Abstract
Latex paints are widely used, and many researchers pointed out that the film formation process depends on the deformability of dispersed polymer particles. However, the relationship between the film formation process and drying rate has not been totally understood due to the lack of accurate data on drying rate throughout the drying process. In the present study, we measured the drying rate of latex coating by the temperature change method proposed by Imakoma in convective drying. We revealed that the drying process significantly depends on particle deformability, especially in the former stage of the falling drying rate period. At a low drying temperature, the close-packed structure of polymer particles is formed throughout the film at the end of the constant drying rate period. On the other hand, partially deformed soft particles due to wet sintering inhibit the drying rate even under high moisture content at high drying temperatures. In either case, after forming the closest-packed structure, the shrinkage of the gap space between particles due to capillary deformation decreases the drying rate, proportional to the moisture content.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coating films obtained by drying the coating layer of paint over the material have long been used widely, such as in buildings, automobiles, and ships. The paint is categorized into solvent and aqueous types. The choice and combination of solvents enable producing any polymer solution, and its mixture with various functional particulate materials has good usability. Therefore, solvent-based paints have been popular rather than water-based paints. However, due to the environmental impact of solvents, paint systems gradually shift to high-solids or aqueous systems these days.
Latex paints are aqueous dispersions of nano-sized polymer particles and attract attention to reduce organic solvent usage compared to solvent-based paints. In the drying process of the coating layer of latex paint, polymer nanoparticles form the packing layer and deform to produce a homogeneous, nonporous film [1,2,3,4,5]. In recent years, many concepts have been reported to improve the mechanical properties of latex coating films. for example, designing a core-shell structure, where polymer nanoparticles are covered by the other polymer having different \(T_{\mathrm {g}}\) [6, 7] or using cross-linked polymer nanoparticles [8, 9]. Furthermore, it is reported that the addition of polymer nanoparticles to dispersions of solid particles can inhibit crack formation [10, 11].
A thorough understanding of the film formation behaviors of latex coatings during drying is indispensable in designing novel and environmentally friendly paints. Latex paints have been studied for more than half a century, and the drying process has been proposed by many researchers, for example, Vanderhoff [1] and Croll [2]. In general, the drying process of latex coating is qualitatively described in three stages (Fig. 1). During the first stage, polymer nanoparticles are dispersed homogeneously in the coating layer, and water evaporates from the latex at the same rate as pure water under the same drying condition (Stage 1). Subsequently, as the evaporation of water progresses, nanoparticles form the packing layer at the drying surface of the coating layer (Stage 2). Finally, after the entire coating layer becomes a packing layer of polymer nanoparticles, the particles deform and coalesce to form a uniform polymer layer (Stage 3). David et al. applied the Cryo-SEM technique to the film formation process of latex paints and revealed that polymer nanoparticles firstly form hexagonal packing structure, change to the honeycomb structure, and eventually fuse each other to form uniform film [12]. Carter et al. used a nuclear magnetic resonance profiling technique (GARField) to measure the vertical distribution of the moisture content in the latex coating layer during drying and clarified the formation of the condensed layer at the top [13].
In the case of drying the coating layer of particle dispersion, Péclet number, Pe is usually used to explain the vertical distribution of particles [14,15,16,17,18]. Péclet number is defined as the ratio of the evaporation rate to particle diffusion rate. The diffusion coefficient of a particle in a liquid with a viscosity \(\eta \) is given by the Stokes-Einstein equation as
where d is the diameter of polymer nanoparticle, k is the Boltzmann constant, and T is the temperature. Then, the Péclet number is defined by Eq. (2).
E is evaporation rate, and H is initial film thickness. When the evaporation rate is high and \(\mathrm Pe\) is much larger than unity, non-sedimentary particles accumulate at the drying surface of the coating layer. In the case of latex paint drying, the Péclet number is usually much larger than unity due to the nanometer order in particle size, and polymer nanoparticles are mainly accumulated beneath the drying surface.
In the drying process of latex coating, Routh and Russel studied the deformation of polymer nanoparticles [19, 20]. They defined a dimensionless parameter \({\bar{\lambda }}\) being the ratio of the evaporation rate to deformation rate to classify the deformation mechanism. The characteristic time for a polymer particle having a diameter d deformed by viscous flow is proportional to its zero-shear rate viscosity, \(\eta _{0}\), which resists deformation and is inversely related to the relevant interfacial tension, \(\gamma _{\mathrm {wa}}\), which drives deformation [21]. The characteristic time for drying is calculated by dividing the initial thickness of a latex coating layer H by the initial decreasing rate of coating layer thickness E. Various equations describing the temperature dependency of polymeric viscosity have been proposed, such as the WLF equation [22]. Taking the glass transition temperature of a polymer as a reference temperature in the WLF equation, the viscosity of various polymers becomes the unique function of the ambient temperature and decreases by several orders of magnitude as the temperature increases. \({\bar{\lambda }}\) is defined by the following equation.
Furthermore, Routh and Russel proposed a map that classifies deformation modes using \({\bar{\lambda }}\) and Péclet number, \(\mathrm{Pe}\). In the case of \({\bar{\lambda }}\) smaller than unity, where the particle deformation rate is large relative to the evaporation rate, particles deform by wet sintering. Conversely, if the rate of particle deformation is much lower than that of evaporation and \({\bar{\lambda }}\) is on the order of \(10^{4}\) or more, particles deformation dominated by dry sintering progresses after water evaporation. In the middle \({\bar{\lambda }}\) ranging from \(10^{0}\) to \(10^{2}\), capillary force causes deformation. The validity of this classification was confirmed by using the GARField technique [13, 23, 24].
The scenario is valid when the drying rate is sufficiently low and non-sedimentary polymer particles are distributed homogeneously in the coating layer throughout the drying experiment. Practically, however, the drying process of the coating layer requires to complete in a short time, and then convective drying using hot air is frequently adopted. As drying temperature or drying rate increases, dispersed particles are accumulated at the drying surface due to the relatively small effect of Brownian diffusive motion. Although the Péclet number becomes much larger than unity under the drying conditions, the deformation map proposed by Russel and Routh is limited around Pe \(\sim \) 1. Most of the reports on the drying rate of latex coating are conducted at room temperature or small Pe [25,26,27]. Under a significantly large Pe, however, how polymer particles deform to produce a homogeneous film has not been thoroughly studied.
Since particle deformation occurs after forming the particle packing layer locally or overall, the accurate measurement of drying rate is required even at the latter part of drying or in the falling drying period. The drying rate of latex coating at high temperature under an infrared drying process was measured by the mass change [28]. However, accurate measurement seems to be conducted only in the former part of drying. Although the mass change method is the most common for measuring the drying rate, the instability of the balance and the disturbances caused by airflow deteriorate the measurement accuracy. Therefore, this method is unsuitable for measuring a small drying rate in convective drying because of a small mass decrease at the end of drying. In contrast, a temperature change method can measure the drying rate even at the end of drying when hot air flows over the sample [29, 30]. In this method, the drying rate is calculated from the temperature change over time in the coating layer and energy balance. In the falling drying rate period, the temperature of the coating layer significantly changes with drying. Therefore, a higher level of measurement accuracy can be expected compared to the conventional mass change method. In this study, the drying rate is measured using the temperature change method and investigates the effect of drying temperature on polymer nanoparticle packing and deformation processes. The simple models to explain the experimental results will be proposed.
2 Experiment
2.1 Sample
An aqueous dispersion of acrylic latex (50 wt.%, specific density 1.17, glass transition temperature, \(T_{\mathrm {g}}=288\, \mathrm {K}\), particle diameter, \(d=220\, \mathrm {nm}\), Japan Coating Resin Corp.) was coated on a copper substrate by a blade coating technique. The cupper substrate has a width of 1.5 cm and a length of 10 cm, and the length and initial thickness of the coating layer are 7.5 cm and \(750 \,\upmu \hbox {m}\), respectively, for all experiments in the present study. The actual value of the initial layer thickness was calculated from the initial volume fraction of water, and the mass difference of the latex-coated copper substrate before and after drying, which is the amount of water evaporated in the drying experiment.
2.2 Apparatus and method for measuring drying rate
Although the drying rate is generally measured by the mass change of a coated substrate using an electric balance, it is not easy to apply it in convective drying because of the airflow. Furthermore, the film formation of latex paint is mainly performed in the latter stage of drying, and the drying rate at this stage is too small to detect by the electric balance. Instead, Imakoma et al. proposed the method to measure the drying rate of the coating layer based on the temperature change [29, 30]. The temperature of the coating layer is stable in a former constant drying rate period, where the heat flow from the surrounding is balanced with the latent heat of the evaporation of water. In the latter falling drying rate period, a higher temperature is required to compensate for reducing the mass flux of water through a semi-dried coating layer. As a result, the heat flow from the surroundings is partially used to increase the coating layer temperature, and then the drying rate decreases from that in the constant drying rate period. As drying progresses, the mass transfer resistance of the semi-dried coating layer increases, and the drying rate decreases further. Therefore, the drying rate can be estimated from the temperature change of the coating layer and the energy balance equation. The detail to estimate the drying rate will be explained in Sect. 2.3.
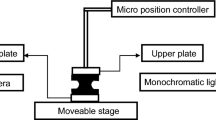
The experimental apparatus for measuring the drying rate is shown in Fig. 2. The latex-coated copper substrate is placed in a drying chamber, the top and both sides of which are surrounded by double insulating walls. One of the open sides of the drying chamber is connected to a hot air generator (HAS-11, Kansai Electric Heat Corp.) using an aluminum duct covered with thermal insulation. The temperature, relative humidity, and flow rate of the hot air in the drying chamber are measured by an anemometer (CLIMOMASTER 6501-00, KANOMAX JAPAN INC.). The hot air temperature in the chamber was controlled at 313–353 K. The relative humidity and flow rate were roughly constant at 50% RH and 1.8 m/s, respectively.
The sample is placed so that the surface was flush with the bottom of the drying chamber not to disturb the airflow. We hollowed out a part of the bottom plate to place the sample and inserted insulation into the space below the sample to neglect the heat flow from the bottom. Furthermore, the sample is placed perpendicular to the airflow direction to ensure uniform drying throughout the coating layer. The uncoated part of the substrate is fixed to a sample holder, and the surface temperature of the coating layer is measured by a radiation thermometer (CTL-CF1-C3 MICRO-EPSILON) installed at the top wall of the drying chamber. Due to rapid drying at the edge area, the drying rate is not entirely uniform over the coating layer. In the present study, to minimize the edge effect on the drying rate measurement, the thermometer is fixed so that the measuring area, which is the circle having a diameter of 0.5 cm, is located at the middle of the coating layer, as shown in Fig. 2c. The thermometer is connected to a PC to measure the temperature continuously at the time interval of 0.05 s. By averaging the measured temperature every 2.5 s, the deviation of the measurement could suppress to the order of 0.1 K, which is less than 1/10 of the raw data.
2.3 Temperature change method
The drying process of thin layers is usually categorized into three types: conduction, radiation, and convection styles [31]. The most efficient and hence most popular drying method is based on convective drying. In this method, a heated carrier gas is forced to pass over the wet thin layer. The convective method can simultaneously increase the coefficients of heat transfer and mass transfer. Estimating the boundary heat transfer coefficient is inevitable in the temperature change method to solve the energy balance equation.
In the drying process of a thin layer coated over a substrate, the heat to evaporate water enters from the top and bottom, but water usually evaporates only from the top. When the bottom of the coated substrate is insulated, the areas for heat transfer and evaporation of water are the same. For thin layers with high effective thermal conductivity, the temperature of the coating layer is assumed to be uniform in the drying direction and identical with that of the substrate. Since the heat flow from the outside heats the coating layer and substrate or evaporates water, the energy balance equation during convective drying can be expressed by Eq. (4)
where, \(C_{\mathrm {W}}\), \(C_{\mathrm {P}}\), and \(C_{\mathrm {S}}\) are the specific heat capacities of water, dry polymer (acrylic resin), and substrate, respectively. \(W_{\mathrm {W}}\), \(W_{\mathrm {P}}\), and \(W_{\mathrm {S}}\) are the mass of water, dry polymer, and substrate, respectively. t is the drying time, and A is the evaporation area. \(T_{\mathrm {a}}\) is the hot air temperature, \(T_{\mathrm {m}}\) is the temperature of the coating layer or substrate, which is measured by the radiation thermometer in the present study and referred to as the sample temperature hereafter. h is the boundary heat transfer coefficient at the surface of the coating layer. \(L_{\mathrm {W}}\) is the latent heat of vaporization of water at \(T_{\mathrm {m}}\). The latent heat of vaporization at any temperature was calculated using a reference temperature, the constant pressure specific heats of vapor and water, and the temperature difference. They were correlated as a linear function of \(T_{\mathrm {m}}\): \(L_{\mathrm {W}} = 3.177 \times {10}^{6} -2.47 \times {10}^{3}\times T_{\mathrm {m}}\) [J/kg] [29].
Assumed that the boundary heat transfer coefficient h is constant during drying, an instantaneous mass change of water, \({\mathrm {\Delta }W}_{\mathrm {W}}\), within a time interval, \(\mathrm {\Delta }t\), is expressed by Eq. (5), which is obtained from the discretized form of Eq. (4). \(X_{\mathrm {W}}\) is the mean moisture content relative to the mass of the dry polymer, \(X_{\mathrm {W}} = W_{\mathrm {W}}/W_{\mathrm {P}}\), and calculated by Eq. (6) with the initial moisture content, \(W_{\mathrm {W}}^{0}\), which is calculated from the mass difference of the coting latex layer before and after drying. The time derivation of the moisture content is used to calculate an instantaneous drying rate \(R_{\mathrm {W}}\) as shown in Eq.(7).
If the boundary heat coefficient is available, the change of the moisture content of the coating layer and the drying rate can be calculated simply by measuring the temperatures of the coating layer and hot air during the drying. Integrating Eq. (4) over the entire drying process gives the boundary heat coefficient in Eq. (8).
where, the superscription of 0 and F denote the initial and final values, respectively. Note that this equation ignores the sensible heat required for the evaporated steam to rise from the evaporation temperature to the hot air temperature. Due to a large flow rate, the evaporated steam is quickly expelled from the drying chamber, and the heat supplied is much larger than the sensible heat.
3 Results & discussion
3.1 Drying rate measurement
We performed the drying experiment of latex coating layers at different drying temperatures, \(T_{\mathrm {a}}\). Figure 3 shows the sample temperature change in the entire drying experiments conducted at different hot air temperatures. The sample temperature rises immediately after drying and takes a steady value for a specific duration, corresponding to a constant drying period. We decide \(T_{\mathrm {c}}\) at the point where the slope of the temperature change becomes zero. At high drying temperatures, \(T_{C}\) is the temperature at which the slope takes the minimum value. The following increasing trend means the transition to a falling drying rate period. The sample temperature eventually reaches the hot air temperature, indicating the end of drying.
Figure 4 shows the change of the moisture content of the coating layer calculated by the temperature change method using the results shown in Fig. 3. Since the initial weight fraction of water and polymer is 1:1, the initial moisture content is unity. Under steady sample temperature, the decreasing slope of the moisture content is constant, which is the initial evaporation rate, \(E_{0}\). At the lowest drying temperature, the constant drying rate period lasts for more than \(10^{3}\) s. After the constant evaporation period, the decreasing rate of moisture content slows to zero. The initial layer thickness, \(H_{0}\), is calculated from the mass of evaporated water, suggesting that the coating thickness is roughly constant for all experiments. These parameters are used to calculate the dimensionless numbers, Pe and \({\bar{\lambda }}\). In the calculation, we used \(T_{a}\) as the sample temperature and estimated the physical properties of resin particles at the temperature. Table 1 summarizes the experimental parameters. Except for the very early stage, where the coating layer temperature is raised to \(T_{\mathrm {c}}\), it is found that the coating layer temperature is largely higher than the glass transition temperature of the polymer (288 K). Therefore, dispersed polymer particles are finally fused to form uniform films at the end of drying under all experimental conditions.
Note that the boundary heat coefficient h is determined to satisfy the energy balance equation in the temperature change method and should be the same under the stable airflow around the coating sample in the sufficiently insulated drying chamber. Table 1 suggests that all experiments are conducted under the stable convective drying condition.
3.2 Change of drying rate at different drying temperatures
The transition from a constant drying rate period to a falling drying rate period is usually caused by changing the governing process from gas-liquid phase equilibrium to mass transfer through the coating layer. In other words, the change of drying rate must relate to the internal structural change in the coating layer. Figure 5 shows the drying rate curve, which is the relationship between the drying rate and the moisture content for different drying temperatures. The drying rate is constant at any temperature until the moisture content becomes smaller than the critical value, \(X_{\mathrm{WC}}\), and the drying rate decreases after that. The moisture content corresponding to the volume fraction of polymer in the latex coating was calculated by Eq. (9) using the densities of water and polymer. In general, when solid spheres fill a container, the solid volume fraction ranges from 55 vol.% (random loose packing with zero gravity) to 64 vol.% (random close packing) [32]. More dense particle packing is known as 68 vol.% (face-centered structure) or 74 vol.% (body-centered structure or maximum close packing) in the crystal structure [33, 34]. These volume fractions correspond to the moisture contents of 0.7, 0.48, 0.40, and 0.30, respectively, unless air enters the packing layer. At the lowest drying temperature of \(T_{\mathrm {a}} = 313 \hbox {K}\), the critical moisture content, \(X_{\mathrm{WC}}\) \(\sim \) 0.4, indicates that the acrylic latex particles form a closest-packed structure at the end of the constant drying rate period. Niga et al. measured the drying rate of the latex coating layer by the thickness change [35] and reported that the particle volume fraction of well-dispersed latex paint attains 70 vol.% at the end of the constant drying rate period, which agrees well with the current result. At higher drying temperatures of \(T_{\mathrm {a}} = 333\) and 353 K, the critical moisture content was larger than 0.5, suggesting that polymer particles cannot form close-packed structures at the onset of the falling drying rate period. The decrease in drying rate is usually explained by the increase in mass transfer resistance due to insufficient residual water. However, the semi-dried coating layer at the moment still contains a considerable amount of water. We will discuss this discrepancy in the last section.
At the lowest drying temperature, the drying rate decreases linearly to the moisture content at \(X_{\mathrm{W}}<X_{\mathrm{WC}}\). This regime corresponds to the falling drying rate period, and the decreasing behavior is changed in this regime at higher drying temperatures. In other words, the decreasing slope of the drying rate gradually decreases in the first stage (concave upward curve) but becomes constant in the second stage as in the low drying temperature case. Furthermore, it is revealed that the decreasing behaviors of drying rate in the second stage at higher temperatures coincide with that at the low temperature. At the transition from the first to the second stage at the higher temperatures, the moisture content, \(X_{\mathrm{WT}}\), is approximately 0.3, indicating that polymer nanoparticles form the closest-packed structure. The constant slope in the relationship between the drying rate and moisture content means that the decreasing rate of moisture content has a linear relationship with the current moisture content.
We considered that the residual water in the gap space of the closest-packed layer of polymer particles forms the network structure at the transition moisture content. The rearrangement of the packing structure rarely occurs, and polymer particles deform due to capillary force so that water always fills the gap space. Therefore, it is assumed that the network structure is equivalent to a bunch of tubes filled with water penetrating from the bottom to the surface, and the inner radius, a, and length, l, of each tube will decrease as water evaporates without changing the number of tubes, n. Therefore, the moisture content can be expressed by the following equations.
In the last stage of the drying process of the latex coating layer, the film thickness is almost the same as the final value, but the pore size eventually attains zero. Therefore, we consider that the cross-sectional area of the water tube is proportional to the moisture content. On the other hand, the evaporation rate of water is the product of the effective drying surface area, the product of the cross-sectional area and number of the water tubes, and the mass transfer flux. Therefore, since the drying rate is proportional to the cross-sectional area of the tube, a proportional relationship between the drying rate and moisture content was observed in the second falling drying rate period.
3.3 Categorization of particle packing process in terms of \({\bar{\lambda }}\)
To clarify the mechanism of how hot air temperature affects drying behavior in the constant and the first falling drying rate periods in terms of the internal structure of latex coating layer, two dimensionless numbers, Péclet number, Pe and \({\bar{\lambda }}\) are calculated. Péclet number is the ratio of layer shrinking rate to diffusion rate of polymer particle. The dispersed particles are accumulated beneath the drying surface in the case of particularly large Pe. On the other hand, \({\bar{\lambda }}\) classifies the deformation mechanism of polymer particles. We calculated the Péclet number and \({\bar{\lambda }}\) for each drying condition as summarized in Table 2. Although the sample temperature is not constant through the drying experiment, the zero-shear viscosity of acrylic resin at the temperature of the constant drying rate period, \(T_{\mathrm {C}}\), was calculated using the WLF equation expressed by Eq.(11) [22]. The glass transition temperature, \(T_{\mathrm {g}}\) is a reference temperature and \(\eta _{\mathrm {s}}\) is a prefactor for the temperature.
At all drying conditions, the Péclet number is on the order of \(10^{2}\), indicating that polymer particles accumulate near the drying interface during drying to form a packed layer. At the drying temperature of \(T_{\mathrm {a}} = 313 \,\hbox {K}\), \({\bar{\lambda }}>1\) indicates that polymer particles show strong resistance against shear deformation due to their high viscosity. The deformation of polymer particles is dominated by the capillary force arising in the gap between close-packed particles, referred to as capillary deformation. On the other hand, the polymer viscosity is significantly reduced and \({\bar{\lambda }}\) become much smaller than unity at \(T_{\mathrm {a}} = 333\) or 353 K. Therefore, even when water fills the gap between particles, the deformation of polymer particles progresses by the interfacial tension at the polymer-water interface, known as wet sintering. At the drying temperature of 323 K, the intermediate packing and deformation process will occur because of \({\bar{\lambda }}\sim 1\). In the following section, the differences in the transition from the constant to falling drying rate period and the decreasing behavior of drying rate in the falling drying rate period will be discussed, taking into account the particle deformation mechanisms.
3.4 Effect of particle deformation on the formation of packing structure
Although the drying rate in the constant drying rate period differed with drying conditions, the drying rate curves coincide below the critical moisture content of \(X_{\mathrm{WT}}\) (\(\sim \) 0.3). To highlight the difference in the decreasing trends of drying rate for each drying temperature, the drying rates normalized by that in the constant drying rate period, \(R_{\mathrm{W0}}\), as shown in Fig. 6. It is confirmed again from this figure that the drying rate has a linear relationship with the moisture content at \(X_{\mathrm{W}}<\) 0.3 regardless of drying temperatures. Additionally, at the lowest drying temperature, the critical moisture content is in the range of 0.3 \(< X_{\mathrm{W}}<\) 0.5, and the drying rate decreases after the structure of the coating layer forms random close packing. The drying rate after the critical moisture content has a linear relationship with the moisture content. In other words, at the low drying temperature, the drying period is divided into two periods, the constant drying rate period and the falling drying rate period.
It is thus presumed that the different trend of drying rate in the first stage reflects the difference in particle packing processor \({\bar{\lambda }}\). At 323 K, no clear transition from the first to the second falling drying rate period was observed, probably due to the intermediate drying condition.
The drying rate curve fitted by the proposed models and the schematic diagram of the changes in packing structure with the progress of drying (\(T_{\mathrm {a}}= 313 \,\hbox {K}\)). a: Accumulation of polymer particles beneath the drying surface. b: Formation of the close-packed layer. Partial deformation of particles due to capillary force decreases drying rate. c: Transition to a honeycomb structure due to capillary deformation. Polymer particles eventually coalesce with each other to form a uniform film
3.4.1 Particle packing with capillary deformation
Figure 7 shows the normalized drying rate curve and the schematic illustration of the change of particle packing structure during drying at 313 K, where capillary deformation dominates particle deformation. The drying rate curve indicates the transition from a constant drying rate period (red line) to a falling drying rate period (green line) with the critical moisture content \(X_{WC}\) of approximately 0.4. The criteria will depend on the deformability of polymer particles. For example, rigid spheres having a large \(T_{\mathrm {g}}\) should be packed tightly and show a smaller critical moisture content.
In the early drying stage, the particle-packed layer developed from the surface to the bottom due to the large Peclet number, keeping the drying rate constant(Fig. 7a). It turns out that the air-water equilibrium governs the drying rate, and the particle packing does not noticeably suppress the transport of water. However, the drying rate started decreasing at a moisture content of approximately 0.6, which is much larger than \(X_{\mathrm{WC}}\). Since the moisture content of 0.7 and 0.48 correspond to a random loose packing and a random close packing, respectably, as mentioned in Sect. 3.2, it was revealed that polymer particles produce the random packing structure at the onset of the falling drying rate period.
After forming the close-packed structure at the critical moisture content, a meniscus existing on the surface of the coating layer induces capillary pressure, which deforms polymer particles. We proposed the water tube model to explain the shrinking process of the residual water with particle deformation, as described in Sect. 3.2. Equation (10) expressing the relationship between the drying rate and moisture content must be valid at the moisture content below \(X_{\mathrm{WC}}\) (\(\sim \) 0.4) illustrated in Fig. 7b. However, the actual drying rate was smaller than the model in the transition regime ranging \(X_{\mathrm{W}}\) from 0.6 to 0.3, which may correspond to the gradual increase in the fraction of polymer particles to be deformed. Therefore, as the drying progresses, the drying rate approaches the model, and they coincide at the moisture content of the closest-packing structure (Fig. 7c).
3.4.2 Particle packing with wet sintering
In the last stage of the drying process, the shrinkage of the closest packing structure causes the linear relationship between the drying rate and moisture content regardless of \({\bar{\lambda }}\). For \({\bar{\lambda }}>>\) 1, hard polymer particles produce a packed layer downward, and the transition from random packing to the closest packing occurs during the first falling drying rate period. This section describes how soft polymer particles form the closest packing structure for \({\bar{\lambda }} <<\) 1 with the consideration of drying rate change in the first falling drying rate period.
The drying rate curve fitted by the proposed models and the schematic diagram of the changes in packing structure with the progress of drying (\(T_{\mathrm {a}}= 353\, \hbox {K}\)). a: Accumulation of polymer particles beneath the drying surface as the water evaporates. b: Deformation of polymer particles in the accumulated layer due to wet sintering. The decrease of evaporation area decreases the drying rate. c: Formation of the closest-packed structure of deformed particles. The pores between the particles filled with water shrink. d: Transition to a honeycomb structure due to capillary deformation
As already explained, polymer particles produce the packed layer beneath the drying interface. Polymer particles can deform by the interfacial tension between water and polymer because of sufficiently low viscosity. However, once the polymer particles completely fuse each other, the particle-packed layer changes to a uniform film without pores, resulting in a drastic reduction in drying rate. Since the drying rate decreases gradually and eventually coincides with that at lower drying temperatures, it is considered that slightly deformed polymer particles form a closest-packed layer, and the packed layers accumulate continuously, producing a uniformly packed structure. When polymer particles generate a single layer of the closest packing structure beneath the drying interface, the effective drying surface area considerably decreases. In the second falling drying rate period or the first falling drying rate period of hard polymer nanoparticles, particle packing structure is formed throughout the semi-dried coating layer. In this case, however, the particle packing layer is too thin to decrease the drying rate, which is still dominated by air-water equilibrium. Therefore, the constant drying rate period exists despite particle accumulation at the top. As increasing the number of layers, the resistance to mass transfer flux gradually increases and eventually dominates the drying rate. This is the onset of the falling drying rate period. Therefore, the mass flux passing through n-layer packed structure is reduced by a factor of n compared to the monolayer case. On the other hand, a constant volume of water evaporates during forming a single packed layer. The decrease of the moisture content, \(X_{\mathrm {W0}}\mathrm {-}X_{\mathrm {W}}\), is proportional to the number of packed layers. Consequently, the proposed model means that the drying rate has an inversely proportional relationship with the moisture content decrease \(X_{\mathrm {W0}}\mathrm {-}X_{\mathrm {W}}\) in this stage. Like Figs. 7, 8 plots the normalized drying rate curve at \(T_{a}= 353 \,\hbox {K}\). The model proposed in this study can reasonably explain the actual drying rate change, as represented by the pink and green dashed curves.
4 Conclusion
Many publications pointed out that the deformation of polymer nanoparticles is indispensable for producing a homogeneous film from latex paint. However, although polymer particles are more deformable at high drying temperatures, the relationship between particle deformability and drying rate has not been well studied under practical drying conditions, such as convective drying. One of the reasons is the difficulty of accurately measuring the slow drying rate with air flowing around the sample. In the present study, applying the temperature change method, we measure drying rate accurately even in the falling drying rate period and investigated the effect of drying temperature on the drying rate and deformation process of particle packed structure.
In all experimental conditions, due to large Péclet numbers, the accumulation of polymer particles beneath the drying interface progressed in the constant drying rate period. However, “capillary deformation” or “wet sintering” induces particle deformation in the accumulated layer depending on the drying temperature, and the different packing behavior significantly affects the decreasing trend of drying rate in the falling drying rate period. The random packing structure of polymer particles is formed throughout the sample at the end of the constant drying period at a low drying temperature and then changes to the closest packing structure by capillary force accompanied by the decrease in drying rate. In contrast, the closest-packed layers of deformable polymer particles are developed from the drying surface to the bottom at high drying temperatures. As progressing particle accumulation, the increase in the resistance of water transport decreases the drying rate. In addition, we proposed a model where the moisture content decrease has an inverse proportional relationship with the drying rate. After forming the closest packed structure, the drying rate decreased linearly to the moisture content regardless of drying temperatures. Assuming that the effective drying area is proportional to the moisture content and the capillary force dominates the water transport to the drying surface, we could explain the linear relationship between drying rate and moisture content.
The result revealed that the film formation process of latex paint is significantly different depending on the deformability of polymer particles, especially in the former part of falling drying rate periods. We provided insights for a deeper understanding of the film formation process in convective drying and proposed the mechanism explaining different drying rate changes. At higher drying temperatures, note that more deformable polymer particles may fuse and form a skin layer. This process is out of the scope of the present study, but the temperature change method is a promising technique to clarify the detail of this process.
References
J.W. Vanderhoff, E.B. Bradford, W.K. Carrington, The transport of water through latex films. J. Polym. Sci. C Polym. Symp. 41(1), 155–174 (1973)
S.G. Croll, Drying of latex paint. J. Coat. Technol. 58(734), 155–174 (1986)
J. Keddie, Film formation of latex. Mater. Sci. Eng. R Rep. 21, 101–170 (1997)
M.A. Winnik, Latex film formation. Curr. Opin. Colloid Interface Sci. 2(2), 192–199 (1997)
J. Keddie, A.F. Routh, Fundamentals of Latex Film Formation: Processes and Principles (Springer, New York, 2010)
K. Price, W. Wu, K. Wood, S. Kong, A. McCormick, L. Francis, Stress development and film formation in multiphase composite latexes. J. Coat. Technol. Res. 11, 827–839 (2014)
A. Cobaj, H.S. Mehr, Y. Hu, M.D. Soucek, The influence of a non-isocyanate urethane monomer in the film formation and mechanical properties of homogeneous and core-shell latexes. Polymer 214, 123253 (2021)
H. Wahdat, M. Gerst, M. Rückel, S. Möbius, J. Adams, Influence of delayed, ionic polymer cross-linking on film formation kinetics of waterborne adhesives. Macromolecules 52, 271–280 (2019)
H. Parsimehr, S. Pazokifard, Ambient temperature cross-linkable acrylic latexes: effect of cross-link density, glass transition temperature and application temperature difference on mechanical properties. Polym. Bull. (2021)
C.C. Roberts, L.F. Francis, Drying and cracking of soft latex coatings. J. Coat. Technol. Res. 10(4), 441–451 (2013)
S. Lim, S. Kim, K.H. Ahn, S.J. Lee, The effect of binders on the rheological properties and the microstructure formation of lithium-ion battery anode slurries. J. Power Sources 299(20), 221–230 (2015)
Y. Ma, H.T. David, L.E. Scriven, Microstructure development in drying latex coatings. Prog. Org. Coat. 52, 46–62 (2005)
F.T. Carter, Radoslaw M. Kowalczyk, I. Millichamp, M. Chainey, J.L. Keddie, Correlating particle deformation with water concentration profiles during latex film formation reasons that softer latex films longer to dry. Langmuir 30, 9672–9681 (2014)
J.P. Gorce, D. Bovey, P.J. McDonald, P. Palasz, D. Taylor, J.L. Keddie, Vertical water distribution during the drying of polymer films cast from aqueous emulsions. Eur. Phys. J. E 8, 421–429 (2002)
P. Ekanayake, P.J. McDonald, J.L. Keddie, An experimental test of the scaling prediction for the spatial distribution of water during the drying of colloidal films. Eur. Phys. J. Spec. Top. 166, 21–27 (2009)
C.M. Cardinal, Y.D. Jung, K.H. Ahn, L.F. Francis, Drying regime maps for particulate coatings. AIChE J. 56, 2769–2780 (2010)
A.F. Routh, W.B. Zimmerman, Distribution of particles during solvent evaporation from films. Chem. Eng. Sci. 59, 2961–2968 (2004)
R.E. Trueman, Domingues E. Lago, S.N. Emmett, M.W. Murray, J.L. Keddie, A.F. Routh, Autostratification in drying colloidal dispersions: experimental investigations. Langmuir 28, 3420–3428 (2012)
A.F. Routh, W.B. Russel, A process model for latex film formation: limiting regimes for individual driving force. Langmuir 15(22), 7762–7773 (1999)
A.F. Routh, W.B. Russel, Deformation mechanisms during latex film formation: experimental evidence. Ind. Eng. Chem. Res. 40(20), 4302–4308 (2001)
R.E. Dillon, L.A. Matheson, E.B. Bradford, Sintering of synthetic latex particles. J. Colloid Sci. 6(2), 108–117 (1951)
M.L. Williams, R.F. Landel, J.D. Ferry, The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J. Am. Chem. Soc. 77, 3701–3707 (1955)
B. Voogt, H. Huinink, B. Erich, J. Scheerder, P. Venema, O. Adan, Water mobility during drying of hard and soft type latex: systematic GARField 1H NMR relaxometry studies. Prog. Org. Coat. 123, 111–119 (2018)
B. Voogt, H.P. Huinink, S.J.F. Erich, J. Scheerder, P. Venema, J.L. Keddie, O.C.G. Adan, Film formation of high Tg latex using hydroplasticization: explanations from NMR Relaxometry. Langmuir 35, 12418–12427 (2019)
S. Erkselius, L. Wadso, O.J. Karlsson, Drying rate variations of latex dispersions due to salt induced skin formation. J. Colloid Interface Sci. 317(1), 83–95 (2008)
P. Ekanayake, P. McDonald, J. Keddie, An experimental test of the scaling prediction for the spatial distribution of water during the drying of colloidal films. Eur. Phys. J. Spec. Top. 166, 21–27 (2009)
S. Kiil, Drying of latex films and coatings: reconsidering the fundamental mechanisms. Prog. Org. Coat. 57(3), 236–250 (2006)
E. Gonzalez, M. Paulis, M.J. Barandiaran, J.L. Keddie, Use of a routh-russel deformation map to achieve film formation of a latex with a high glass transition temperature. Langmuir 29(6), 2044–2053 (2013)
N. Nishimura, T. Takigawa, H. Iyota, H. Imakoma, Measurement of drying rate from temperature data during drying of slab materials. Kagaku Kogaku Ronbunshu 33(6), 586–592 (2007). (in Japanese)
T. Yamamoto, E. Aizawa, H. Imakoma, H. Omura, Measurement of drying rate of glue coating by modified temperature change method. Kagaku Kogaku Ronbunshu 35(2), 246–251 (2009). (in Japanese)
R.B. Keey, Introduction to Industrial Drying Operations (Pergamon, Oxford, 1978)
F. Zamponi, Packings close and loose. Nature 453, 606–607 (2008)
G.D. Scott, D.M. Kilgour, The density of random close packing of spheres. J. Phys. D Appl. Phys. 2, 863 (1969)
J.A. Neil, Sloane, Kepler’s conjecture confirmed. Nature 395, 435–436 (1998)
Y. Komoda, K. Niga, H. Suzuki, Effect of shear strain applied in coating and colloidal stability on the drying process of latex dispersions. J. Chem. Eng. Jpn. 48(1), 87–93 (2015)
Acknowledgements
The authors are grateful to Japan Coating Resin Corporation for providing the latex paint.
Author information
Authors and Affiliations
Contributions
HT, YK and HI were involved in study design. All authors contributed to the experiment analysis and discussed the results. HT wrote the manuscript. YK edited the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, H., Komoda, Y., Horie, T. et al. Drying rate of latex coating affected by the deformability of resin particles in convection drying. Eur. Phys. J. E 45, 2 (2022). https://doi.org/10.1140/epje/s10189-021-00155-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epje/s10189-021-00155-1