Abstract
Slow-controlled release fertilizers are experiencing a popularity in rice cultivation due to their effectiveness in yield and quality with low environmental costs. However, the underlying mechanism by which these fertilizers regulate grain quality remains inadequately understood. This study investigated the effects of five fertilizer management practices on rice yield and quality in a two-year field experiment: CK, conventional fertilization, and four applications of slow-controlled release fertilizer (UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer). In 2020 and 2021, the yields of UF and SCU groups showed significant decreases when compared to conventional fertilization, accompanied by a decline in nutritional quality. Additionally, PCU group exhibited poorer cooking and eating qualities. However, BBF group achieved increases in both yield (10.8 t hm−2 and 11.0 t hm−2) and grain quality reaching the level of CK group. The adequate nitrogen supply in PCU group during the grain-filling stage led to a greater capacity for the accumulation of proteins and amino acids in the PCU group compared to starch accumulation. Intriguingly, BBF group showed better carbon–nitrogen metabolism than that of PCU group. The optimal nitrogen supply present in BBF group suitable boosted the synthesis of amino acids involved in the glycolysis/ tricarboxylic acid cycle, thereby effectively coordinating carbon–nitrogen metabolism. The application of the new slow-controlled release fertilizer, BBF, is advantageous in regulating the carbon flow in the carbon–nitrogen metabolism to enhance rice quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Rice (Oryza sativa L.) is a crucial staple food crop globally, but its production is challenged by two key factors: grain development and grain quality [1,2,3]. With the rapid economic development, there is a growing demand for high-quality rice in developing countries across Asia [4, 5]. Grain quality characteristics are essential for determining market value and include factors such as nutritional value, physical appearance, cooking and sensory properties, and milling recovery [6]. Fertilization plays a critical role in regulating the grain quality of rice as it affects the content and composition of starch and protein in the grain [7]. Over the past few decades, conventional fertilizers in rice cultivation face challenges such as low efficiency, high wastage, and labor-intensive application methods, causing environmental pollution and economic losses [8]. To address these issues, the application of slow-controlled release fertilizers has been proven to be a more efficient method of fertilization, supplying crops with required nutrients in a single basal application throughout the growth period [9, 10]. The slow-controlled release fertilizer has a dramatic influence on optimizing grain quality and nitrogen use efficiency, and are widely adopted to minimize economic losses and reduce environmental pollution [11, 12].
A large number of slow-controlled release fertilizers, including urea formaldehyde (UF), sulfur-coated urea (SCU), polymer-coated urea (PCU), and controlled-release bulk blending fertilizer (BBF), have been developed to meet rice’s nutrient demands, enhance yield and quality [10]. UF and SCU are slow-release fertilizers made from easily decomposable organic materials. UF fertilizer is produced by combining urea with formaldehyde, resulting in quick nitrogen release during the early growth stage but limited release during the middle and late stages [13]. In contrast, SCU fertilizer involves coating urea granules with a layer of sulfur, enabling the nutrients to be slowly released over time; however, the release rate is insufficient during the middle and late stages [14]. PCU and BBF are two types of controlled-release fertilizers that provide a regulated-release of nutrients to plant. The PCU fertilizer is an enhanced version of SCU, designed to minimize the impact of microorganisms on nutrient release through coating urea granules with a polymer material. BBF is created by blending various types of slow-release and controlled-release fertilizers, allowing for a consistent release of nitrogen nutrients throughout the entire rice growth cycle [15]. Given the growing demand for grain quality and efficient fertilizer use, further research is needed to examine the relationship between grain quality and the application of slow-controlled release fertilizers.
Grain filling is vital for rice quality and is highly influenced by fertilizer use. Accumulation of lipids, starch, and storage proteins during grain filling stage is crucial for grain quality, and is strongly influenced by nitrogen availability [16,17,18]. The improper use of fertilizer can negatively impact the physical appearance of rice grain, leading to the formation of chalkiness as a result of an unbalanced carbon–nitrogen metabolism [17, 19]. The carbon-to-nitrogen (C: N) balance in rice grains refers to the optimal ratio of protein and starch in the grain, which is critical for determining the nutritional value and other rice qualities [20]. The changes in nitrogen and carbon supply can alter numerous central metabolites involved in carbon and nitrogen metabolism in parallel [12]. To support the synthesis of amino acids and ensure adequate growth in rice grain, an appropriate nitrogen content is needed to drive the production of carbon skeletons and provide enough amino acids [17, 21].
The inorganic nitrogen of soil is absorbed by roots and can then be assimilated into glutamine and glutamate in grain, which serves as a source of organic nitrogen [22]. Glutamine synthetase (GS), encoded by OsGS2, is a crucial enzyme for assimilating NH4+ in rice. Maintaining a stable GS level is essential for maintaining the carbon–nitrogen balance during rice growth [23]. The key enzymes of the GS and GOGAT (glutamate synthase) cycle play key roles in nitrogen assimilation of rice grain, GOGAT transfers the amide group to 2-oxoglutarate for producing glutamate [24, 25]. Additionally, nitrogen assimilation requires energy and carbon skeletons, which are derived from sucrose, glucose, other glycolysis-derived carbohydrates (pyruvate) and the tricarboxylic acid (TCA)-derived organic acids (eg., cetoglutarate and oxaloacetate) [22]. The carbon and nitrogen metabolites are also involved in the oxidative pentose phosphate pathway (OPPP), which is crucial for generating the energy and reducing power required for rice grain growth [17, 26, 27]. The TCA cycle is essential for providing 2-oxoglutarate, which is necessary for the production of glutamate and glutamine involved in nitrogen assimilation [28]. The intermediates of the glycolysis pathway, like pyruvate, play an important role in the TCA cycle and nitrogen assimilation in rice [29].
The primary product of photosynthesis, sucrose, plays a crucial role in supplying the carbon skeletons required for synthesizing a variety of macromolecules, such as proteins, nucleic acids, lipids, and starch [16, 30]. This is facilitated by the transport pathways of OsCIN4 (invertase) and OsSUT2 (sucrose transporter) during grain filling [31, 32]. The major function of SuSase is to breakdown sucrose to supply carbohydrates for the process of OPPP and glycolysis/TCA cycle, and the enzymes AGPase, GBSS and SBE play major roles in catalyzing the synthesis of starch to promote grain filling [17, 33]. However, the mechanisms linking carbon and nitrogen metabolism to rice grain growth under the influence of slow-controlled release fertilizer are not yet fully understood.
In this study, a two-year field experiment was conducted to examine the impact of various slow-controlled release fertilizers on grain quality of rice. To gain a deeper understanding of the nitrogen assimilation process in rice grain under the influence of slow-controlled release fertilizers, we also analyzed the underlying carbon–nitrogen mechanisms during grain filling. Our findings will provide a solid foundation for improving the quality and yield of rice through the use of slow-controlled release fertilizers.
Materials and methods
Study site and N sources
Field experiments were conducted over two rice growing seasons (2020 and 2021) in Yanling Town of Danyang city, Jiangsu Province, China (31°54′31″N, 119′28′21″E). The meteorological data came from the meteorological station (Watch Dog 2900ET, SPECTRUM, USA) installed 100 m from the experimental station (Figure.S1). The soil properties were classified as Orthic Acrisol [34]. The soil had the following characteristics: pH = 6.4, organic matter = 18.32 g·kg−1, total N = 1.27 g·kg−1, Olsens-P = 16.85 mg·kg−1 and NH4OAc-extractable K = 139.63 mg·kg−1. The slow-controlled release fertilizers used in this study were: UF (35% N), SCU (37% N), PCU (43% N), and BBF (40% N). The UF, SCU, PCU and BBF were provided by Hanfeng Slow Release Fertilizer Co., Ltd. (Jiangsu, China). Conventional urea (46% N) fertilizers were used as CK group for comparison.
Experimental design
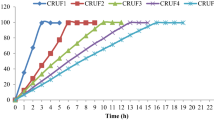
For analyzing effects of different controlled-release fertilizers on grain quality of rice, the experiment was conducted by rice cultivation of Ningjing 8th (Oryza sativa L.), a high-yielding japonica rice cultivar bred by Nanjing Agricultural University (NAU). This experiment included treatments as follows: a conventional fertilization (CK, four spilt applications of urea), and a single basal application of slow-controlled release fertilizer (SCU, PCU, UF, and BBF). The N release rate of the slow-controlled release fertilizer was shown in Fig. 1, which was determined by buried bag method [35]. The conventional urea fertilization was split among basic fertilizer, tiller fertilizer, spikelet-promoting fertilizer, and spikelet-developing fertilizer. Each treatment was applied nitrogen at 210 kg·ha−1, and for all treatments, P and K fertilizers were applied as basal dressings at 135 kg·ha−1 (P2O5) and 216 kg·ha−1 (K2O). The field plots, measuring 7.2 m × 20 m, were arranged in a randomized complete block design with three replicates per treatment. Seeds were sown in the nursery beds on May 26 in 2020 and May 24 in 2021, which was transplanted to the fields after 17 days. In each replicate, a total of approximately 1600 panicles with similar growth patterns were labeled and monitored for their flowering date. This was done in September, when about 50% panicles had fully emerged from the flag leaf sheath.
Nitrogen release rate (A) and appearance feature (B) of different types of slow-controlled release fertilizer. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer
Sampling and analysis
Harvesting and grain quality measurements
At maturity stage, all necessary plants (excluding edge-row plants) from each plot were harvested. The grain yield was determined by multiplying each yield component, which had been measured after removing impurities and adjusting moisture content to 14.5%. Before harvesting, the number of effective tillers per hill was determined by selecting 50 plants from each plot. At maturity, approximately 30 marked plants were used to analyze the 1000-grain weight, seed setting rate, and grain number per panicle.
At maturity, the rice grain quality was analyzed. For rice milling quality, the brown rice yield and milled yield were analyzed according to the method of rice measurement standards (NY147-88; Ministry of Agriculture, PR China, 1988). To test the appearance quality of rice, the chalk characteristics of brown rice were determined by the cleanliness test-bed according to Tang et al. [36]. Rice cooking and eating quality was analyzed by the Rapid Visco Analyser (RVA, Starchmaster 2, Perten Instruments of Australia Pty Ltd., Sydney, New South Wales, Australia) to obtain profile characteristics according to Standard Method AACC61-02. Viscous profile characteristics of RVA were expressed as peak viscosity, hot viscosity, cool viscosity, breakdown viscosity, setback (difference between final viscosity and peak viscosity), and consistency. Rice taste characteristics were analyzed by an STA-1A device (Satake Corp., Hiroshima, Japan) according to the method of Jin et al. [37].
Grain weight and grain growth rate
To analyze the grain filling process, 90 tagged panicles of each replicate plot were sampled every 7 days post anthesis (DPA) from 7 to 42 DPA (7, 14, 21, 28, 35, and 42 DPA). All spikelets were deactivated at 105℃ for 60 min and then dried at 80℃ to determine the grain dry weight (DW). Grain filling processes were fit to Richards’s growth equation [38].
Grain weight (mg), W; Grain filling rate, R; Final grain weight (mg), A; Time after anthesis (days), t; and B, k, and N are coefficients established from the regression of the equation.
Analyze of sugar, starch and storage protein composition
To analyze the sugar and starch content, all samples were first ground into a fine powder and then passed through a 100-mesh sieve. The sugar and starch were extracted using a modified version of the method described by Yang et al. [39]. Approximately 100 mg of the ground sample was mixed with 8 mL of 80% (v/v) ethanol at 80℃ for 30 min. After cooling, the tube was centrifuged at 5000 g for 15 min, and the supernatant was collected. The extraction process was repeated three times, and the sugar extract was diluted with distilled water to a final volume of 50 mL. After the sugar extraction, the residues in the tubes were dried at 80℃ to extract starch using HCLO4 according to Yang's method. The amylose and amylopectin contents were measured using the Megazyme Amylose/Amylopectin Assay Kit (Megazyme International Ireland Ltd, Bray, Ireland).
The total protein content was measured using an AutoKjeldahl Unit K-370 nitrogen analyzer (Büchi Labortechnik, Flawil, Switzerland) with AACC International Approved Method 46–30.01. Different protein components including albumin, globulin, prolamin, and glutelin were extracted in a stepwise manner using different solvents such as distilled water, dilute hydrochloric acid, ethanol, and dilute alkali. The glutelin content was determined using the biuret colorimetric method, while the remaining protein components were measured using the Coomassie brilliant blue colorimetric method.
Measurements of amino acid composition
To hydrolyze the rice powder samples, approximately 10 mg of the sample was mixed with 1 mL of 6 N HCl (Sigma, USA) in a 2 mL screw-cap tube. After heating at 110℃ for 24 h, the samples were further treated for 6 h at 65℃. The resulting residue was dissolved in 1 mL of Na-S™ buffer, and the mixture was centrifuged at 1600 × g for 10 min. The supernatant was filtered through a 0.45 μm nylon membrane syringe filter (Pall Life Sciences, USA), and about 10 nmol of L-( +)-norleucine (Wako Pure Chemicals, Japan) was added. Amino acid analysis was performed using a Hitachi L-8900 amino acid analyzer (Hitachi Corp, Japan) based on the national standard of the People’s Republic of China (Ministry of Agriculture PR China, 1988). The amino acids tested included aspartic acid (Asp), glutamic acid + glutamine (Glu), glycine (Gly), histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), phenylalanine (Phe), serine (Ser), threonine (Thr), tyrosine (Tyr), and valine (Val). Total amino acid content was the sum of all amino acid content in the same period. All experiments were conducted at least three biological replicates in each sample, and the HPLC data were normalized to the level of L- ( +)-norleucine per sample.
Enzyme extraction and analysis
For enzyme extraction, the grains collected at 7, 14, 21, 28, 35, and 42 DPA were ground into fine powder in liquid nitrogen. The activities of several enzymes including sucrose synthase (SuSase), adenine diphosphoglucose pyrophosphorylase (AGPase), starch branching enzyme (SBE), and granule-bound starch synthase (GBSS) were determined using the method described by Yang et al. [40]. In addition, the activities of glutamine synthetase (GS) and glutamate synthetase (GOGAT) were analyzed according to the protocols outlined by Tang et al. [41].
RNA extraction and qRT-PCR
Total RNA was extracted from frozen rice grains using a previously described method [42]. Gene transcription levels of the relevant genes were analyzed using RNase-free DNase I treatment, cDNA synthesis, and quantitative real-time polymerase chain reaction (qRT-PCR). The RNA-prep pure PLANT Kit (DP432, Tiangen Biotek, Beijing, China) was used to isolate total RNA from the rice grains, which was then reverse-transcribed into first-strand cDNA using the Prime-Script-TM RT Reagent Kit (RR036, Takara, Kyoto, Japan). The quantitative real-time polymerase chain reaction was performed using the ABI 7300 sequencer and SYBR Premix Ex Taq-TM (RR420, Takara, Kyoto, Japan) according to the manufacturer's protocol. Each sample was replicated three times to test the expression of genes (OsSUT2, OsCIN4, and OsGS2). The primers used in this study are listed in the Supporting Information (Table S1).
Statistical analysis
Excel 2019 (Microsoft Corp. Redmond, WA, USA), SPSS version 20.0. (SPSS Statistics, SPSS Inc., Chicago, USA), and Origin 2021 (OriginLab, Northampton, MA, USA) were used for data visualization. Data was performed the analysis by using two-way analysis of variance (ANOVA) and principal component analysis (PCA). Means were compared based on the least significant difference at P = 0.05 (LSD0.05).
Results
Influence of slow-controlled release fertilizer on rice yield
Compared to the conventional fertilizer’s yield (10.2 t hm−2 and 11.2 t hm−2) in 2020 and 2021, the rice yields decreased to 8.4 t hm−2 and 9.7 t hm−2 after application of urea formaldehyde fertilizer (UF), in consistent with the decline in yield (9.6 t hm−2 and 8.6 t hm−2) of sulfur-coated urea fertilizer (SCU) (Table 1). The groups of PCU and BBF showed similar or even higher yield parameters compared with CK group (Table 1). In 2020, the yield of the BBF group reached 10.8 t hm−2, marking the highest yield among all types of fertilizers. Similarly, in 2021, the BBF yield increased to 11.0 t hm−2, significantly surpassing the yields of the UF and SCU groups. The application of BBF could made the agronomic traits of rice reaching the level of CK group. Such as, panicle number, spikelets number, and grain weight.
The application of UF, SCU, PCU, and BBF all resulted in a significant improvement in grain weight compared with the CK group in 2019 and 2020 (Table 1). The differences in grain weight among the various fertilizer group were even more conspicuous in 2021, particularly with the application of BBF and UF significantly promoting grain weight accumulation in comparison to CK group (Fig. 2). Concurrently, the grain filling rate of BBF and UF groups was significantly higher than that of other treatments at the middle grain-filling stage (14 DPA). These results demonstrate that the use of suitable slow-controlled release fertilizers, such as BBF, can enhance grain growth and rice yield to the level of conventional fertilizer.
Dynamics of rice grain growth after the heading stage under different types of slow-controlled release fertilizer in 2020 and 2021. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer
Effects of slow-controlled release fertilizer on rice quality
In 2020 and 2021, the application of UF and SCU showed no significant inhibition to the physical appearance, as well as cooking and eating qualities in rice grain (Table 2). The composition of protein and amino acids greatly contributes to the nutritional value of rice grain [43]. Compared to the CK group, the application of UF and SCU resulted in a lower content of nitrogen metabolites in rice grains (Table 3), indicating that the use of UF and SCU was unfavorable to the nutritional value of rice grain. The taste values for the PCU group were noticeably lower at 65 and 73 in 2020 and 2021, respectively, compared to the CK group's values of 72.7 and 75 (Table 2). Additionally, the PCU group displayed notably lower cool viscosity and breakdown than the CK group (Table 2). The taste value is critical for cooking and eating qualities and is tightly correlated with the RVA value [44]. These results indicate that the poor taste value and RVA value resulted in poor cooking and eating qualities in PCU group. Intriguingly, BBF significantly improved the physical appearance, cooking and eating qualities, as well as the nutritional value of rice, reaching levels comparable to those of the CK group (Tables 2 and 3).
Carbon metabolism of rice grain
The carbon metabolism pathway plays a crucial role in rice quality by facilitating the synthesis of starch and protein [17, 45]. The sugar content of all slow-controlled release fertilizer treatments reached or even exceeded the level of CK group at grain-filling stage in 2021 (Fig. 3), while the PCU group showed significantly lower accumulation of starch and amylopectin compared to the CK treatment during grain-filling period (Table 3, Fig. 3). The application of slow-controlled release fertilizers obviously affected the activity of key enzymes involved in carbon–nitrogen metabolism in rice grains (Table S2). The PCU group did not exhibit the lowest enzyme activities related to carbon metabolism (SuSase, AGPase, GBSS, and SBE) in grains among all treatments, but the UF group demonstrated lower enzyme activities during grain filling (Fig. 4). However, the starch content in the UF group was not the lowest (Table 3, Fig. 3). The carbon metabolite content and the related key enzyme activity of BBF were neither the highest nor the lowest among these treatments (Figs. 3 and 4), leading proper contents of carbon and nitrogen metabolites in rice grain (Table 3).
Effects of different types of slow-controlled release fertilizer on the changes in carbohydrates in rice grain during the filling stage in 2020 and 2021. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer; Significant differences at each time point are indicated by different letters (P < 0.05) as determined by Duncan’s test
Effects of different types of slow-controlled release fertilizer on the activity of starch synthesis-related enzymes in rice grain during the filling stage in 2020 and 2021. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer; Significant differences at each time point are indicated by different letters (P < 0.05) as determined by Duncan’s test
Nitrogen metabolism of rice grain
The formation of amino acids and proteins through nitrogen metabolism process is crucial for the growth of rice grains [46]. During grain filling, the UF group had significantly lower accumulation of total protein and amino acid in the grain compared to the CK group, whereas the PCU group's content reached or surpassed the level of CK group in both 2020 and 2021 (Fig. 5). Relative to the CK group, the UF group exhibited significantly lower activities in key enzymes (GS and GOGAT) associated with nitrogen assimilation, whereas the PCU group demonstrated comparable activities in GS and GOGAT to those of the CK group (Fig. 6). During grain filling, the key metabolites and enzyme activities associated with nitrogen metabolism in the grain of both BBF and SCU groups were not significantly higher than those in the PCU group, nor were they lower than those in the UF group (Figs. 5 and 6).
Effects of different types of slow-controlled release fertilizer on the content of total protein and amino acids in rice grain during the filling stage in 2020 and 2021. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer; Profiling of total amino acids presented as a heat map calculated by log2 fold change (red meaning increased and blue meaning decreased); Significant differences at each time point are indicated by different letters (P < 0.05) as determined by Duncan’s test
Effects of different types of slow-controlled release fertilizer on changes in nitrogen metabolism-related enzyme activities in rice grain during the filling stage in 2020 and 2021. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer; Significant differences at each time point are indicated by different letters (P < 0.05) as determined by Duncan’s test
The crosstalk of carbon and nitrogen metabolism in rice grain
In the dynamic analysis, profiles of sugar-unloading pathway and carbon–nitrogen metabolism were tested during grain filling (Fig. 7). During grain filling, the gene expression of OsSUT2 and OsCIN4, which are related sugar unloading [17, 47], did not significantly decrease in the groups of UF, PCU and BBF compared to the CK group. This suggested that the application of these fertilizers did not limit the supply of sucrose. The PCU group showed the strongest accumulation of 12 amino acids in rice grain compared to other slow-controlled release fertilizers, and reached the level of the CK group (Figs. 7 and S2). Meanwhile, the UF group showed the lowest accumulation of amino acids among all fertilizer treatments. The accumulation of the BBF group was not higher than that of the PCU group, but not lower than the UF group. The OsGS2 has a significant impact on both the nitrogen transportation in rice and the metabolism of amino acids in grains [48]. During grain filling, the gene expression of OsGS2 was higher in the groups of PCU and BBF than that of UF and SCU groups. Thus, the increased nitrogen supply in groups of PCU and BBF might enhance nitrogen assimilation and led to an increase in amino acid synthesis of rice grains at the grain filling stage (Table S3, Figs. 1 and S2).
Metabolic profiling corresponding to the accumulation of starch and amino acids in rice grains during the filling stage in 2021. CK, conventional fertilization with four spilt applications of urea; UF, urea formaldehyde; SCU, sulfur-coated urea; PCU, polymer-coated urea; BBF, controlled-release bulk blending fertilizer; DPA, days post anthesis; Values of the heat map calculated by log2 fold change (red meaning increased and blue meaning decreased)
Discussion
Rice yield and quality are improved by the application of optimal slow-controlled release fertilizer
Compared to the conventional fertilizer, the UF and SCU groups exhibited a significantly lower number of spikelets per panicle and area, which notably inhibited rice yield (Table 1). Applying an optimal nitrogen source close to the grain-filling stage can effectively promote yield formation [49]. Compared to the UF and SCU fertilizers, the PCU and BBF fertilizers consistently released nitrogen throughout the growth stage, providing more nitrogen for panicle differentiation and grain filling (Fig. 1). Intriguingly, the rice yield of PCU and BBF groups did not show significant differences compared to CK group, and the application of slow-controlled release fertilizers obviously improved grain weight (Table 1, Fig. 2). In 2020, the BBF group achieved the highest yield of 10.8 t hm−2 among all fertilizers, surpassing 0.6 t hm−2 in yield than CK group (Table 1). In 2021, the BBF yield increased to 11.0 t hm−2, significantly surpassing nearly 12%-20% of UF and SCU groups’ yields (Table 1).
Variations in nitrogen supply significantly impact the distribution and buildup of starch and protein during grain filling, ultimately affecting grain quality [18, 50]. Compared to conventional fertilizer, the application of UF and SCU fertilizers led to a significant reduction in nutritional value, while the PCU group exhibited lower cooking and eating quality (Tables 2 and 3). The low nutritional value observed in the UF and SCU groups might be attributed to insufficient nitrogen supply during the grain filling stage (Fig. 1), based on the report that inadequate nitrogen reduced synthesis of protein and amino acids [50]. In addition, the cooking and eating quality of rice can be adversely reduced by high nitrogen application [51], the low quality of the PCU group might be attributed to the release of excess nitrogen during the grain filling stage (Table 2, Fig. 1). Conversely, the application of BBF fertilizer led to good grain quality in terms of physical appearance, cooking and eating qualities, and nutritional value (Tables 2 and 3). This was achieved due to its optimal nitrogen release during grain filling (Fig. 1), with each quality-related index reaching those of CK group and surpassing other fertilizer treatments (Tables 2 and 3). Hence, to ensure a high rice yield and quality, it is crucial to employ an optimal slow-controlled release fertilizer, such as BBF.
The carbon and nitrogen balance in rice grains is influenced by the type of slow-controlled release fertilizer
The accumulation of starch, protein, and amino acids determines grain quality, and this process requires significant amounts of carbon and nitrogen metabolites [17, 46, 52]. The application of slow-controlled release fertilization did not hinder sucrose accumulation but led to a decrease in starch and amylopectin content in the PCU group compared to the CK group (Table 3, Fig. 3). Meanwhile, the PCU group showed no reduction in enzyme activity related to carbon metabolism (Fig. 4). In contrast, the UF group decreased the activities of SuSase, AGPase, and SBE, but did not show lower starch and amylopectin content compared to the PCU group (Table 3, Figs. 3 and 4). Carbon metabolism is crucial in nitrogen metabolism as it provides the carbon skeletons necessary for amino acid synthesis, including pyruvate, oxaloacetate, and alpha-ketoglutarate [53]. Thus, the complex results observed in this study might be linked to the interplay between carbon and nitrogen metabolism [54, 55].
The PCU and BBF fertilizers had a higher nitrogen-release ability during grain filling compared to UF and SCU fertilizers [15, 21]. Intriguingly, the application of UF and SCU fertilizers resulted in a significant reduction in accumulation of protein and amino acids compared to conventional fertilizer, whereas PCU fertilizers achieved levels similarly to those of the CK group during grain filling (Fig. 5). The GS/GOGAT cycle plays a key role in nitrogen assimilation [56, 57]. During grain filling, the PCU group showed increased activity in GS and GOGAT enzymes, reaching levels comparable to those of the CK group, whereas the groups of UF and SCU fertilizers showed significantly lower activity in these enzymes (Figs. 5 and 6). These results indicated that increased nitrogen supply mainly leads to increased synthesis of protein and amino acids in rice grains rather than increased accumulation of storage starch. However, the BBF group showed a better partition in starch and protein content, which was neither the highest nor the lowest compared to others (Figs. 3, 4, 5 and 6). This phenomenon may be related to the appropriate nitrogen release ability of BBF fertilizer during grain filling (Fig. 1). Despite the complex relationship between protein production and carbon–nitrogen metabolism, the dynamic changes in protein synthesis are significantly related to the application of slow-controlled release fertilizers (Table S3, Figure. S2).
Key steps for promoting carbon–nitrogen crosstalk in developing grain under the application of slow-controlled release fertilizer
Maintaining a balance between carbon and nitrogen metabolism is crucial for grain filling of rice, as nitrogen, functioning as a signaling molecule, influences plant metabolism and physiology through changes in gene expression [58]. During the grain-filling stage, rice grain undergoes dynamic metabolic adjustments to meet its nutritional demands [30, 59]. An analysis of the primary changes in amino acid metabolism revealed clear differences in the carbon and nitrogen metabolism among various slow-controlled release fertilizers (Fig. 7).
The expression of genes (OsSUT2 and OsCIN4) related to sucrose unloading [60], as well as sucrose content, in the UF, PCU, and BBF groups were not lower than that of CK group (Fig. 3 and 7), suggesting that the availability of carbohydrates was not restricted by these application of slow-controlled release fertilizers. N-nutrient/metabolites and carbon metabolism are intricately interconnected [46]. Carbon metabolism plays a critical role in incorporating nitrogen into cell metabolism, primarily through the oxidative pentose phosphate pathway (OPPP) and glycolysis/ the tricarboxylic acid (TCA) cycle [17, 27, 61, 62]. When comparing various types of slow-controlled release fertilizers, it was found that the application of PCU and BBF fertilizers led to a higher capacity for sucrose unloading and nitrogen assimilation (Figs. 7 and S2). In the metabolic analysis, the increased carbon flux was primarily directed towards the glycolysis and TCA cycle for amino acid synthesis in the PCU and BBF groups (Figs. 6 and S2). As the increase of nitrogen supply in PCU and BBF (Fig. 1), the GS/GOGAT cycle was significantly promoted in rice grain at the filling stage (Table S2, Figs. 7 and S2). The PCU group demonstrated significantly higher enzyme activity in carbon and nitrogen metabolism and a lower starch content in rice grain, compared to the UF and SCU groups (Figs. 3, 4, 5 and 6). A high supply of nitrogen can increase the sugar unloading and metabolic utilization [63, 64]. Consequently, the PCU group's high capacity for nitrogen assimilation lead to efficient utilization of carbohydrates for amino acid synthesis during grain filling. Interestingly, the BBF group showed a relatively balanced performance in key enzymes of carbon and nitrogen metabolism, as well as in the levels of starch, protein, and amino acids (Figs. 3, 4, 5 and 6). These results suggest that a suitable supply of nitrogen promotes both grain filling and maintains an optimal carbon–nitrogen state in the rice grain. Therefore, the application of slow-controlled release fertilizer like BBF is a practical solution for manipulating the balance between carbon and nitrogen in rice.
Overall, these observations suggest that an increase in nitrogen supply not only enhances nitrogen metabolism in grain, but also improves carbon flow (Table S2, Figs. 7 and S2). Our study proposes a model in which nitrogen supply regulates the networks of carbon–nitrogen metabolism, and the regulation of starch and protein synthesis under high nitrogen conditions is linked to nitrogen assimilation and the glycolysis/TCA cycle (Table S3, Fig. 7 and S2).
Conclusions
The application of BBF fertilizer not only increased rice yield but also enhanced grain quality compared to other slow-release fertilizers, such as UF, SCU, and PCU. Moreover, it has the potential to reach or even exceed the levels achieved with conventional fertilization. This can be primarily attributed to BBF fertilizer's superior regulation of the carbon–nitrogen balance in rice grains compared to other slow-controlled release fertilizers. The application of BBF fertilizer appropriately increases nitrogen assimilation (amino acid synthesis and nitrogen transport) in the grain by modulating carbon flow in the carbon metabolism of grain (e.g., glycolytic metabolism and the TCA cycle). Our research provides new insight into the relationship among the nitrogen supply, metabolism pathway of carbon and nitrogen, and grain growth to finely promote grain quality under the application of slow-controlled release fertilizer in rice.
Availability of data and materials
Data sets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CK:
-
Conventional fertilization
- UF:
-
Urea formaldehyde fertilizer
- SCU:
-
Sulfur-coated urea fertilizer
- PCU:
-
Polymer-coated urea fertilizer
- BBF:
-
Controlled-release bulk blending fertilizer
- TCA:
-
The tricarboxylic acid
- NUE:
-
Nitrogen use efficiency
- GS:
-
Glutamine synthetase
- GOGAT:
-
Glutamate synthase
- OPPP:
-
Oxidative pentose phosphate pathway
- SuSase:
-
Sucrose Synthase
- AGPase:
-
ADP-glucose pyrophosphorylase
- GBSS:
-
Granule-bound starch synthase
- SBE:
-
Starch branching enzyme
- Glu:
-
Glutamine
- Gly:
-
Glycine
- His:
-
Histidine
- Ile:
-
Isoleucine
- Leu:
-
Leucine
- Lys:
-
Lysine
- Phe:
-
Phenylalanine
- Ser:
-
Serine
- Thr:
-
Threonine
- Tyr:
-
Tyrosine
- Val:
-
Valine
- DPA:
-
Days post-anthesis
- RVA:
-
Rapid viscosity analyzer
- DW:
-
Dry weight
References
Thakur AK, Rath S, Patil DU, Kumar A. Effects on rice plant morphology and physiology of water and associated management practices of the system of rice intensification and their implications for crop performance. Paddy Water Environ. 2011;9:13–24.
Wang F, Peng SB. Yield potential and nitrogen use efficiency of China’s super rice. J Integr Agric. 2017;16:1000–8.
Custodio M, Cuevas R, Ynion J, Laborte A, Velasco M, Demont M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists?. Trends Food Sci Technol. 2019;92:122–37.
Adjao R, Staatz J. Asian rice economy changes and implications for sub-Saharan Africa. Glob Food Sec. 2015;5:50–5.
Concepcion J, Ouk M, Zhao D, Fitzgerald M. The need for new tools and investment to improve the accuracy of selecting for grain quality in rice. Field Crop Res. 2015;182:60–7.
Fitzgerald M, McCouch S, Hall R. Not just a grain of rice: the quest for quality. Trends Plant Sci. 2009;14:133–9.
Gu J, Chen J, Chen L, Wang Z, Zhang H, Yang J. Grain quality changes and responses to nitrogen fertilizer of japonica rice cultivars released in the Yangtze River Basin from the 1950s to 2000s. Crop J. 2015;3:285–97.
Chen X, Cui Z, Fan M, Vitousek P, Zhao M, Ma W, Wang Z, Zhang W, Yan X, Yang J, Deng X, Gao Q, Zhang Q, Guo S, Ren J, Li S, Ye Y, Wang Z, Huang J, Tang Q, Sun Y, Peng X, Zhang J, He M, Zhu Y, Xue J, Wang G, Wu L, An N, Wu L, Ma L, Zhang W, Zhang F. Producing more grain with lower environmental costs. Nature. 2014;514:486–9.
Naz MY, Sulaiman SA. Slow release coating remedy for nitrogen loss from conventional urea: a review. J Control Release. 2016;225:109–20.
Wu Q, Wang Y, Ding Y, Tao W, Gao S, Li Q, Li W, Liu Z, Li G. Effects of different types of slow- and controlled-release fertilizers on rice yield. J Integr Agric. 2021;20:1503–14.
Ke J, Xing X, Li G, Ding Y, Dou F, Wang S, Liu Z, Tang S, Ding C, Chen L. Effects of different controlled-release nitrogen fertilisers on ammonia volatilisation, nitrogen use efficiency and yield of blanket-seedling machine-transplanted rice. Field Crop Res. 2017;205:147–56.
Gao Y, Xu Z, Zhang L, Li S, Wang S, Yang H, Liu X, Zeng D, Liu Q, Qian Q, Zhang B, Zhou Y. MYB61 is regulated by GRF4 and promotes nitrogen utilization and biomass production in rice. Nat Commun. 2020;11:5219.
Shaviv A. Advances in controlled-release fertilizers. Adv Agron. 2001;71:1–49.
Sheehy JE, Dionora MJA, Mitchell PL. Spikelet numbers, sink size and potential yield in rice. Field Crop Res. 2001;71:77–85.
Xu X, Ma H, Nin Y, Wang J, Zhang Y. Effects of slow-released nitrogen fertilizers with different application patterns on crop yields and nitrogen fertilizer use efficiency in rice-wheat rotation system. J Plant Nutr Fertilizer Sci. 2016;22:307–16.
Ye Y, Liang X, Chen Y, Liu J, Gu J, Guo R, Li L. Alternate wetting and drying irrigation and controlled-release nitrogen fertilizer in late-season rice. Effects on dry matter accumulation, yield, water and nitrogen use. Field Crop Res. 2013;144:212–24.
Jiang Z, Chen Q, Chen L, Yang H, Zhu M, Ding Y, et al. Efficiency of sucrose to starch metabolism is related to the initiation of inferior grain filling in large panicle rice. Front Plant Sci. 2021;12:732867.
Wang M, Perez-Garcia MD, Daviere JM, Barbier F, Oge L, Gentilhomme J, Voisine L, Peron T, Launay-Avon A, Clement G, Baumberger N, Balzergue S, Macherel D, Grappin P, Bertheloot J, Achard P, Hamama L, Sakr S. Outgrowth of the axillary bud in rose is controlled by sugar metabolism and signalling. J Exp Bot. 2021;72:3044–60.
Xu C, Yang F, Tang X, Lu B, Li Z, Liu Z, et al. Super rice with high sink activities has superior adaptability to low filling stage temperature. Front Plant Sci. 2021;12:729021.
Zhao M, Zhao M, Gu S, Sun J, Ma Z, Wang L, Zheng W, Xu Z. DEP1 is involved in regulating the carbon–nitrogen metabolic balance to affect grain yield and quality in rice (Oriza sativa L.). Plos one. 2019;14:e0213504.
Dou Z, Tang S, Li G, Liu ZH, Ding C, Chen L, et al. Application of nitrogen fertilizer at heading stage improves rice quality under elevated temperature during grain-filling stage. Crop Sci. 2017;57:2183–92.
Gutiérrez R, Lejay L, Dean A, Chiaromonte F, Shasha D, Coruzzi G. Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol. 2007;8:1–13.
Huang X, Zhang Y, Wang L, Dong X, Hu W, Jiang M, Chen G, An G, Xiong F, Wu Y. OsDOF11 affects nitrogen metabolism by sucrose transport signaling in rice (Oryza sativa L.). Front Plant Sci. 2021;12:703034.
Bao A, Zhao Z, Ding G, Shi L, Xu F, Cai H. The stable level of glutamine synthetase 2 plays an important role in rice growth and in carbon-nitrogen metabolic balance. Int J Mol Sci. 2015;16:12713–36.
Luo H, Wang Z, Chen Y, Zheng A, Chen Y, Du P, Mao T, Meng S, Tang X. The effects of different temperatures on the biosynthesis of grain protein in rice at filling stage. Appl Ecol Environ Res. 2018;16:8017–27.
Poucet T, González-Moro MB, Cabasson C, Beauvoit B, Gibon Y, Dieuaide-Noubhani M, Marino D. Ammonium supply induces differential metabolic adaptive responses in tomato according to leaf phenological stage. J Exp Bot. 2021;72:3185–99.
Li M, Li D, Feng F, Zhang S, Ma F, Cheng L. Proteomic analysis reveals dynamic regulation of fruit development and sugar and acid accumulation in apple. J Exp Bot. 2016;67:5145–57.
Yang X, Nian J, Xie Q, Feng J, Zhang F, Jing H, Zhang J, Dong G, Liang Y, Peng J, Wang G, Qian Q, Zuo J. Rice Ferredoxin-dependent glutamate synthase regulates nitrogen–carbon metabolomes and is genetically differentiated between japonica and indica subspecies. Mol Plant. 2016;9:1520–34.
Nunes-Nesi A, Fernie AR, Stitt M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant. 2010;3:973–96.
Masumoto C, Miyazawa S-I, Ohkawa H, Fukuda T, Taniguchi Y, Murayama S, Kusano M, Saito K, Fukayama H, Miyao M. Phosphoenolpyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. Proc Natl Acad Sci. 2010;107:5226–31.
Jiang Z, Yang H, Zhu M, Wu L, Yan F, Qian H, He W, Liu D, Chen H, Chen L, Ding Y, Sakr S, Li G. The inferior grain filling initiation promotes the source strength of rice leaves. Rice. 2023;16(41):1–15.
Wang E, Wang J, Zhu X, Hao W, Wang L, Li Q, Zhang L, He W, Lu B, Lin H, Ma H, Zhang G, He Z. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat Genet. 2008;40:1370–4.
Deng Y, Yu Y, Hu Y, Ma L, Lin Y, Wu Y, et al. Auxin-mediated regulation of dorsal vascular cell development may be responsible for sucrose phloem unloading in large panicle rice. Front Plant Sci. 2021;12:630997.
Xu C, Lu B, Liang L, Yang F, Ding C, Yan F, Zhou Y, Jiang Z, Liu Z, Ding Y, Li W, Li G. Delayed sowing date improves rice cooking and taste quality by regulating the quantity and quality of grains located on secondary branches. Agronomy Basel. 2022;12:1316.
Falsone G. Keys to Soil Taxonomy 2010. 2013.
Yang Y, Zhang M, Zheng L, Cheng D, Liu M, Geng Y. Controlled release urea improved nitrogen use efficiency, yield, and quality of wheat. Agron J. 2011;103:479–85.
Tang S, Zhang H, Liu W, Dou Z, Zhou Q, Chen W, Wang S, Ding YJFC. Nitrogen fertilizer at heading stage effectively compensates for the deterioration of rice quality by affecting the starch-related properties under elevated temperatures. Food Chem. 2019;277:455–62.
Jin Z, Jiang W, Chin J, Koh H. Analysis on the combining ability of taste meter value and starch RVA properties in Indica rice. Europe PMC. 2004;30:1210–4.
Richards F. A flexible growth function for empirical use. J Exp Bot. 1959;10:290–301.
Yang J, Zhang J, Wang Z, Zhu Q. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J Exp Bot. 2001;52:2169–79.
Yang J, Zhang J, Wang Z, Zhu Q, Liu L. Activities of enzymes involved in sucrose-to-starch metabolism in rice grains subjected to water stress during filling. Field Crop Res. 2003;81:69–81.
Tang S, Chen W, Liu W, Zhou Q, Zhang H, Wang S, Ding Y. Open-field warming regulates the morphological structure, protein synthesis of grain and affects the appearance quality of rice. J Cereal Sci. 2018;84:20–9.
Yasuda H, Tada Y, Hayashi Y, Jomori T, Takaiwa F. Expression of the small peptide GLP-1 in transgenic plants. Transgenic Res. 2005;14:677–84.
Amagliani L, O’Regan J, Kelly A, O’Mahony J. Composition and protein profile analysis of rice protein ingredients. J Food Compos Anal. 2017;59:18–26.
Kesarwani A, Chiang P, Chen S. Rapid visco analyzer measurements of japonica rice cultivars to study interrelationship between pasting properties and farming system. Int J Agron. 2016;2016:3595326.
Dai Z, Léon C, Feil R, Lunn J, Delrot S, Gomès E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J Exp Bot. 2013;64:1345–55.
Yamakawa H, Hakata M. Atlas of rice grain filling-related metabolism under high temperature: joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010;51:795–809.
Ji X, Raveendran M, Oane R, Ismail A, Lafitte R, Bruskiewich R, Cheng S, Bennett J. Tissue-specific expression and drought responsiveness of cell-wall invertase genes of rice at flowering. Plant Mol Biol. 2005;59:945.
Wang W, Wang W, Pan Y, Tan C, Li H, Chen Y, Liu X, Wei J, Xu N, Han Y, Gu H, Ye R, Ding Q, Ma C. A new gain-of-function OsGS2/GRF4 allele generated by CRISPR/Cas9 genome editing increases rice grain size and yield. Crop J. 2022;10:1207–12.
Nasielski J, Deen B. Nitrogen applications made close to silking: Implications for yield formation in maize. Field Crop Res. 2019;243:107621.
Wang X, Wang K, Yin T, Zhao Y, Liu W, Shen Y, et al. Nitrogen fertilizer regulated grain storage protein synthesis and reduced chalkiness of rice under actual field warming. Front Plant Sci. 2021;12:715436.
Shi S, Zhang G, Chen L, Zhang W, Wang X, Pan K, et al. Different nitrogen fertilizer application in the field affects the morphology and structure of protein and starch in rice during cooking. Food Res Int. 2023;163:112193.
Liu W, Miao T. Mixture of controlled-release and conventional urea fertilizer application changed soil aggregate stability, humic acid molecular composition, and maize nitrogen uptake. Sci Total Environ. 2021;789:147778.
Xu G, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012;63:153–82.
Baunsgaard L, Lütken H, Mikkelsen R, Glaring MA, Pham TT, Blennow A. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J. 2005;41:595–605.
MacNeill G, Mehrpouyan S, Minow M, Patterson J, Tetlow I, Emes M. Starch as a source, starch as a sink: the bifunctional role of starch in carbon allocation. J Exp Bot. 2017;68:4433–53.
Gao Z, Wang Y, Chen G, Zhang A, Yang S, Shang L, Wang D, Ruan B, Liu C, Jiang H. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat Commun. 2019;10:5207.
Hou W, Xue X, Li X, Khan MR, Yan J, Ren T, Cong R, Lu J. Interactive effects of nitrogen and potassium on: Grain yield, nitrogen uptake and nitrogen use efficiency of rice in low potassium fertility soil in China. Field Crop Res. 2019;236:14–23.
Xin W, Zhang L, Zhang W, Gao J, Yi J, Zhen X, Li Z, Zhao Y, Peng C, Zhao C. An integrated analysis of the rice transcriptome and metabolome reveals differential regulation of carbon and nitrogen metabolism in response to nitrogen availability. Int J Mol Sci. 2019;20:2349.
Jiang Z, Chen Q, Chen L, Liu D, Yang H, Xu C, Hong J, Li J, Ding Y, Sakr S, Liu Z, Jiang Y, Li G. Sink strength promoting remobilization of non-structural carbohydrates by activating sugar signaling in rice stem during grain filling. Int J Mol Sci. 2022;23:4864.
Sekhar S, Kumar J, Mohanty S, Mohanty N, Panda RS, Das S, Shaw BP, Behera L. Identification of novel QTLs for grain fertility and associated traits to decipher poor grain filling of basal spikelets in dense panicle rice. Sci Rep. 2021;11:13617.
Bussell J, Keech O, Fenske R, Smith S. Requirement for the plastidial oxidative pentose phosphate pathway for nitrate assimilation in Arabidopsis. Plant J. 2013;75:578–91.
Jiang Z, Wang M, Nicolas M, Ogé L, Pérez-Garcia M-D, Crespel L, Li G, Ding Y, Le Gourrierec J, Grappin P, Sakr S. Glucose-6-phosphate dehydrogenases: the hidden players of plant physiology. Int J Mol Sci. 2022;23:16128.
Zhang L, Sun S, Liang Y, Li B, Ma S, Wang Z, et al. Nitrogen levels regulate sugar metabolism and transport in the shoot tips of crabapple plants. Front Plant Sci. 2021;12:626149.
Acknowledgements
The authors thank Maria-Dolores Pérez-Garcia, and Laurent Ogé for kindly providing guidance and Gaoya Sun for providing technical assistance. Thanks for the help of Jiangsu Province Rice Industry Cluster Project.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2021YFD1700803), the Province Key Research and Development Program of Jiangsu, China (D21YFD17008), and the China Scholarships Council (no. 202106850042).
Author information
Authors and Affiliations
Contributions
ZJ and QC: Conceptualization, Software, Formal analysis, Investigation, Writing - Original Draft and Visualization; DL, WT, SG, and JL: Validation and Investigation. CL and MZ: Data Curation; YD, and WL: Resources and Supervision; GL and SS: Conceptualization and Revising - Original Draft; LX: Draft revision, Project administration and Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Z., Chen, Q., Liu, D. et al. Application of slow-controlled release fertilizer coordinates the carbon flow in carbon-nitrogen metabolism to effect rice quality. BMC Plant Biol 24, 621 (2024). https://doi.org/10.1186/s12870-024-05309-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05309-9