Abstract
The dissemination of cancer cells is one of the main reasons for treatment failure. During the process of establishing distant metastases, cancer cells must migrate through narrow environments, such as intercellular junctions, extracellular matrix, and basement membranes. The deformability of the cell nucleus is a limiting factor for migration through narrow environments; therefore, as the largest and hardest organelle, the nucleus is a key factor in crossing restrictive spaces. Nesprin-1/2 provides mechanical linkage between the nucleus and the cytoskeleton, but the specific mechanism by Nesprin-1/2 regulating tumor cell nuclear deformation is unclear. Our study found that knocking down Nesprin-1/2 significantly weakens cell migration ability, and knocking down Nesprin-1/2 makes the nucleus becoming more easily deformed. Meanwhile, the knockdown of Nesprin-1/2 leads to a decrease in Lamin A/C levels. To explore whether Lamin A/C protein undergoes degradation, we treated cells with caspase-6 inhibitor Z-VEID-FMK, autophagy inhibitor 3-methyladenine (3-MA), or broad-spectrum proteasome inhibitor MG132, and found that knockdown of Nesprin-1/2 led to the degradation of Lamin A/C via the proteasome pathway. Through immunofluorescence experiments, we observed F-actin distribution in the process of pore migration, and found that knockdown of Nesprin-1/2 weakened the pushing force of the nuclear tail, making it unable to propel the nucleus forward. In conclusion, this study demonstrates that Nesprin-1/2 regulates nuclear deformation and reorganizes the cytoskeleton, which together affects cell pore migration and provides a theoretical reference for the study of nuclear deformation mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cell migration is a complex physicochemical process that results in the displacement of cells in a two-dimensional surface or three-dimensional environment [1,2,3]. Cell migration in the body requires crossing complex extracellular matrix (ECM). The main way for migrating cells to overcome these restrictions is through hydrolyzing ECM to widen gaps or altering the cell's elastic properties [4]. The nucleus is the largest and hardest organelle in cells, composed of stable structural proteins, enabling it to resist significant shape changes [5]. For migration through small pores or 3D scaffolds, the nucleus can become a rate-limiting organelle [6]. The shape and size of nuclei vary greatly between different cell types and even within cells. Nevertheless, most cells imaged in situ or cultured in 3D matrices have oval or spherical nuclei with diameters of 5–15 μm [7]. In 2D culture, nuclear distribution is often obvious, with diameters of 10–20 μm and heights of around 10 microns. However, in a few cell types that frequently move at high speeds, including myeloid and cancer cells, the nucleus can be bean-shaped, lobulated, or segmented, potentially forming larger deformations [8]. Whether protein hydrolysis or non-protein migration through 3D tissue, the ability of the nucleus to deform is closely associated with the cell's ability to traverse restrictive spaces.
Cells can adapt to the structure of the extracellular tissue, which is necessary for establishing and maintaining tissue function. During the process of cell migration through basement membrane or 3D interstitial tissues, external signals may cause significant changes in the cell nucleus. Nuclear deformation is caused by several parallel parameters, including tissue porosity, tissue shape discontinuity, traction force generated by moving cells, and matrix degradation. In loose tissues, the nucleus maintains its original ellipsoidal shape during displacement but may still cause local compression when crossing narrow areas of dense tissue. In this process, the changes in nuclear shape are transient. However, nuclear deformation in extreme spaces has a significant impact on cell viability. Nuclear deformation may result in the conformational changes of related genes or chromatin [9], the accessibility of transcription mechanisms [10], the conformation of nuclear skeleton proteins [11], and the contractibility of cells [12, 13]. The mechanisms underlying cell deformability are still poorly understood, but one accepted way to explain mechanical force transduction is force-induced conformational change [14]. Recent studies had shown that the stretching of endoplasmic reticulum can be driven by nucleus stretch and unfolding of the inner nuclear membrane. This allows calcium from the endoplasmic reticulum to be released into the cytoplasm, and the unfolded nuclear membrane provides more protein-binding sites than the folded nuclear membrane. This activates the production of cytosolic phospholipase A2 (cPLA2) and arachidonic acid (AA), a metabolite that regulates myosin II activity, thus improving the cell's ability to pass through confined pores [12]. Therefore, nucleus deformation and cell contractility are particularly important when tumor cells invade the complex tumor microenvironment. Nuclear deformation is also associated with self-structural molecules, such as nuclear lamina proteins and chromatin, which affect the nucleus's ability to deform.
The nuclear lamina protein (Lamins), as part of the nuclear membrane, further participates in anchoring and positioning of the nucleus, and nuclear size and shape [15,16,17]. Lamins include Lamin A/C and Lamin B, which form different but overlapping networks at the nuclear membrane, with a unique impact on nuclear shape and rigidity. Fibroblast nuclei lacking Lamin B1 exhibit chromatin protrusions (nuclear blebs), while cells lacking Lamin A and/or Lamin C have irregular nuclei [18]. Besides their structural functions, Lamins also interact with transcription factors, including retinoblastoma protein (RB), SREBP-1, and c-Fos. In healthy tissue cells, the presence of Lamin A is primarily associated with cell quiescence or differentiation, and de-differentiation can induce a reduction in Lamin A/C levels. Reduced expression of nuclear lamina proteins has been reported to be associated with tumor invasiveness [19]. However, in colorectal and prostate cancers, levels of Lamin A/C are elevated and cell invasion capability increases. Changes in Lamin A levels are closely linked to the occurrence and development of cancer cells. Therefore, changes in Lamins are closely related to cancer, and Lamin expression may be associated with malignant ability of tumor cells through the nucleus.
The LINC complex is a nuclear membrane protein that includes Nesprins and SUN proteins, acting as a bridge between the nuclear and cytoskeletal skeletons. The Nesprin family has four known isoforms: Nesprin-1 and -2 bind to actin, Nesprin-3 binds to intermediate filaments (via Plectin) and Nesprin-4 binds to microtubules [20]. Nesprin-1 and -2 were first identified in the screening of novel smooth muscle cell differentiation markers. The typical structure of Nesprin-1 and -2 consists of three main domains: a C-terminal KASH domain targeting the nuclear membrane, an N-terminal Calponin homology (CH) domain binding to the actin cytoskeleton, and a rod-like structure composed of multiple spectrin repeat (SR) sequences [21]. Nesprin-1 and -2 provide mechanical connections from the cytoplasm to the nucleus, and are closely associated with Lamins [22, 23]. The LINC complex has been found to maintain the position of the nucleus and movement during cell migration and differentiation [24,25,26]. Studies have shown that myosin causes significant deformation of the cell nucleus and affects cell migration efficiency in breast cancer cells under restricted space conditions [27]. Nesprins are associated with nuclear movement and positioning during development of fibroblasts and muscle cells, outer hair cells, as well as retina and neuron cells [28, 29]. Fascin provides additional connections between actin and Nesprin-2, regulating nuclear displacement and deformation in a narrow environment [30, 31]. Thus, nuclear deformation is closely related to the LINC complex and Nesprins may further regulate the motility of tumor cells through Lamins.
Overall, the deformation of the cell nucleus is closely related to the environment and the composition of its own molecules, such as nuclear Lamins. Nesprin-1/2 provides mechanical connection between the nucleus and the cytoskeleton, playing a significant role in regulating cell movement, nuclear positioning and deformation in narrow environments. However, the specific mechanism by which Nesprin-1/2 regulates nuclear deformation in cancer cells is unclear. This study mainly investigates the mechanisms of Nesprin-1/2 regulates breast cancer cell migration via nuclear deformation and mechanical transmission.
2 Materials and methods
2.1 Antibodies and reagents
Cell culture medium of L-15, penicillin, streptomycin, and newborn calf serum (NCS) was purchased from Gibco (USA). Antibodies against Lamin A/C and GAPDH were from Abcam (UK). DAPI was purchased from MedChemExpree (CN). Phalloidin 555 was from ATTbio (CN). 3-MA, Z-VEID-FMK, and MG132 were purchased from Selleck (USA). All other reagents were used as received without additional purification unless otherwise noted.
2.2 Cell culture
The triple-negative human breast cancer cell MDA-MB-231 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in L-15 medium composed of 10% newborn calf serum and 1% penicillin and streptomycin. For culturing, we used a 37℃ humidified incubator.
2.3 Transwell assay
After resuscitation, the human breast cancer cell line MDA-MB-231 were incubated with 10% fetal bovine serum, penicillin (100 μg/mL), and streptomycin (100 μg/mL) in L-15 medium. When the cells grew to 70% of the culture bottle area, they were subcultured. After three times of subculture, the cells were used for experiments. Inoculate cells at a density of 1 × 105/cm2 in a transwell chamber and migrate under normal culture conditions for 24 h using serum induction. Intracellular F-actin were imaged by Z-stack (0.3 μm/step) stack scan with a confocal microscope (Zeiss, LSM800, 63/1.4-NA objective).
2.4 Plasmids and transfection
The plasmids for Nesprin-1/2 shRNA were amplified by primers as follows: Nesprin-1 shRNA: 5´-CAGAAGTGCTGGTAGCATAAG-3´, Nesprin-2 shRNA: 5´-GATGGAAACAATCAATCATA-3´, Sc RNA: 5´-TTCTCCGAACGTGTCACGT- 3´. The PCR product was first cloned and the insertion was recombined into PCDNA3.1(+) vector. Cells were transfected with Lipofetamine® LTX (Invitrogen, USA) according to the manufacturer’s protocol. Transfer MDA-MB-231 cells (1.5 × 105) overnight in a 6-well plate and add 2.5 μg DNA and 500 μL volume dilution medium. Then add 5 μL Plus Reagent in a 2 mL EP tube and incubate at 37 ℃ for 10 min. Continue with 5 μL Lipofetamine® LTX Reagent was added to the above mixed liquid and incubated at 37 ℃ for 30 min. Finally, the transfection reagent and culture medium with serum but without double antibodies were added to the well plate for co-incubation.
2.5 Immunofluorescence staining
Transwell membrane was fixed in 4% formaldehyde for 15 min, permeated with 0.25% Triton-X for 10 min, sealed with 5% BSA, and incubated overnight in various primary antibodies: Lamin A/C, and 555 Phalloidin. Finally, label the first antibody with the corresponding second antibody for 1.5 h. For F-actin staining, after adding of phalloidin, the samples were incubated at room temperature for 2 h. Stain DNA with DAPI for 15 min. The images were collected by the use of confocal Zeiss 800 system. Various image quantization and processing are completed using Image J software.
2.6 Western blot analysis
MDA-MB-231 was trypsinized and washed in cold PBS, and then cleaved in RIPA lysis buffer containing a cocktail of protease and phosphatase inhibitors. The sample was run on 8–12% SDS-PAGE and transferred to a PVDF membrane. The membrane was blocked in TBST buffer (10 mM Tris HCl, 100 mM NaCl, 0.1% Tween-20) at room temperature for 1 h in 5% skimmed milk, and then detected with the primary antibodies of Lamin A/C and GAPDH. After placing at 4 ℃, gently shake and incubate with the corresponding secondary antibody (1:500) at room temperature for 2 h. Visualize and capture protein bands using Western blot reagent in chemiluminescence imaging system.
2.7 Statistical analysis
To eliminate errors, each experiment was repeated at least three times. Data were collected as means ± SDs. A t test was used to analyze the data with two distribution tails. A value of p > 0.05 (ns) was considered that there was no significant differences, a value of p < 0.01(**) was used to determine statistically significant differences, and a value of p < 0.001(****) was considered to be remarkably statistically significant. The results were analyzed with GraphPad Prism software 6.0.
3 Results
3.1 Knocking down Nesprin-1/2 inhibits pore migration of breast cancer cells
During the process of tumor cell migration, especially through narrow environments such as cell–cell junctions, extracellular matrix, and basement membranes, the nucleus of the cell needs to undergo deformation to adapt to confined spaces. Nesprin-1/2 serves as a bridge molecule between the nucleus and the cytoskeleton, providing mechanical connections from the cytoplasm to the interior of the nucleus. Nesprin-1/2 may be involved in tumor cell migration and nuclear deformation. To investigate whether Nesprin-1/2 regulates cell migration in restrictive environments, we constructed Nesprin-1/2 (knockdown group) and Scramble (control group) plasmids, and evaluated the migration of the three groups of cells in a Transwell chamber with 3 μm pore size (Fig. 1A–C). Crystal violet staining showed that the number of migrating cells in the Sc shRNA group was significantly higher than that in the Nesprin-1/2 shRNA group (Fig. 1D, E), indicating that knocking down Nesprin-1/2 significantly reduces pore migration capacity and suggesting that Nesprin-1/2 is an important regulatory molecule for this process.
Knocking down Nesprin-1/2 significantly reduces transcellular migration capacity of breast cancer cells. A, B Detection of Nesprin-1/2 expression levels in cells transfected with ShRNA using protein immunoblotting. C Transwell schematic diagram of pore migration. D Transwell experiment measuring cell migration after 24 h. Scale bar is 100 μm. E Statistical analysis of cell migration ability in (D) using Image J software. **p < 0.01; ****p < 0.001
3.2 Knocking down Nesprin-1/2 makes breast cancer cell nuclei more deformable
To explore whether Nesprin-1/2 regulates tumor cell nuclear deformation, we conducted experiments using cells transfected with shRNA (scramble group) or Nesprin-1/2 knockdown plasmids. We used immunofluorescence imaging to quantify nuclear area, height, and volume (Fig. 2A–D) to characterize nuclear deformation. The results showed that knocking down Nesprin-1/2 significantly reduced nuclear area compared to the scramble group, but nuclei displayed a higher degree of height advantage, while total nuclear volume did not change. Therefore, we concluded that knocking down Nesprin-1/2 makes nuclei more prone to deformation and that Nesprin-1/2 plays an important role in regulating nuclear deformation.
Knockdown of Nesprin-1/2 leads to nuclear deformation. A Immunofluorescence image of cell nuclei. Cell nucleus images obtained through layer scanning by confocal microscopy were processed by ZEN software to obtain YZ and XZ projections of the cell nucleus. Blue represents the nucleus. Scale bar is 10 μm. B–D Twenty cells were randomly selected in each group, and the height, area, and volume of the cell nucleus were statistically analyzed using confocal software and image J (ns no significant difference, ****p < 0.001)
3.3 Knockdown of Nesprin-1/2 downregulates the expression of Lamin A/C
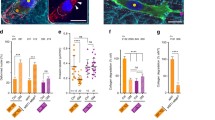
The nuclear lamina, as a supporting network of the nucleus structure, is closely related to stabilize the nuclear membrane, and maintain the position of nuclear pores and spatial structure during interphase. Lamin A/C, as the main component of the nuclear lamina, plays an important role in maintaining the shape of the nucleus. To investigate whether the nuclear deformation regulated by Nesprin-1/2 is related to the expression of Lamin A/C, we detected the expression level of Lamin A/C by immunofluorescence (Fig. 3A, B). Compared with the control group, the expression level of Lamin A/C in the nucleus was significantly reduced after knocking down Nesprin-1/2. However, there was no significant change in Lamin A/C mRNA levels (Fig. 3C), which may suggest that Lamin A/C underwent degradation at the protein level.
Knockdown of Nesprin-1/2 leads to downregulation of Lamin A/C protein expression. A Immunofluorescence confocal image of Lamin A/C. Green represents Lamin A/C, and blue represents the cell nucleus. Scale bar is 5 μm. B Statistical analysis of Lamin A/C expression intensity in 20 randomly selected cells in each group. C Statistical analysis of Lamin A/C mRNA expression (ns no significant difference, ****p < 0.001
3.4 Knockdown of Nesprin-1/2 promotes the degradation of Lamin A/C through the proteasome pathway
In previous experiments, we confirmed that knocking down Nesprin-1/2 led to degradation of Lamin A/C. We treated cells with translation inhibitor cycloheximide (CHX) and found that the expression level of Lamin A/C gradually decreased in the Sc RNA group. As the drug treatment time increased, the protein expression level of Lamin A/C in the knockdown group remained consistently low (Fig. 4A). This experiment confirmed that after knocking down Nesprin-1/2, the unstable Lamin A/C underwent degradation, resulting in downregulation of its protein expression level. We treated experimental group cells with Caspase-6 inhibitor Z-VEID-FMK or autophagy inhibitor 3-methyladenine (3-MA) and compared them with control group cells without drug treatment. The treatment with both inhibitors did not result in upregulation of Lamin A/C protein levels (Fig. 4B). This phenomenon indicates that the degradation of Lamin A/C is not mediated by the caspase-6 or autophagy pathways. Next, we investigated whether the proteasome pathway regulates the degradation of Lamin A/C. We treated experimental group cells with broad-spectrum proteasome inhibitor MG132 and compared them with control group cells without drug treatment. The protein level of Lamin A/C was upregulated (Fig. 4C), confirming that knocking down Nesprin-1/2 leads to degradation of Lamin A/C through the proteasome pathway.
Knockdown of Nesprin-1/2 leads to the degradation of Lamin A/C. A Cells were treated with cycloheximide (50 μg/mL), and the protein expression level of Lamin A/C in transfected cell lines was detected by Western blot. B, C Detection of Lamin A/C degradation pathways in transfected cell lines treated with Z-VEID-FMK (20 μM, 2 h), 3-MA (5 mM, 2 h), and MG132 (20 μM, 6 h). The protein levels of Lamin A/C were detected by western blot
3.5 Nuclear migration requires the propelling force of nuclear envelope actomyosin contraction
As a bridge molecule between the nuclear skeleton and the cytoskeleton, it is unclear whether the knockdown of Nesprin-1/2 affects the distribution of the cytoskeleton and the mechanism by which Nesprin-1/2 transmits force to the nucleus. To further investigate whether Nesprin-1/2 regulates the cytoskeleton, we used immunofluorescence to detect the distribution of F-actin (Fig. 5A–E). The results showed that knocking down Nesprin-1/2 led to a decrease in the degree of F-actin accumulation at the nuclear tail, due to the lack of actomyosin contractile force to propel its movement, ultimately resulting in decreased migration ability. In conclusion, nuclear migration requires the propelling force of nuclear envelope actomyosin contraction.
The thrust provided by F-actin is needed during the cell passing through the pore. A–C Fluorescence images of intracellular F-actin on both sides of the membrane in transwell chamber. The XZ (below the XY image) and YZ (right side of XY image) side views show the position which dashed crossed line in the XY images. Scale bar is 50 µm. D, E The distribution and mean intensity of actin on both sides of the membrane in XZ and YZ image in Sc shRNA, Nesprin-1 shRNA, and Nesprin-2 shRNA
4 Discussion
During the process of migration and invasion in vivo, cells need to pass through complex and narrow microenvironments, including extracellular matrix (ECM) networks and neighboring cells. Cell migration requires passage through pores ranging in size from 0.1 to 30 μm, some of which are significantly smaller than the size of the cell or nucleus. To better simulate the restrictive environment of cell migration in vivo, we used Transwell chambers with pore sizes of 3 μm to observe cell migration. Other studies have also explored cell migration in other restrictive environments, such as using microfluidic devices with 2, 3, 5 μm width restrictive channels and 15 μm width non-restrictive channels [32]. These microfluidic devices can measure important parameters such as cell migration rate and distance, and can be improved in the future. Nesprin-1/2 acts as a cytoskeletal and nuclear physical link; it plays an important role in transmitting mechanical signals to the cell nucleus. Recent studies have shown a significant correlation between Nesprin-2 and the transport of the nucleus during cellular movement [33]. Knockdown or incomplete structure of Nesprin-1/2 leads to a lack of force transmission, resulting in decreased nucleus transport and cell migration abilities. Knockdown of Nesprin-1 has been found to increase the number of focal adhesions and matrix traction, leading to a decrease in cell migration speed without changes in non-muscle myosin II activity [34]. Therefore, the impact of Nesprin-1/2 on cell migration in a restricted environment (3 μm pores) was explored and the results showed that knockdown of Nesprin-1/2 decreased cell migration ability, potentially due to defects in nuclear localization and cell migration caused by the inability of the cytoskeleton to act on the nucleus.
The migration environment determines cell adaptability and affects migration efficiency. The process of ECM remodeling in tumors has been extensively studied, but the mechanism of nuclear deformation, especially in adapting to small usable tissue space during migration, remains an unresolved issue. The cell nucleus is a major mechanical sensing and integrating factor. Therefore, future goals may involve exploring in-depth the roles of the nuclear membrane and actin structures that help facilitate nuclear movement and deformation to reduce resistance during migration in three-dimensional structures. Compared to other organelles, the large volume and high stiffness of the cell nucleus pose a significant physical challenge for cells moving in narrow three-dimensional environments. Rac1 and actin organization have a promoting effect on nuclear deformation and invasive abilities. When ROCK or myosin II is suppressed, phenotypes of nuclear deformation and dendritic cells moving forward through dense collagen matrices disappear as tumor and immune cells move forward in 3D confined spaces [35]. This suggests that myosin plays an important role in nuclear deformation. Changes in nuclear morphology and mechanical plasticity during cell differentiation, tumor transformation, pathological invasion, and recirculation may be determining factors for cell migration. As a bridging molecule between nuclear and cytoskeletal frameworks, Nesprin-1/2 can regulate nuclear deformation and transport.
Nesprin-1/2 provides mechanical linkage between the nucleus and the cytoskeleton by binding to the SUN protein in the nucleus via the KASH domain. The SUN protein passes through the nuclear membrane and binds to Lamin inside the nucleus. Through the CH domain linking to F-actin, Nesprin provides mechanical connection from the cytoplasm to the interior of the nucleus. Nesprin-2 cooperates with actin/myosin IIb to regulate restricted collagen gel migration. We observed significant deformation of nuclear shape, decreased area, increased height, and no change in volume after knocking down Nesprin-1/2. The nucleus is influenced by the cytoskeleton within the cell, such as the myosin cap on the top of the nucleus significantly affecting its shape, such as flattening, inhibiting the formation of the myosin cap with inhibitors weakens the force acting on the top of the nucleus, increasing its height [36]. As a mechanosensor, the disappearance of Nesprin-1/2 may result in the inability of biologic forces mediated by the cytoskeleton to be transmitted to the nucleus, thus affecting nuclear deformation.
As part of the nuclear envelope, the nuclear lamina protein plays an important role in maintaining nuclear stability and shape, further participating in anchoring and positioning of the nucleus, nuclear size and shape, and nuclear hardness. The lamina includes A-type nuclear lamina proteins (mainly Lamin A and C) and B-type nuclear lamina proteins. We found that the decrease in Lamin A/C protein level by Western Blot experiments and immunofluorescence experiments further illustrated that Nesprin-1/2 knockdown caused a reduction in Lamin A/C expression levels, making the nucleus more susceptible to deformation, confirming the importance of Lamin A/C in regulating nuclear deformation. Studies have also shown that cells with low Lamin A/C expression levels have softer nuclei, making them more prone to deformation in narrow environments, promoting cell migration [37, 38]. However, the nucleus cannot be too soft, as it affects cell lifespan [39].
The deformability of the cell nucleus is a limiting factor for cell migration through narrow environments. It is unclear whether knocking down Nesprin-1/2, as a bridging molecule between the nuclear and cytoskeletal network, disrupts the distribution of the cytoskeleton and the mechanism by which Nesprin-1/2 transmits force to the nucleus, whether it is pushing or pulling. We investigated whether Nesprin-1/2 affects the expression level of F-actin. The results showed that knockdown of Nesprin-1/2 resulted in the disappearance of F-actin accumulation at the tail of the nucleus, indicating that Nesprin-1/2 transmits pushing forces at the tail of the nucleus, promoting nuclear movement and regulating cell migration. Other studies have shown that nuclear deformation and perinuclear actomyosin are essential for cell migration through small pores, independent of proteolytic enzymes. Nuclear deformation and cytoskeletal mechanics are inseparable for cell migration across confining spaces [40]. In this study, we linked Nesprin-1/2 regulation of nuclear deformation and mechanical transmission, which has important implications for future research on cell migration in restricted spaces.
In summary, our study found that Nesprin-1/2 regulates cell migration by affecting nuclear deformation and cytoskeletal distribution. The specific molecular mechanism is that Nesprin-1/2 can regulate the expression of Lamin A/C. Loss of Nesprin-1/2 leads to the ubiquitination-proteasome-dependent degradation of Lamin A/C. Stable expression of Nesprin-1/2 maintains the accumulation of F-actin at the tail of the nucleus, thereby transmitting pushing forces at the tail of the nucleus and promoting cell migration across confining spaces (Fig. 6).
Scheme cartoon to summarize the process of cell pore migration in the presence of Nesprin-1/2 expressions. Nesprin-1/2 regulates the expression of Lamin A/C. Loss of Nesprin-1/2 leads to the ubiquitination-proteasome-dependent degradation of Lamin A/C, resulting in nuclear deformation. Stable expression of Nesprin-1/2 maintains the accumulation of F-actin at the tail of the nucleus, thereby transmitting pushing forces at the tail of the nucleus for the efficient pore migration
Data availability
Data will be made available on reasonable request.
References
A.J. Ridley, M.A. Schwartz, K. Burridge, R.A. Firtel, M.H. Ginsberg, G. Borisy, J.T. Parsons, A.R. Horwitz, Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003)
J.P. Campanale, D.J. Montell, Who’s really in charge: diverse follower cell behaviors in collective cell migration. Curr. Opin. Cell Biol. 81, 102160 (2023)
J. Chen, D. Yan, Y. Chen, Understanding the driving force for cell migration plasticity. Biophys J. 122, 1–7 (2023)
P. Friedl, Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 16, 14–23 (2004)
M. Krause, F.W. Yang, M. Te Lindert, P. Isermann, J. Schepens, R.J.A. Maas, C. Venkataraman, J. Lammerding, A. Madzvamuse, W. Hendriks, J. Te Riet, K. Wolf, Cell migration through three-dimensional confining pores: speed accelerations by deformation and recoil of the nucleus. Philos. Trans. R Soc. Lond. B Biol Sci. 374, 20180225 (2019)
A. Fruleux, R.J. Hawkins, Physical role for the nucleus in cell migration. J. Phys. Condens. Matter 28, 363002 (2016)
D.E. Jaalouk, J. Lammerding, Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63–73 (2009)
D. Feng, J.A. Nagy, K. Pyne, H.F. Dvorak, A.M. Dvorak, Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 187, 903–915 (1998)
M.M. Nava, Y.A. Miroshnikova, L.C. Biggs, D.B. Whitefield, F. Metge, J. Boucas, H. Vihinen, E. Jokitalo, X. Li, J.M. Garcia Arcos, B. Hoffmann, R. Merkel, C.M. Niessen, K.N. Dahl, S.A. Wickstrom, Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181, 800-817e822 (2020)
A. Tajik, Y. Zhang, F. Wei, J. Sun, Q. Jia, W. Zhou, R. Singh, N. Khanna, A.S. Belmont, N. Wang, Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 15, 1287–1296 (2016)
J. Swift, I.L. Ivanovska, A. Buxboim, T. Harada, P.C. Dingal, J. Pinter, J.D. Pajerowski, K.R. Spinler, J.W. Shin, M. Tewari, F. Rehfeldt, D.W. Speicher, D.E. Discher, Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013)
V. Venturini, F. Pezzano, F. Catala Castro, H.M. Hakkinen, S. Jimenez-Delgado, M. Colomer-Rosell, M. Marro, Q. Tolosa-Ramon, S. Paz-Lopez, M.A. Valverde, J. Weghuber, P. Loza-Alvarez, M. Krieg, S. Wieser, V. Ruprecht, The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370, eaba2644 (2020)
A.J. Lomakin, C.J. Cattin, D. Cuvelier, Z. Alraies, M. Molina, G.P.F. Nader, N. Srivastava, P.J. Saez, J.M. Garcia-Arcos, I.Y. Zhitnyak, A. Bhargava, M.K. Driscoll, E.S. Welf, R. Fiolka, R.J. Petrie, N.S. De Silva, J.M. Gonzalez-Granado, N. Manel, A.M. Lennon-Dumenil, D.J. Muller, M. Piel, The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894 (2020)
B.D. Hoffman, C. Grashoff, M.A. Schwartz, Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011)
A. Katiyar, J. Zhang, J.D. Antani, Y. Yu, K.L. Scott, P.P. Lele, C.A. Reinhart-King, N.J. Sniadecki, K.J. Roux, R.B. Dickinson, T.P. Lele, The nucleus bypasses obstacles by deforming like a drop with surface tension mediated by Lamin A/C. Adv. Sci. (Weinh) 9, e2201248 (2022)
N.L. Ovsiannikova, S.V. Lavrushkina, A.V. Ivanova, L.M. Mazina, O.A. Zhironkina, Kireev, II, Lamin A as a determinant of mechanical properties of the cell nucleus in health and disease. Biochemistry (Mosc) 86, 1288–1300 (2021)
G. Lee, S.B. Han, D.H. Kim, Cell-ECM contact-guided intracellular polarization is mediated via lamin A/C dependent nucleus-cytoskeletal connection. Biomaterials 268, 120548 (2021)
J. Lammerding, P.C. Schulze, T. Takahashi, S. Kozlov, T. Sullivan, R.D. Kamm, C.L. Stewart, R.T. Lee, Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113, 370–378 (2004)
F. Roncato, O. Regev, S.W. Feigelson, S.K. Yadav, L. Kaczmarczyk, N. Levi, D. Drago-Garcia, S. Ovadia, M. Kizner, Y. Addadi, J.C. Sabino, Y. Ovadya, S.F. de Almeida, E. Feldmesser, G. Gerlitz, R. Alon, Reduced Lamin A/C does not facilitate cancer cell transendothelial migration but compromises lung metastasis. Cancers (Basel) 13, 2383 (2021)
G.E. Morris, K.N. Randles, Nesprin isoforms: are they inside or outside the nucleus? Biochem. Soc. Trans. 38, 278–280 (2010)
D. Rajgor, C.M. Shanahan, Nesprins: from the nuclear envelope and beyond. Expert Rev. Mol. Med. 15, e5 (2013)
E. Antmen, U. Demirci, V. Hasirci, Micropatterned surfaces expose the coupling between actin cytoskeleton-Lamin/Nesprin and nuclear deformability of breast cancer cells with different malignancies. Adv. Biol. (Weinh) 5, e2000048 (2021)
A. Matsumoto, M. Hieda, Y. Yokoyama, Y. Nishioka, K. Yoshidome, M. Tsujimoto, N. Matsuura, Global loss of a nuclear lamina component, lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med. 4, 1547–1557 (2015)
I. Dupin, S. Etienne-Manneville, Nuclear positioning: mechanisms and functions. Int. J. Biochem. Cell Biol. 43, 1698–1707 (2011)
M. Almonacid, M.E. Terret, M.H. Verlhac, Nuclear positioning as an integrator of cell fate. Curr. Opin. Cell Biol. 56, 122–129 (2019)
C. Bruno, Nuclear positioning: a matter of life. Semin. Cell Dev. Biol. 82, 1–2 (2018)
D.G. Thomas, A. Yenepalli, C.M. Denais, A. Rape, J.R. Beach, Y.L. Wang, W.P. Schiemann, H. Baskaran, J. Lammerding, T.T. Egelhoff, Non-muscle myosin IIB is critical for nuclear translocation during 3D invasion. J. Cell Biol. 210, 583–594 (2015)
B. Cadot, V. Gache, E.R. Gomes, Moving and positioning the nucleus in skeletal muscle - one step at a time. Nucleus 6, 373–381 (2015)
B. Burke, Chain reaction: LINC complexes and nuclear positioning. F1000Res 8, 136 (2019)
A. Jayo, M. Malboubi, S. Antoku, W. Chang, E. Ortiz-Zapater, C. Groen, K. Pfisterer, T. Tootle, G. Charras, G.G. Gundersen, M. Parsons, Fascin regulates nuclear movement and deformation in migrating cells. Dev. Cell 38, 371–383 (2016)
K. Pfisterer, A. Jayo, M. Parsons, Control of nuclear organization by F-actin binding proteins. Nucleus 8, 126–133 (2017)
P.M. Davidson, A. Battistella, T. Dejardin, T. Betz, J. Plastino, N. Borghi, B. Cadot, C. Sykes, Nesprin-2 accumulates at the front of the nucleus during confined cell migration. EMBO Rep. 21, e49910 (2020)
R. Zhu, C. Liu, G.G. Gundersen, Nuclear positioning in migrating fibroblasts. Semin. Cell Dev. Biol. 82, 41–50 (2018)
T.J. Chancellor, J. Lee, C.K. Thodeti, T. Lele, Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 99, 115–123 (2010)
T. Lammermann, B.L. Bader, S.J. Monkley, T. Worbs, R. Wedlich-Soldner, K. Hirsch, M. Keller, R. Forster, D.R. Critchley, R. Fassler, M. Sixt, Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008)
J.K. Kim, A. Louhghalam, G. Lee, B.W. Schafer, D. Wirtz, D.H. Kim, Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 8, 2123 (2017)
S.J. Heo, K.H. Song, S. Thakur, L.M. Miller, X. Cao, A.P. Peredo, B.N. Seiber, F. Qu, T.P. Driscoll, V.B. Shenoy, M. Lakadamyali, J.A. Burdick, R.L. Mauck, Nuclear softening expedites interstitial cell migration in fibrous networks and dense connective tissues. Sci. Adv. 6, eaax5083 (2020)
T. Fischer, A. Hayn, C.T. Mierke, Effect of nuclear stiffness on cell mechanics and migration of human breast cancer cells. Front. Cell Dev. Biol. 8, 393 (2020)
T. Harada, J. Swift, J. Irianto, J.W. Shin, K.R. Spinler, A. Athirasala, R. Diegmiller, P.C. Dingal, I.L. Ivanovska, D.E. Discher, Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J. Cell Biol. 204, 669–682 (2014)
A. Das, A. Barai, M. Monteiro, S. Kumar, S. Sen, Nuclear softening is essential for protease-independent migration. Matrix Biol. 82, 4–19 (2019)
Acknowledgements
This work is supported, in part or in whole, by the National Natural Science Foundation of China (32071304, U19A2006, 12132004, 11972111, 32171309, 12272086), the China Postdoctoral Science Foundation (2019T120821), the Sichuan Science and Technology Program (2023NSFSC1233, 2022NSFSC0048, 2022NSFSC0686, 2023YFSY0038), and the Joint Funds of Center for Engineering Medicine (ZYGX2021YGLH017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no potential conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, X., Chen, K., Wang, M. et al. Nesprin-1/2 facilitates breast cancer cell pore migration via nucleus deformation. Eur. Phys. J. Spec. Top. 232, 2739–2749 (2023). https://doi.org/10.1140/epjs/s11734-023-00930-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjs/s11734-023-00930-5