Abstract
Hybrid organic-inorganic membranes based on tetraethoxysilane and orthophosphoric acid-doped copolymers of 4-vinylpyridine (4-VP) and 2-hydroxyethyl methacrylate (HEMA) have been formed by the sol-gel synthesis method. The membranes are characterized by high values of exchange capacity and proton conductivity. An increase in the proton conductivity of hybrid organo-inorganic membranes compared to the initial copolymer can be associated with the generation of crystallization water during the formation of a silicon dioxide fragment, which follows from quantum-chemical modeling of the local structure of the membrane. The latter includes an organic part from the copolymerization product of 4-VP with HEMA (44 atoms) and an inorganic part of 27 atoms, repeating the structure of the silicon dioxide block.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Ion-exchange materials and membranes are widely used in chemical technology, biotechnological processes, mechanical and microelectronic devices, medicine, ecology, etc. Ion-exchange membranes of various types, including organo-inorganic (hybrid) membranes, are especially widespread in the energy sector, as they are used as part of the membrane-electrode unit of solid polymer fuel cells (SPFCs), which is primarily due to the widest possibility of modifying their conductive, mechanical, and chemical properties by changing the type of components included in their structure.
These membranes have high thermal stability and mechanical strength [1], and provide significant proton conductivity [2]. At the same time, according to a number of indicators, for example, mechanical strength and the ability to control the hydrophilic component, these membranes are superior to traditional commercial membranes such as Nafion [3, 4]. In addition, they may differ in lower cost. At present, ion-conducting polymeric membranes are produced on the basis of polyimides [3], polyether ether ketones [5], polyarylene ether ketones [6], polypyrroles [7], epoxy-containing polymers [8], triazole-containing polymers [9], etc.
To improve the mechanical properties, increase the thermal stability, water resistance, and proton conductivity of polymer membranes, inorganic fillers are introduced, such as silicon and zirconium oxides [10], cerium and titanium oxides [11], carbon materials, for example, nanotubes, fullerenes, or graphene derivatives [12], heteropolyacids (such as polyantimonic acid [13], polytungstic acid [14]).
Among the inorganic components available for use on an industrial scale, silicon dioxide appears to be the most preferred due to its lower cost. Doping membranes with silicon dioxide increases their thermal, chemical, and mechanical stability, as well as ionic conductivity [15].
It has been shown that the main part of inorganic precursors mainly affects the structure formation of membranes and does not contain chemically active groups capable of generating protons or intercalating dopant acids into the membrane [20].
Previously, we have obtained and studied a wide range of hybrid membranes based on tetraethoxysilane (TEOS) and binary copolymers of various nature doped with orthophosphorus or sulfonated with sulfuric acids: 4-vinylpyridine–2-hydroxyethyl methacrylate, 2-methyl-5-vinylpyridine–vinyl chloride, 2‑methyl-vinylpyridine–vinyl acetate, vinylpyrazole–vinyl chloride, vinylpyrazole–methyl methacrylate, vinylpyrazole–vinyl acetate, styrene–allyl glycidyl ether. At the same time, it was shown [16–19] that the proton conductivity of a number of membranes formed from a hybrid composite is more than an order of magnitude higher than the conductivity of membranes obtained on the basis of copolymers in the absence of additional proton-conducting groups in them. An increase in the proton conductivity of hybrid membranes occurs due to an increase in porosity and, consequently, an increase in the total volume of channels providing proton transfer [20].
The purpose of this work is to explain the experimental values of the proton conductivity of hybrid organic-inorganic membranes based on 4-VP-HEMA copolymers and the hydrolytic polycondensation product of TEOS in comparison with organic 4-VP-HEMA copolymers using quantum chemical calculations.
COMPUTATIONAL DETAILS
Quantum-chemical evaluation of the thermodynamic stability of the local structure of the composite. Using the ORCA 4.2.0 software package [21] and the Becke-PerdewBP86 density functional method, consisting of the B88 exchange functional and the PW86 correlation functional [22, 23], the thermodynamic stability of molecular systems simulating different conformations of copolymer constituents was evaluated using the def2-SVP basis set [24]. We used the approach implemented [25], where for a similar atomic–molecular system, the choice of the method and basis for computer simulation was substantiated. To calculate the one-point energy by the self-consistency procedure, we used the DIIS convergence algorithm with an energy convergence criterion of 10–8 Hartree with the integration grid parameter Grid5. The calculations were carried out using the Split-RI-J integrals approximation method. As a result of computer simulation, the values of the internal energy \(U_{0}^{0}\) at T = 0 K were obtained for all the structures under consideration. Next, the Hessian of the energy was calculated analytically, from which the harmonic vibration frequencies were obtained. Based on the values of the oscillation frequencies, we obtained the values of the Gibbs function G at p = 1 atm and T = 298.15 K using the algorithms of the ORCA software package based on the methods of statistical thermodynamics. The obtained values of the thermodynamic functions \(U_{0}^{0}\) and \(G_{{298}}^{0}\) were used to assess the thermodynamic stability of the atomic-molecular structures of the copolymer unit. This assessment was carried out by calculating the change in the thermodynamic function of formation of a given structure as the difference between the corresponding value of the energy of the model structure and the sum of the energies of the inorganic and organic components.
RESULTS AND DISCUSSION
Copolymers of 4-vinylpyridine (4-VP) with 2‑hydroxyethyl methacrylate (HEMA) were obtained under conditions of free radical initiation by the action of azobisisobutyric acid dinitrile at a temperature of 60°C in a DMF solution [17].
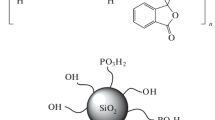
Hybrid membranes were synthesized according to the methods developed earlier [26, 27]. Sol–gel synthesis with the participation of tetraethoxysilane (TEOS) [28, 29] was used to obtain hybrid membranes consisting of a polymer matrix in which particles of hydrated silicon dioxide are uniformly distributed (Scheme 1). To impart ion-conducting properties, the membranes were doped with a solution of 9 M H3PO4.

Scheme 1 .
Hybrid 4-VP-HEMA-SiO2 membranes have been studied by physicochemical methods (elemental analysis, IR spectroscopy, TGA and DSC, ion-exchange capacity, proton conductivity, and mechanical properties) [17]. The synthesized hybrid membranes have the following characteristics: ion-exchange capacity, 2.1 meq/g; proton conductivity, (0.9–1.9) × 10–2 S/cm (Table 1); activation energy, 12 ± 2 kJ/mol in the range 298–363 K; heat resistance, up to 412°С; tensile strength, 55.5 MPa [17].
Table 1 data analysis shows that the proton conductivity of hybrid membranes is more than an order of magnitude higher than the proton conductivity of polymer membranes. To explain this fact, a quantum-chemical assessment of the thermodynamic stability of the model structure of the composite was carried out.
Thermodynamic Characteristics of Model Structures
The structure of the siloxane fragment used in this simulation was shown [25]. Figure 1 shows various model structures simulating one unit of a 4-VP-HEMA copolymer with a phosphoric acid molecule attached to the pyridine ring.
The geometry was optimized for two molecular structures (Model 1 and Model 2) and two covalent structures (Model 3 and Model 4) that differ in the relative position of the siloxane fragment. In Model 1 and Model 2, the bond between the siloxane and organic parts of the composite is due to intermolecular hydrogen bonds. Models 3 and 4 take into account the possibility of the formation of a Si–O–C covalent bond during the interaction between the hydroxyl groups of the siloxane and the organic component of the copolymer.
In Model 1, the water molecule released due to the closing of the siloxane ring and having three hydrogen bonds with it forms an additional intermolecular bond with the hydroxyl group of the copolymer. Thus, it has a coordination number of 4. The phosphoric acid molecule associated with the pyridine ring forms a hydrogen bond with the hydroxyl of the inorganic fragment of the siloxane on the opposite side of the first intermolecular bond. In Model 2, the siloxane fragment is turned with respect to the organic block of the composite by the opposite side of the ring. Water arising from the inorganic part of the composite and phosphoric acid do not form intermolecular links between the organic and inorganic parts of the composite. Here, two hydroxyls of the siloxane form hydrogen bonds with the hydroxyl group of the copolymer. The third hydroxyl group of the siloxane fragment interacts with the oxygen atom of the carbonyl group of the copolymer.
In Model 3, the second water molecule formed by the interaction of two hydroxyl groups enters into a Coulomb interaction with the hydroxyl group of the siloxane fragment and the carbonyl oxygen of the copolymer. Water, previously included in the inorganic part of the composite, and phosphoric acid do not form intramolecular links between the fragments of the composite. The conformation of Model 3 is similar to that of Model 2. In Model 4, the phosphoric acid molecule associated with the pyridine ring formed an additional three hydrogen bonds with the hydroxyls of the inorganic fragment. The water molecule released during the formation of a covalent bond between the organic and inorganic components of the composite formed one intermolecular bond with the oxygen of the Si–O–C group. The orientation between organic and inorganic blocks in the composite of the fourth model is similar to the orientation in Model 2 and Model 3, when the first water molecule is stabilized by an inorganic fragment and does not take part in the formation of intermolecular bonds.
Table 2 compares the energies of the metastable states of the organo-inorganic composite 4-VP-HEMA–SiO(2 – k)(OH)2k in various conjugation models.
It can be seen from the calculations (Table 2) that the difference between the energies of all four structures is small. The structures of models 2 and 4 are the most stable. From the standpoint of statistical thermodynamics, it can be assumed that in the real structure of the copolymer, all types of conformations of units of the corresponding models can be present, which is confirmed by the low intensity of vibrations of the Si–O–C group in the IR spectrum. However, Model 3 has the highest energy in comparison with Model 4. Therefore, according to the relative energy, the following series can be distinguished in terms of the frequency of unit conformations in the copolymer: 3 < 1 < 2 < 4.
All models can be grouped according to conformational features. Scheme 2 shows the principle of calculating the total energy (E) and the change in the Gibbs function (ΔG) of the formation reaction of composite structures according to the equation Eform (composite) = E (composite) – E (org.) – E (inorg.).

Scheme 2.
Figure 2 shows the changes in thermodynamic functions (internal energy ΔE at T = 0 K and Gibbs function ΔG at T = 298.15 K) calculated using the Hess law and Scheme 2. All values are relative to those for Model 4.
From Fig. 2 it is clear that the difference between the energies of the structures is small. Two minima correspond to the most stable structural Models 2 and 4. At the same time, Model 4 is characterized by the maximum number of hydrogen bonds (9), the siloxane fragment is held by the Si–O–C covalent bond, and three hydrogen bonds formed with the participation of a phosphoric acid molecule.
Models 1 and 2 have the same number of hydrogen bonds (8), while the first is higher in energy than the second. This can be explained by the increase in interelectronic repulsion due to the tight conformation, and also by the fact that the inorganic part is retained only by one hydrogen bond formed between the water molecule and the OH group of the copolymer, as well as by one hydrogen bond from phosphoric acid. Model 2 is lower in energy. The inorganic part is held by two hydrogen bonds from Si–OH to HO–C and one from Si–OH to O=C.
The calculation of the change in the Gibbs functions for the formation of model structures makes it possible to refine the series according to the Boltzmann probability of meeting certain structures of copolymer units: 3 < 1 < 2 < 4. It repeats the series obtained from the analysis of the total single-point energy of structures at T = 0 K, but shows a more differentiated distribution, demonstrating a relatively low probability of meeting supramolecular structure 1 and covalent structure 3.
Additionally, the strength of the intermolecular interaction of the phosphoric acid molecule with the nitrogen atom of the pyridine group was estimated by subtracting the sum of free acid molecules and the organic component from the total energy of the structure of the organic component associated with phosphoric acid. A value of 70 kJ/mol was obtained, which indicates the presence of a strong hydrogen bond between phosphoric acid and the pyridine group.
CONCLUSIONS
Thus, it was established that during the formation of the 4-VP-HEMA–SiO2 composite, the phosphoric acid molecule is bound to the nitrogen atom by a fairly strong hydrogen bond (70 kJ/mol). In the most stable Model 4, the H3PO4 molecule has one interaction-free hydrogen atom. It can easily split off due to the electron density shift and participate in the proton transfer by the Grotthus mechanism. An increase in the proton conductivity of hybrid membranes in comparison with membranes formed only from an organic copolymer can be associated with the participation in the transport of hydrogen ions not only of phosphoric acid molecules but also of water molecules released during the formation of the dendritic structure of the siloxane fragment in the composite structure.
The structural features of hybrid membranes containing silicon dioxide found by a numerical method explain the increase in their electrical conductivity compared to membranes based on 4-VP-HEMA copolymers.
REFERENCES
J. Honma, H. Nakayama, O. Nishikawa, T. Sugimoto, and S. Nomura, Solid State Ionics 162–163, 237 (2003).
A. B. Yaroslavtsev, Russ. Chem. Rev. 85, 1255 (2016).
V. S. Ivanov, A. S. Yegorov, G. R. Allakhverdov, and V. V. Men’shikov, Oriental J. Chem. 34, 255 (2018).
Guizhen Guo, Sun Youyi, Fu Qiang, MaYibing, Zhou Yaya, Xiong Zhiyuan, and Liu Yaqing, Int. J. Hydrogen Energy 44, 6103 (2019).
Zhang Xiaoyu, Shiyuan Yu, Qian Zhu, and Lianhua Zhao, Int. J. Hydrogen Energy 44, 6148 (2019).
Wang Yuanyuan, Xu Jingmei, Zang Huan, and Wang Zhe, Int. J. Hydrogen Energy 44, 6136 (2019).
K. Brijesh, K. Bindu, Shanbhag Dhanush, and H. S. Nagaraja, Int. J. Hydrogen Energy 44, 757 (2019).
M. V. Markova, D. M. Mognonov, L. V. Morozova, A. I. Mikhaleva, and B. A. Trofimov, Polym. Sci. Ser. B 56, 229 (2014).
S. Roy, S. Saha, A. G. Kumar, A. Ghorai, and S. Banerjee, J. Appl. Polym. Sci. 137, 48514 (2020).
E. Yu. Safronova, A. V. Parshina, K. Yu. Yankina, E. A. Ryzhkova, A. A. Lysova, O. V. Bobreshova, and A. B. Yaroslavtsev, Pet. Chem. 57, 327 (2017).
S. A. Makulova, Yu. A. Karavanova, I. I. Ponomarev, I. A. Stenina, and Yu. A. Volkova, Pet. Chem. 58, 304 (2018).
I. A. Prikhno, E. Y. Safronova, and A. B. Ilyin, Pet. Chem. 57, 1228 (2017).
F. A. Yaroshenko and V. A. Burmistrov, Pet. Chem. 58, 249 (2018).
K. Brijesh and K. Bindu, Int. J. Hydrogen Energy 44 (2019).
H. Pan, Ya. Zhang, H. Pu, and Z. Chang, J. Sources Energy 263, 195 (2014).
O. V. Lebedeva, Y. N. Pozhidaev, and E. I. Sipkina, Adv. Mater. Res. 749, 71 (2013).
O. V. Lebedeva, E. I. Sipkina, and Yu. N. Pozhidaev, Pet. Chem. 56, 401 (2016).
A. I. Emelyanov, O. V. Lebedeva, E. A. Malakhova, T. V. Raskulova, Y. N. Pozhidaev, Y. A. Verkhozina, L. I. Larina, S. A. Korzhova, G. F. Prozorova, and A. S. Pozdnyakov, Membr. Membr. Technol. 3, 147 (2021).
A. Chesnokova, O. V. Lebedeva, Y. N. Pozhidaev, E. A. Malakhova, T. V. Raskulova, V. Kulshrestha, A. V. Kuzmin, and A. S. Pozdnyakov, Int. J. Hydrogen Energy 45, 18716 (2020).
A. K. Osipov, I. A. Prikhno, and A. B. Yaroslavtsev, Pet. Chem. 58, 1129 (2018).
F. Neese, Comput. Mol. Sci. 2, 73 (2012).
A. D. Becke, Phys. Rev. A 38, 3098 (1988).
J. P. Perdew, Phys. Rev. B 33, 8822 (1986).
F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys. 7, 3297 (2005).
L. V. Fomina, E. A. Malakhova, O. V. Lebedeva, Yu. N. Pozhidaev, S. A. Beznosyuk, A. S. Fomin, and T. V. Raskulova, Vest. Angarsk. Gos. Tekhn. Univ. 13, 81 (2019).
O. V. Lebedeva, Yu. N. Pozhidaev, N. S. Shaglaeva, A. S. Pozdnyakov, and S. S. Bochkareva, Khim. Tekhnol. 11, 20 (2010).
A. N. Chesnokova, O. V. Lebedeva, Y. N. Pozhidaev, N. A. Ivanov, and A. E. Rzhechitskii, Adv. Mater. Res. 884–885, 251 (2014).
G. I. Dobryanskaya, Yu. L. Zub, M. Barchak, and A. Dabrowski, Colloid. J. 68, 548 (2006).
K. A. Andrianov, Silicone Compounds (Goskhimizdat, Moscow, 1955) [in Russian].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Lebedeva, O.V., Raskulova, T.V., Beznosyuk, S.A. et al. Structural Features of 4-VP-HEMA-SiO2 Hybrid Membranes and Their Proton Conductivity. Membr. Membr. Technol. 5, 92–97 (2023). https://doi.org/10.1134/S251775162302004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S251775162302004X