflated were set by the surgeon. Sevoflurane inhalation and a remifentanil pump were used to maintain general anesthesia (BIS: 40–60). The PETCO2 was maintained between 35 and 45 mmHg by adjusting the respiratory rate and tidal volume. If the heart rate was less than 50 bpm, 0.5 mg of atropine was injected intravenously; when the heart rate exceeded 100 bpm, 10 mg of esmolol was injected intravenously, which could be repeated if necessary. When the systolic blood pressure was lower than 90 mmHg or the mean arterial pressure was lower than 65 mmHg, 9 mg ephedrine was injected intravenously, and when the systolic blood pressure exceeded 20% above preoperative baseline, 10 mg urapidil was injected intravenously.
All operations were performed by the same operation group. Before and after the implantation of the prosthesis, the surgeons administered periarticular local infiltration anesthesia (100 mg ropivacaine with 5 mg dexamethasone diluted to 50 ml with normal saline). Ondansetron (4 mg) and parecoxib sodium (40 mg) were given intravenously when the articular cavity was irrigated. After the knee capsule was closed, the tourniquet was released, and the patient-controlled intravenous analgesia pump (fentanyl 1 mg, flurbiprofen axetil 100 mg, ondansetron 8 mg prepared to 100 ml, background dose set to 1 ml/h, 2 ml PCA at a lock-time of 15 min) was connected. Of course, there was an option to turn off the PCA pump whenever they think they didn’t need it. The endotracheal tube was removed when the end-expiratory sevoflurane concentration was less than 0.2%, spontaneous breathing was restored, and the eyes were opened as instructed.
Postoperative regimen
After extubation, patients were transferred to the postoperative anesthesia care unit (PACU). In the PACU, if the patient’s NRS score at rest was greater than or equal to 4 points, 5 mg of dezocine was injected intravenously. Patients were discharged from the PACU and transferred to the ward when the modified Aldrete Scale score was > = 9. The NRS scores at rest and during activity were recorded before the patients left the PACU.
During the first 3 days after surgery, intravenous PCA was applied in conjunction with an oral celecoxib capsule (200 mg/12 h) and an acetaminophen oxycodone tablet (containing 5 mg oxycodone and 325 mg paracetamol) every 12 h in the ward. When the resting NRS score was greater than or equal to 4, 5 mg of dezocine was injected intramuscularly as rescue analgesia. Thereafter, the dose of acetaminophen oxycodone decreased to half a tablet every 12 h for the next 3 days postoperatively and then stopped. The consumption of fentanyl within 24 h postoperatively was recorded. The functional recovery of the knee joint was measured by the range of motion and the strength of the quadriceps femoris. The range of motion of the joint was measured with a protractor at 8 h, 24 h, 48 h, and 72 h postoperatively. The quadriceps muscle strength was evaluated using the MRC scale (0–5), which is a manual muscle strength test [19]: the patient was placed in a supine position, with the knee and hip flexed, and then the calf was straightened. The evaluator resisted this movement and touched the contracted muscle in the thigh to measure its muscle strength. A score of 0 indicated no muscle contraction, 1 indicated muscle contraction but no joint movement, 2 indicated joint movement but no gravity resistance, 3 indicated gravity resistance, 4 indicated gravity resistance and partial reaction resistance, and 5 indicated normal joint function.
At discharge, patients were asked to evaluate their satisfaction with pain management (NRS: 0–10; 0 as dissatisfied, 10 as greatest satisfied) and were instructed to continue medication for controlling their pain.
The subjects were followed up by telephone contact at 2 weeks, 1 month, and 3 months after the operation. The main purpose of follow-up was to record the pain intensity and the incidence of pain during rest, followed by asking the patients about their readmission within 30 days associated with the operation and whether they were imaged or re-examined. The interviews were conducted by a researcher who was unaware of the groups.
Outcome
The primary outcome was the incidence of postoperative chronic pain in patients who were followed up by telephone contact 3 months after the operation. We defined postoperative chronic pain as an NRS score greater than or equal to 1 at rest.
Secondary outcomes included pain intensity at 2 weeks, 1 month, and 3 months after the operation; the resting and active NRS scores at 4 h, 8 h, 16 h, 24 h, 48 h, and 72 h after the operation; the time to first rescue analgesia; the 24-hour opioid consumption; and the intraoperative dosage of opioids (fentanyl, remifentanil). The range of motion of the knee; muscle strength of the quadriceps femoris at 8 h, 24 h, and 72 h after the operation; CRP and IL-6 levels on the first and third days after the operation; length of postoperative hospital stay; and cost of hospitalization and postoperative complications, including nausea and vomiting, urinary retention, nerve injury, local anesthetic toxicity, incision infection, postoperative falls, and 30 days after readmission, were also recorded.
Sample size calculation
Our pilot study revealed that the estimated incidence of chronic pain was 58% in patients without ACB + IPACK at 3 months after TKA and 29% in patients with ACB + IPACK. Assuming a 50% lower rate of chronic pain incidence in the nerve block group than in the control group, a difference was detected at 80% efficacy and 0.05% significance, and sample size calculations revealed that 45 subjects were required in each group. Considering a possible dropout rate of up to 10%, we enrolled 50 subjects per group.
Data analysis
Normally distributed data are represented by the mean ± standard deviation, and nonnormally distributed data are expressed as the median and interquartile range. The data were analyzed by SPSS v.26.0 software (IBM, Chicago, IL). Student’s t test was used to compare continuous variables such as weight, age, height, body mass index, operation time, drug dosage, and postoperative hospitalization between the two independent samples, and the χ2 test was used to compare the sex ratio between the two groups. ROM, CRP, and IL-6 were analyzed by repeated measures of variance using an unstructured variance‒covariance matrix, and time × group interaction tests were performed on each dataset. Independent sample Mann‒Whitney U tests, sample-dependent Friedman analysis of variance, and pairwise comparisons were applied to evaluate pain intensity. We evaluated the incidence of chronic pain using the χ2 test, Cochrane Q test, and pairwise comparisons of dependent samples. Logistic regression was used to examine the interactions between the confounding factors that may affect the incidence of chronic pain at 3 months between the two groups. P < 0.05 was considered to indicate statistical significance.
Results
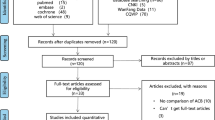
A total of 130 people were approached to participate, and 100 patients were included in the study. Among them, 5 patients withdrew informed consent, and 4 patients failed to complete the follow-up. The remaining 91 patients in the study completed the trial (Fig. 1). There was no significant difference in age, body mass index, height, weight, operation time, tourniquet time, or intraoperative bleeding between the ropivacaine group and the saline group (Table 1).
Our primary outcome (Fig. 2), that is, the incidence of chronic pain three months after TKA in the ropivacaine group, was lower than that in the placebo group (30.4% vs. 51.1%, P < 0.05), and there was also a significant difference in the incidence of pain one month after the operation (47.8% vs. 68.9%, P < 0.05).
Pain intensity at rest on the right side of the image, presented as box plots. In the ropivacaine group, pain intensity decreased significantly at 1 and 3 months compared to 2 weeks after surgery. The incidence of pain at rest on the left side of the image is presented as a bar. The data are expressed as frequencies (%). Measurements were recorded at 2 weeks, 1 month, and 3 months postoperation. The Mann‒Whitney U test, Friedman analysis of variance, and pairwise comparisons were applied to evaluate pain intensity. We evaluated the incidence of chronic pain using the χ2 test and Cochrane Q test. P < 0.05 was considered to indicate statistical significance. The median (horizontal line), interquartile range (box), and range of pain scores (NRS) at rest in both groups are presented as box plots. NRS: Numerical Rating Scale
Regarding the intensity of postoperative pain in the acute stage, as shown in Fig. 3, the analgesic effect of ropivacaine was greater than that of saline at 4 h, 8 h, 16 h, and 24 h postoperation under conditions of rest and movement of the anterior knee (P < 0.05). At rest, the NRS scores in the posterior area of the knee at 4 h, 8 h, 16 h, and 24 h postoperation in the ropivacaine group were significantly lower than those in the saline group (P < 0.05). In the movement state, the NRS scores in the posterior area of the knee at 4 h, 8 h, 16 h, 24 h, and 48 h postoperation were significantly lower in the ropivacaine group than in the saline group (P < 0.05). There were no significant differences in pain scores between the two groups at discharge from the PACU or 24 h after discharge, whether anteriorly or posteriorly to the knee, at rest, or during movement.
Pain scores for early postoperative rest and mobilization (anterior and posterior to the knee). NRS: Numerical Rating Scale. The pain score is presented as a line chart. Independent sample Mann‒Whitney U test, sample-dependent Friedman analysis of variance, and pairwise comparison were applied to evaluate pain intensity. * was represented for statistically significant differences
Opioid consumption
The dose of remifentanil in the ropivacaine group was significantly lower than that in the saline group (599.0 ± 88.3 vs. 656.1 ± 99.0, P < 0.05), and the fentanyl consumption recorded by the PCA pump within 24 h after the operation was significantly lower than that in the placebo group (438.5 [403.0-486.5] vs. 470.0 [430.5-503.5], P < 0.05). In terms of remedial analgesia, the first analgesic pump pressing minutes in the ropivacaine group was significantly longer than that in the saline group (695.0 [540.0-795.0] vs. 510.0 [422.5–550.0], P < 0.05). Moreover, the 24-hour consumption of dezocine (mg) in the ropivacaine group was significantly lower than that in the saline group (0 [0–5] vs. 5 [0–5], P < 0.05) (Table 2).
Regarding knee function and muscle strength, knee flexion (degrees) at 8 h (96.2 ± 8.0 vs. 87.1 ± 10.9, P < 0.001) and 24 h (99.9 ± 7.1 vs. 91.1 ± 10.2, P < 0.001) after the operation was greater in the ropivacaine group than in the saline group, but there was no difference between the two groups at 48 h and 72 h after the operation, and an intragroup comparison of both groups revealed that the range of motion of the knee increased over time. There was no significant difference in quadriceps muscle strength between the two groups at any time point (Table 3, Appendix 1).
CRP and IL-6 levels were significantly greater on postoperative days 1 and 3 in both groups than preoperatively (P < 0.05, Appendix 2). However, the CRP concentration in the ropivacaine group was significantly lower than that in the saline group on postoperative days 1 (11.6 ± 3.5 vs. 14.1 ± 6.5, P < 0.05) and 3 (18.2 ± 6.6 vs. 24.4 ± 10.7, P < 0.05), and the IL-6 concentration in the ropivacaine group on the first day was significantly lower than that in the saline group (10.6 ± 5.5 vs. 15.4 ± 8.3, P < 0.05) (Table 3).
ACB and IPACK block were all successfully performed with no acute complications (such as, local anesthetic toxicity, infection, or nerve injury). There was no postoperative fall, 30-day readmission, or surgical incision infection in either group. There was no significant difference in systemic complications between the two groups. There was no significant difference in hospitalization costs or pain management satisfaction between the two groups; however, the postoperative hospital stay in the ropivacaine group was significantly shorter than that in the placebo group (5.04 days vs. 6.82 days, P < 0.001) (Table 2).
There was no significant difference in sex, age, body mass index, or other potential confounding factors, such as the duration of the operation, inflammatory factors, or pain catastrophizing scale scores, between the two groups (Appendix 3).
Discussion
This single-center, prospective, randomized, double-blind, placebo-controlled study showed that ACB combined with IPACK reduced the incidence of chronic postoperative pain 3 months after TKA in the context of a multimodal analgesia strategy (primary endpoint of the study).
The high incidence of chronic pain after TKA has always been the focus of attention. Many studies have explored its mechanism and possible influencing factors, but there are few randomized controlled trials on the effect of nerve block on chronic pain after TKA. Our study revealed that pain scores and the incidence of chronic postoperative pain were lower in the ACB + IPACK group at 1 and 3 months after TKA, which we attributed to better early pain control. As shown in Fig. 3, from discharge from the PACU to 24 h after TKA, the NRS scores of the Ropivacaine group were statistically significantly lower than the controls, both in the anterior and posterior of the knee, and both in the rest and movement. It is worth noting that although the NRS score decreased in the Ropivacaine group at 24 h, whether this is truly a clinically beneficial reduction (i.e., a reduction within the minimal clinically important difference, MCID [20, 21]) deserves further investigation. As shown in the past studies, MCID estimates based on different methods can vary widely [22,23,24]. Meanwhile, although there was a statistically significant difference in the incidence of chronic pain between the two groups 3 months after the operation, the 50% reduction we expected was not achieved, which may be due to sampling errors. In addition, even under nerve block, up to 30.4% of the patients in this study developed postoperative chronic pain, which reminds us to think about other influencing factors associated with chronic pain.
The mechanisms underlying postoperative chronic pain are complex [25]. David H et al. reported that there was a strong correlation between postoperative acute pain and chronic pain, which might be related to the continuous pain input of the injured knee joint caused by neuropeptides released by nerve endings during acute pain, leading to hyperalgesia and central sensitization [26]. Therefore, the lower incidence of chronic pain in the ropivacaine group may be due to blocking the transmission of nociceptive nerve impulses and preventing the occurrence of central sensitization. This approach not only effectively controls acute postoperative pain but also has a good effect on long-term pain. Our study revealed that the ropivacaine group had significantly lower pain scores within 24 h than did the placebo group, which also confirmed our hypothesis.
Several randomized controlled trials have demonstrated that ACB + IPACK reduces the consumption of opioids after TKA. In our study, opioid consumption was relatively low in both groups within 24 h after the operation, which may be due to the implementation of a multimodal analgesia strategy. However, a significant reduction in opioid consumption after ACB + IPACK with ropivacaine, a longer time to first press the PCA pump, and fewer remedial analgesic treatments also showed the benefits of ACB + IPACK, consistent with the results of Vander Wielen et al. [27].
The inflammatory response plays an important role in chronic postoperative pain. Studies by Chapman CR et al. showed that a strong postoperative inflammatory response may lead to central sensitization, leading to the transition to chronic pain [28]. Effective control of postoperative acute pain, a reduction in the postoperative inflammatory response, and interruption of nociceptive signals are the most common solutions. Ropivacaine, a type of long-acting amide local anesthetic, mainly blocks the transmission of pain by affecting the movement of sodium ions, has a continuous analgesic effect, and reduces the cascade of inflammatory factors [29]. Our study revealed that the CRP concentration on the 1st and 3rd days and the IL-6 concentration on the 1st day were significantly lower in the ropivacaine group than in the placebo group (Table 3) (Appendix 2), which may be due to the anti-inflammatory effect of ropivacaine through the blockade of nociceptive signal transduction.
Although the pain intensity decreased significantly within 24 h in the ropivacaine group, there was no significant difference in pain scores between the two groups at 2 and 3 days after the operation, which may be related to the pharmacokinetics and pharmacodynamics of local anesthetics. Cummings et al. reported that even with dexamethasone as an adjuvant, the action time of ropivacaine was difficult to exceed 24 h [30]. However, there was no difference in pain score between the two groups at discharge from the PACU, which may be due to the residual effects of analgesics during surgery.
The nerves innervating the knee are relatively complex. A cadaver study showed that the saphenous nerve and nerve to the vastus medialis running in the adductor canal provide substantial innervation to the anteromedial aspect of the knee, including the joint capsule and the medial patellar retinaculum [31]. Another cadaver study showed that the articular branches providing innervation to the posterior knee joint capsule originate from the posterior division of the obturator nerve, sciatic nerve, main common fibular nerve, and tibial nerve [32]. Therefore, ACB can frequently block the saphenous nerve and nerve to the vastus medialis, which innervates the anterior part of the knee, and even the obturator nerve. At the same time, the IPACK blocks sensory nerves that innervate the posterior knee compartment. The combination of ACB and IPACK has been widely used in clinical practice and is favored by surgeons and patients. Therefore, we chose ACB combined with IPACK for postoperative analgesia.
Considering the complexity of knee innervation, anterior and posterior sensory innervation are relatively independent. To better define the blocking effect, we recorded the postoperative pain score of the knee (distinguished between the anterior and posterior sides) in detail to evaluate the analgesic effect of nerve block more objectively. These results were consistent with our expectations; that is, the pain intensity in the ropivacaine group was significantly lower than that in the placebo control group.
Recent data show that chronic pain is associated not only with postoperative early pain intensity but also with the surgical procedure itself and psychosocial and environmental factors [33]. Therefore, when we analyzed the data, we added binary logistic regression analysis to examine the interaction between confounding factors that could affect the incidence of chronic pain in the two groups and eliminated confounding factors such as age, sex, duration of operation, and pain disaster (Appendix 3), which also increases the credibility of our results.
We only applied single-shot ACB in this study. Because elastic bandages are routinely used by surgeons in our research center for a short time after TKA, we abandoned continuous analgesia by ACB to avoid the possible risk of catheter displacement, detachment, or even postoperative catheter-related infection. In addition, previous studies have shown that a single-shot ACB achieves the same early analgesic effect as continuous infusions [34].
Wang Q et al. performed an adductor canal block with different concentrations of ropivacaine and reported that 0.25% ropivacaine had a better analgesic effect and fewer side effects [35]. Therefore, in this study, 0.25% was selected as the investigated concentration for nerve block. Similarly, our results also confirmed that ACB combined with IPACK by 0.25% ropivacaine effectively reduced the anterior and posterior pain scores of the knee within 24 h after TKA and had no adverse effect on lower limb muscle strength.
Several studies have shown that ACB + IPACK enhances the quality of postoperative recovery in patients. Similar to Malige A et al. [36], our study also revealed that ACB + IPACK not only increased the motive range of the knee at 8 h and 24 h after TKA but also had no effect on quadriceps muscle strength or did not increase the risk of falls. There was no significant difference in knee joint motion between the two groups after 24 h (Appendix 1), which may be related to the patients’ functional exercise and the short effective blocking time of the local anesthetics. Moreover, without increasing hospitalization costs, the postoperative hospital stay in the ropivacaine group was significantly shorter than that in the placebo group (5.04 ± 1.1 days vs. 6.82 ± 1.2 days) (Table 2), and this would be of value to somewhat expand on the ambulation, recovery of daily activities.
It is worth mentioning that the subjects in the ropivacaine group needed to receive an injection of approximately 200 mg ropivacaine. Although blood ropivacaine concentrations were not measured, we did not observe any cardiac or neurotoxic adverse events. This finding is similar to a previously published study in which no local anesthetic-related toxic effects were observed when a total of 225 mg of ropivacaine was administered for femoral nerve block, and the peak concentration of ropivacaine in plasma was lower than the prescribed toxicity threshold [37]. Therefore, the safety of 200 mg of ropivacaine can be guaranteed.
There are several limitations of this study: (1) Due to the design of the study, the endpoint of the study was 3 months after the surgery, while the pain after TKA may last longer or disappear after 3 months. Therefore, if possible, we will extend the follow-up time in future studies to obtain more information. (2) In terms of early postoperative recovery quality, we mainly evaluated knee range of motion and quadriceps strength, which may not be general and objective. It would be more comprehensive to use the commonly used quality of recovery scale (Such as QoR-15 or QoR-40) to evaluate [38]. (3) During the follow-up, we recorded only the intensity of the pain but not included other aspects, such as pain-related distress, pain-related interference with activities of daily living, the nature of the pain (whether it is neuropathic pain or complex regional pain syndrome, or anything else), and etc. This topic needs to be improved upon in future research. (4) The sample size included in this study was small, and additional studies with larger sample sizes may be needed in the future to confirm the benefits of nerve block in chronic pain patients. (5) Considering that we used telephone follow-up after discharge, we did not obtain CRP or IL-6 levels at 2 weeks, 1 month, or 3 months after surgery. Obtaining the above information may help provide much more useful information in relation to the occurrence of subacute or chronic pain.
Conclusions
In the context of multimodal analgesia, ultrasound-guided adductor canal block combined with infiltration between the popliteal artery and posterior capsular of the knee decreases the incidence and intensity of chronic postoperative pain 3 months after TKA. In addition, the above combination of peripheral nerve blocks reduces NRS scores at rest and during activity, improves the range of motion of the knee, and reduces the consumption of opioids within 24 h postoperatively.
Availability of data and materials
Data and materials related to this study can be obtained from the corresponding authors.
Abbreviations
- TKA:
-
Total knee arthroplasty
- ACB:
-
Adductor canal block
- IPACK:
-
Infiltration between the popliteal artery and posterior capsular of the knee
- CPSP:
-
Chronic post-surgical pain
- NRS:
-
Numerical rating scale
- HADS:
-
Hospital Anxiety and Depression Scale
- PCS:
-
Pain Catastrophizing Scale
- ROM:
-
Range of motion
- PACU:
-
Postoperative anesthesia care unit
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- MCID:
-
Minimal clinically important difference
References
Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis[J]. Lancet. 2015;386:376–87.
Springer B, Bechler U, Waldstein W, et al. Five questions to identify patients with osteoarthritis of the Knee[J]. J Arthroplasty. 2020;35:52–6.
Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030[J]. Bone Joint Surg Am. 2007;89(4):780–5.
Bayliss Lee E, Culliford David, Monk A, Paul, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study[J]. Lancet. 2017;389(10077):14241430.
Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain[J]. Anesthesiology Clin North Am. 2005;23(1):21–36.
Hebl JR, Kopp SL, Ali MH et al. A comprehensive anesthesia protocol that emphasizes peripheral nerve blockade for total knee and total hip arthroplasty[J]. J Bone Joint Surg Am, 2005:87 Suppl 2:63–70.
Baratta JL, Gandhi K, Viscusi ER. Perioperative pain management for total knee arthroplasty[J]. J Surg Orthop Adv. 2014;23:22–36.
Sugiyama Y, Iida H, Amaya F, et al. Prevalence of chronic postsurgical pain after thoracotomy and total knee arthroplasty: a retrospective multicenter study in Japan (Japanese Study Group of Subacute Postoperative Pain)[ J]. J Anesth. 2018;32(3):434–8.
Schug SA, Lavand’homme P, Barke A, et al. IASP Taskforce for the classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52.
Rice DA, Kluger MT, Mcnair PJ, et al. Persistent postoperative pain after total knee arthroplasty: a prospective cohort study of potential risk factors[J]. Br J Anaesth. 2018;121:804–12.
Buvanendran A, Della Valle CJ, Kroin JS, et al. Acute postoperative pain is an independent predictor of chronic postsurgical pain following total knee arthroplasty at 6 months: a prospective cohort study[J]. Reg Anesth Pain Med. 2019;44(3):e100036.
Thomazeau J, Rouquette A, Martinez V, et al. Predictive factors of chronic post-surgical pain at 6 months following knee replacement: influence of postoperative pain trajectory and genetics[J]. Pain Physician. 2016;19(5):E729–41.
Puolakka PAE, Rorarius MGF, Roviola M, et al. Persistent pain following knee arthroplasty[J]. Eur J Anesthesiol. 2010;27(5):455–60.
Terkawia S, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network Meta-analysis of 170 randomized controlled trials[J]. Anesthesiology. 2017;126(5):923–37.
Pelt CE, Anderson AW, Anderson MB. Et a1. Postoperative falls after total knee arthroplasty in patients with a femoral nerve catheter: can we reduce the incidence[J]. J Arthroplasty. 2014;29(6):1154–115.
Abdullah MA, Abu Elyazed MM, Mostafa SF. The Interspace between Popliteal artery and posterior Capsule of the knee (IPACK) block in knee arthroplasty: a prospective randomized trial[J]. Pain Physician. 2022;25(3):E427–33.
Grevstad U, Mathiesen O,Valentiner LS, et al. Effect of adductor canal block versus femoral nerve block on quadriceps strength, mobilization, and pain after total knee arthroplasty: a randomized, blinded study[J]. Reg Anesth Pain Med. 2015;40(1):3–10.
Wells C, McCormack S. Screening Tools for Chronic Post-Surgical Pain. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2021.
Merlini L. Measuring muscle strength in clinical trials[J]. Lancet Neurol. 2010;9(12):1146.
Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, Dennis A. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state[J]. Br J Anaesth. 2017;118(3):424–9.
Danoff JR, Goel R, Sutton R, Maltenfort MG, Austin MS. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty[J]. J Arthroplasty. 2018;33(7S):S71–S75.e2.
Grönkvist R, Vixner L, Äng B, Grimby-Ekman A. Measurement error, minimal detectable change, and minimal clinically important difference of the short Form-36 health survey, hospital anxiety and depression scale, and pain numeric rating scale in patients with chronic pain[J]. J Pain. 2024;10:104559.
Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale[J]. Eur J Pain. 2004;8(4):283–91.
Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain[J]. Spine (Phila Pa 1976). 2005;30(11):1331–4.
Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms[J]. J Pain. 2017;18(4):359.
Kim DH, Pearson-Chauhan KM, McCarthy RJ, et al. Predictive factors for developing chronic pain after total knee arthroplasty[J]. J Arthroplasty. 2018;33(11):3372–8.
Schnabel A. Acute neuropathic pain and the transition to chronic postsurgical pain[J]. Pain Manag. 2018;8(5):317–9.
Jensen MP, Turk DC. Contributions of psychology to the understanding and treatment of people with chronic pain: why it matters to all psychologists[J]. Am Psychol. 2014;69(2):105–18.
Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications[J]. Acta Anesthesiol Scand. 2006;50(3):265–82.
Cummings KC, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine[J]. Br J Anaesth. 2011;107(3):446–53.
Burckett-St Laurant D, et al. The nerves of the Adductor Canal and the innervation of the Knee[J]. Reg Anesth Pain Med. 2016;41:321–7.
Tran J, Peng PWH, Gofeld M, et al. Anatomical study of the innervation of posterior knee joint capsule: implication for imageguided intervention[J]. Reg Anesth Pain Med. 2019;44:234–8.
Koga K, Li S, Zhuo M. Metabotropic glutamate receptor-dependent cortical plasticity in chronic pain[J]. Curr Neuropharmacol. 2016;14(5):427–34.
Nasir Hussain S, Zhou R, Schroell, et al. Analgesic benefits of single-shot versus continuous adductor canal block for total knee arthroplasty: a systemic review and meta-analysis of randomized trials[J]. Reg Anesth Pain Med. 2023;48:49–60.
Qiuru W. Comparison of different concentrations of ropivacaine used for ultrasound-guided Adductor Canal block + IPACK block in total knee Arthroplasty[J]. J Knee Surg. 2023;36:1273–82.
Malige A, Pellegrino AN, Kunkle K, et al. Liposomal bupivacaine in Adductor Canal blocks before total knee arthroplasty leads to improved postoperative outcomes: a randomized controlled Trial[J]. J Arthroplasty. 2022;37(8):1549–56.
Kazune S, Nurka I, Zolmanis M et al. Systemic ropivacaine concentrations following local infiltration analgesia and femoral nerve block in older patients undergoing total knee Arthroplasty[J]. Local Reg Anesth 2023:16143–151.
Myles PS, Shulman MA, Reilly J, Kasza J, Romero L. Measurement of quality of recovery after surgery using the 15-item quality of recovery scale: a systematic review and meta-analysis[J]. Br J Anaesth. 2022;128(6):1029–39.
Acknowledgements
The authors thank all the patients, nurses, anesthesiologists, physiotherapists, and orthopedic surgeons from the Affiliated Hospital of North Sichuan Medical College who were involved in this study for their support.
Funding
The authors have no sources of funding to declare for this manuscript.
Author information
Authors and Affiliations
Contributions
Substantial contribution to conception and design: Jingyan Lin, Wenqin Yin. Patient recruitment: Wanli Yang, Dan Luo, Wenqin Yin. Study conduct: Wenqin Yin, Wenmei Xu, Dan Luo. Data collection: Wenmei Xu, Dan Luo, Wanli Yang. Data analysis: Shuaiying Jia, Jingyan Lin. Drafting the article: Wenqin Yin, Jingyan Lin. Revising it critically for important intellectual content: Wenmei Xu, Wenqin Yin, Jingyan Lin. Approval of final version: Wenqin Yin, Wenmei Xu, Dan Luo, Wanli Yang, Shuaiying Jia, Jingyan Lin.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (number: 2022ER399-1). The study protocol adheres to the ethical requirements of the 1975 Declaration of Helsinki (6th reversion, 2008) as shown in a priori approval by the institutions’ human research committee. All participants gave written informed consent and completed the questionnaires.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yin, W., Luo, D., Xu, W. et al. Effect of adductor canal block combined with infiltration between the popliteal artery and posterior capsular of the knee on chronic pain after total knee arthroplasty: a prospective, randomized, double-blind, placebo-controlled trial. BMC Anesthesiol 24, 320 (2024). https://doi.org/10.1186/s12871-024-02707-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02707-2