Abstract—The genus Colletotrichum includes a number of plant pathogens of major importance, causing diseases in a broad variety of woody and herbaceous plants. Due to recent molecular analysis, Colletotrichum spp. have undergone many taxonomic changes, i.e., introduction of a significant number of new species and abolition of some old ones. The data on the species diversity, abundance and host specialization of species in this genus on the territory of Russia and neighboring countries are obviously far from being complete, do not correspond to the modern taxonomy of the genus and require substantial revision. In this work, the molecular genetic identification and pathogenicity assessment of 35 isolates, previously identified as Colletotrichum spp., from the European part of Russia, Ukraine, Siberia, and the Russian Far East were carried out. It has been found that 12 isolates obtained from wild plants and crops belong to the species Colletotrichum coccodes. The remaining isolates belong to destructivum (14 isolates) and dematium (9 isolates) species complexes. Among the members of the destructivum complex, it was possible to identify the species C. destructivum and C. lini. C. dematium, C. lineola and Colletotrichum cf. spinaciae were defined among the isolates of the dematium complex. Three isolates of C. destructivum from wild plants of Leningrad oblast and Kamchatka, to our knowledge, are the first findings of this species for Russia. According to the results of the pathogenicity assessment, three isolates assigned to the destructivum species complex may be of interest for the biocontrol of Galinsoga parviflora, and one isolate identified as С. coccodes may be potentially used for the control of Ambrosia artemisiifolia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The genus Colletotrichum Corda (Sordariomycetes, Glomerellales, Glomerellaceae) includes widely distributed phytopathogenic fungi that hold particular significance for agriculture (Zhang et al., 2006; Réblová et al., 2011). The crop diseases caused by these fungi, mainly anthracnoses, blights, and rots, lead to significant economic losses worldwide. The damage caused by fungi of this genus extends to cereals, legumes, vegetables, fruit and berry crops, ornamental and other crops (Cannon et al., 2012). The outbreaks of previously registered diseases such as anthracnoses of lupine, clover, and beans, as well as relatively new ones: oat and strawberry anthracnoses, are not uncommon in Russia (Kotova and Kungurtseva, 2014). There are widespread anthracnoses of solanaceous (Belov et al., 2018) and pumpkin crops (Varivoda and Maslennikova, 2019). It is also known that these fungi cause significant damage in the postharvest period during the storage of agricultural products.
The majority of species in the genus Colletotrichum exhibit a hemibiotrophic type of nutrition, sequentially combining biotrophic and necrotrophic phases, the duration of which varies depending on the particular pathogen, host species, as well as environmental conditions. After colonizing a host, fungi can remain latent or dormant for an extended period, eventually manifesting their pathogenic properties some time later (Jayawardena et al., 2021).
Colletotrichum spp. are often recorded as endophytes. By entering into mutualistic relationships, they are capable of providing plants with disease resistance, drought tolerance, and stimulating their growth (Redman et al., 2001; Busby et al., 2016). However, it should be noted that such relationships from the fungal side can quickly develop into parasitic relationships due to changes in external factors.
Currently, there are about 250 actual species distributed among 14 species complexes (caudatum, graminicola, spaethianum, destructivum, acutatum, dematium, gigasporum, gloeosporioides, boninense, truncatum, orbiculare, dracaenophilum, magnum, and orchidearum), as well as 13 individual species based on molecular genetic data (Damm et al., 2019; Jayawardena et al., 2021). There are references to some Colletotrichum species on wild plants in our country, but they are mostly unsystematized and fragmentary (Gasich et al., 1999, 2015, 2016; Mel’nik et al., 2008; Gannibal et al., 2010). Moreover, the existing data in the literature primarily rely on morphological characteristics of the fungi and their association with host plants. Nowadays, it is difficult to verify and interpret the results of such identification, because the current understanding of species diversity in this genus is based mainly on molecular-genetic data.
The current phytosanitary conditions of agrocenoses and the biological characteristics of Colletotrichum spp. allow them to be categorized into the top 10 phytopathogens most important for science and economy (Dean et al., 2012). In this regard, the range of surveyed plants (and consequently the fungi isolated from them) is biased towards agricultural crops; wild host plants are less frequent in mycological collections, despite being are a rich reservoir of biodiversity of this taxon. The fungi of the genus Colletotrichum and the phytotoxins they produce are considered to be important agents for biological weed control; some Colletotrichum strains have been registered as mycoherbicides (Chakraborty and Ray, 2021). The data on species diversity, occurrence frequency, and specialization of these fungi in Russia and neighboring countries, compared to the accumulated data set of the global level, are insufficient and need to be revised. The present study is devoted to the molecular-genetic identification and assessment of pathogenicity of Colletotrichum spp. isolates (from the collection of pure cultures of micromycetes of the Laboratory of Mycology and Phytopathology, the All-Russian Institute of Plant Protection) collected in the European part of Russia, Siberia, the Far East, and Ukraine.
MATERIALS AND METHODS
Isolation and cultivation of fungi. The isolates tentatively identified as Colletotrichum spp., which were isolated from crops and wild plants in the period from 1995 to 2019 mainly in Russia were used in this work (four samples from Ukraine, Sumy oblast, were studied additionally). To isolate the fungi in pure culture, small fragments of affected plant tissue were washed with tap water, superficially disinfected with 0.1% silver nitrate solution for 1 min, then washed several times in sterile water and transferred to Petri dishes with potato sucrose agar (PSA) (Samson et al., 2000). Isolates were cultured at 24°C; colony diameter was measured on day 7; characterization of the colonies and species identification were performed on day 14. All isolated cultures are currently stored in the microbial collection of the All-Russian Institute of Plant Protection. The origins of the isolates are given in Table 1.
DNA extraction and sequencing. The standard CTAB/chloroform method (Doyle, J.J. and Doyle, J.L., 1987) was used for DNA extraction from Colletotrichum spp. isolates. The subsequent amplification was performed for rDNA internal transcribed spacers (ITS), as well as the β-tubulin (Tub2) and actin (Act) genes with the respective primers ITS1F (Gardes and Bruns, 1993)/ITS4 (White et al., 1990); βtub2Fw/βtub4Rd (Aveskamp et al., 2009); Act-512F/Act-783R (Carbone and Kohn, 1999). Amplification products were separated by electrophoresis in 1% agarose gel stained with ethidium bromide. Fragments of desired length were purified using silicon dioxide powder (Malferrari et al., 2002). Sequencing PCR was performed according to Sanger (Sanger et al., 1977) with a BigDye Terminator kit v. 3.1 Cycle Sequencing Kit (ABI, United States); nucleotide sequences were identified with an ABI PRISM 3500 genetic analyzer. The sequences were corrected with the SeqScape and VectorNTI programs and compared with those deposited in the GenBank using the Blast-n algorithm (Altschul et al., 1990). The sequenced ITS were deposited in the GenBank with accession numbers OL647872–OL647906; the Tub2 and Act sequences were deposited as OL676734–OL676768 and OL676699–OL676733, respectively.
Bioinformatic analysis. Sequence alignment was performed with MegaX software using the Muscle algorithm (Kumar et al., 2018; Edgar, 2004). The search for the optimal nucleotide substitution models for constructing maximum likelihood (ML) phylogenetic trees for all loci individually was conducted in ModelFinder program, using the Bayesian Information Criterion (BIC) (Kalyaanamoorthy et al., 2017). The following optimal models were selected: K2P + R2 (Kimura, 1980) for the ITS locus, TNe + G4 (Tamura and Nei, 1993) for Tub2, and K2P + I (Kimura, 1980) for Act. The concatenation of sequences from three loci was carried out using the SequenceMatrix 1.7.8 software (Vaidya et al., 2011). Reconstruction of the multilocus ML tree and assessment of the reliability with UFBoot topology (Minh et al., 2013) using 10,000 bootstrap iterations was performed with the IQ-tree program (Kimura, 1981; Nguyen et al., 2015).
Pathogenicity assessment. To assess pathogenicity, isolates were grown at room temperature on a liquid soybean medium [KH2PO4, 2 g; (NH4)2SO4, 1 g; MgSO4, 1 g; glucose, 20 g; soybean meal, 10 g; water, 1 L; 50 mL of the medium per 250-mL flask] on an orbital shaker (200 rpm) for four days. The medium was inoculated with three mycelial disks (5 mm) cut out with a drill from 2-week-old colonies grown on PSA. The mycelium was separated from the culture liquid, dried, and grounded. Plant leaf segments were placed into Petri dishes on filter paper moistened with sterile water. A drop of mycelial suspension (10 μL; C = 100 mg/mL) was applied to the center of the previously injured or intact leaf segments on the abaxial or adaxial surface. Leaf segments inoculated with sterile water were used as a negative control. The dishes were incubated on a laboratory bench under natural lighting; necrosis diameters were measured at 4, 7, and 14 day post-treatment. Subsequently, to confirm Koch’s postulates, the pathogen was isolated from infected plants and identified.
The isolates showing the highest pathogenicity on the leaf segments of weeds were assessed on whole plants grown in 250-mL plant containers. The plants were germinated from seeds (5 seeds per vial) under periodic light (12/12 h). By the time of inoculation, the small-flowered galinsoga, the common dandelion and the common purslane were at the stage of two sets of true leaves, while common ragweed was at the stage of three sets of true leaves. Mycelial suspension was prepared as described above. Plants were sprayed with mycelial suspension at 50 mg/mL and placed in wet chambers for 24 or 48 h (dew period). Following this, the plants were transferred to the lighting fixture. The affected surface area of each leaf was determined on day 2, 3 and 7 by a 6-point scale (0, no symptoms; 1, 0–5%; 2, 6–25%; 3, 26–75%; 4, 76–95%; 5, more than 95% of leaf surface with necrosis; 6, leaf dead completely). The affected plant surface area was determined by the formula: (2.5n1 + 15n2 + 50n3 + 85n4 + 97.5n5 + 100n6)/N, where nx is the number of leaves with a given score and N is the total number of leaves (Pfirter and Defago, 1998).
RESULTS
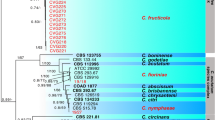
Molecular phylogeny. Molecular genetic identification of fungi from the genus Colletotrichum with respect to three loci (ITS, Act, Tub2) has shown that more than one third of all isolates (34%) belongs to the species C. coccodes (Wallr.) S. Hughes (Fig. 1). This species has been found both on wild plants (small-flowered galinsoga, common ragweed, hemp, common purslane) and crops (rapeseed, beet) from Primorsky krai of Russia to Ukraine. Other isolates under study belonged to the two species complexes: destructivum (40%) and dematium (26%).
Multilocus phylogenetic tree of Colletotrichum spp. isolates reconstructed by maximum likelihood (ML) method based on nucleotide sequences of ITS, Tub2 and Act genes. References belonging to type strains are in bold. Bootstrap support values above 50% are given for studied isolates. Species Colletotrichum phaseolorum was used as outgroup.
Isolates MF-13.20, MF-13.23 and MF-13.24 from the destructivum species complex exhibited full similarity with the type (ex-epitype) strain C. destructivum O’Gara CBS 136228 in the loci under study, which allows them to be assigned to this species. Isolate MF-13.10 can be confidently identified as C. lini (Westerd.) Tochinai. The remaining isolates from the destructivum complex were not as closely to the available references, making their confident identification to the species level difficult. For example, MF-20.1, MF-20.2, MF-20.3 from Amur oblast, isolated from soybean, not only formed a distinct basal group in relation to the species C. destructivum but also exhibited differences among each other. Therefore, in the context of this study, it is considered possible to identify them only as Сolletotrichum cf. destructivum. MF-3.5 isolated, from rapeseed, was assigned to the group formed by the species C. americae-borealis Damm and С. lini, and its final identification is also proves to be challenging. The group of isolates MF-13.2, MF-13.25, MF-13.27 from the destructivum species complex, isolated from Galinsoga collected in Russia (Pskov oblast, Velikoluksky district) and Ukraine (Sumy oblast, Seredino-Budsky district), in all likelihood, represent a new, yet undescribed species. Isolates MF‑13.11 and MF-19.5 from the bitter dock and the common dandelion, respectively, proved to be phylogenetically close to the species C. panacicola Uyeda et S. Takim. and C. utrechtense Damm. Isolate MF-13.13 from the field bindweed was included into the group formed by the species C. antirrhinicola Damm, C. bryoniicola Damm and C. vignae Damm.
Among representatives of the dematium species complex, MF-13.21 isolated from Sosnowsky’s hogweed could be confidently identified as C. lineola Corda. A similar isolate MF-17-018 from soybean, which was included in the group with С. lineola and C. menispermi Chethana, Jayaward., Bulgakov et K.D. Hyde was not identified to a species with respect to the loci used. Isolates MF-17-012, MF-17-013, and MF-13.22 differed by one nucleotide substitution in the Tub2 gene from the type (ex-epitype) strain C. dematium (Pers.) Grove CBS 125.25 but showed complete similarity to another reference strain, С. dematium CBS 125340, which allows these isolates to be assigned to the given species. Isolate MF-16-008 from cabbage was identified as Colletotrichum cf. spinaciae Ellis et Halst. The species affiliation of isolates MF-13.19, MF-19.2 and MF-19.6 included in the dematium species complex could not be determined.
Pathogenicity. Pathogenicity assessment of 20 Colletotrichum spp. isolates from weed plants demonstrated the ability of 11 isolates to induce disease symptoms on the host plants from which they had been isolated (Table 2). The MF-13.17 isolate showed the highest pathogenicity against the common purslane: necrosis around the inoculation sites was already observed in the first days after inoculation; on day 4, large necroses also developed in case of applying the inoculum onto the upper undamaged leaf surface. The early development of symptoms was also observed on the common ragweed (isolate MF-13.14) and the meadow salsify (isolate MF-19.2). On the seventh day after inoculation, isolates MF-13.2, MF-13.25, and MF-13.27 caused the appearance of spots on the upper leaf surfaces of small-flowered galinsoga, and by the 14th day, limited necrosis was observed on the lower leaf surface. Isolates MF-13.13 and MF-19.6 caused limited necroses in the field bindweed and the common dandelion, respectively, which did not extend beyond the area of the drop applied. On the contrary, isolates MF-13.24 on the common dandelion, MF-13.3 and MF-13.26 on small-flowered galinsoga caused the later (day 14) development of symptoms. The remaining isolates did not demonstrate pathogenicity on the tested plant leaves.
In the experiment on the whole plants, isolates MF-13.24 (C. destructivum) and MF-13.17 (C. coccodes) demonstrated rather weak pathogenicity towards the common dandelion and the common purslane, respectively. Symptoms were observed only on day 7, with a 48-h dew period (Table 3). The extent of damage to common ragweed plants by isolate MF-13.14 (C. coccodes) was more than 50%. Isolates MF-13.2, MF-13.25 and MF-13.27 (from the destructivum species complex) showed high pathogenicity towards Galinsoga plants, with more than 70% damage observed on the second day and 100% plant mortality by the seventh day.
The pathogenicity of 15 isolates from beet, soybean, rapeseed, and cabbage, was assessed on these plants (Table 4). All isolates from the beet (MF-16-014, MF-16-015, MF-17-014) showed pathogenicity against this plant. The isolates from the soybean (MF-20.1, MF-20.2, MF-20.3) were characterized by weak pathogenicity and caused the development of limited necrosis on soybean leaf segments; isolate MF-17-018 did not show any pathogenicity towards soybean. Among the isolates from Brassica spp., only MF-3.2 (from rapeseed) and MF-16-008 (from cabbage) caused necrosis on rape leaf segments; other isolates proved to be nonpathogenic.
DISCUSSION
A significant proportion of our isolates collected in East Europe, Siberia, and the Far East belongs to the species C. coccodes. This species is considered to be a cosmopolitan phytopathogen with broad specialization. In addition to the traditionally mentioned plants from the nightshade family, there is a considerable number of plants that can be infected by this fungal species (Farr and Rossman, 2021). Inter alia, there is fragmentary information that this species affects beet (Ginns, 1986), rapeseed (Nitzan et al., 2006), cabbage, and hemp (McPartland et al., 2000). Most reports on isolation of C. coccodes from a variety of plants often rely solely on the culture and morphological data, and the frequency of occurrence of this species may be exaggerated (Jayawardena et al., 2021). Our C. coccodes isolates were also obtained from small-flowered galinsoga, common ragweed and the common purslane, which have not been previously reported as host plants in the literature. The isolated strains exhibited pathogenic properties against the latter three plant species.
The rest of the isolates belonged to the two species complexes: dematium and destructivum. The latter includes precisely the species C. destructivum, as well as 16 closely related species that are mainly plant pathogens. C. destructivum (similar to C. coccodes) occurs on a wide range of hosts from the families Asteraceae, Convolvulaceae, Fabaceae, Magnoliaceae, Menispermaceae, Lamiaceae, Poaceae, Polygonaceae, and Solanaceae (Damm et al., 2014). The three isolates identified in this study as C. destructivum and isolated from wild plants collected in Leningrad oblast and Kamchatka krai also indicate that this species is not specialized and has a wide geographical distribution, although, according to the available data, its occurrence in Russia has not been recorded previously. This is apparently due to the difficulty of its identification without the involvement of molecular genetic methods. In view of the above, we should additionally mention the group of phylogenetically similar isolates (Colletotricum cf. destructivum) from soybean in Amur oblast. Another species from the destructivum complex, C. lini, originally isolated from flax, often infects plants of the legume family (Medicago sativa, Trifolium sp.) and, in our case, was isolated from the meadow vetchling.
The dematium species complex currently includes 18 species, some of them being highly specialized and associated with a single plant species. Among the isolates of this species complex, three species—Colletotrichum dematium, C. lineola, and C. spinaciae— have been reliably identified in the present work. The species C. dematium has been reported as a pathogen, an endophyte, and a saprotroph with broad substrate specialization (Damm et al., 2009). In the pathogenicity tests that we have performed, the isolates of this species were unable to induce disease symptoms.
The fungus C. lineola is represented within a considerable range of host plants (including Heracleum sp.) and occurs almost everywhere in temperate zones (Damm et al., 2009; Jayawardena et al., 2021). In our case, the isolate from Sosnowsky’s hogweed did not show pathogenicity on the respective plant.
The species C. spinaciae was originally isolated from the prickly seeded spinach, but later was also isolated from other plants (lamb’s quarters, alfalfa, common purslane, Damm et al., 2009). In the present study, an isolate of this species was obtained from cabbage and could induce necroses in the pathogenicity test.
Some isolates (MF-3.5, MF-13.2, MF-13.11, MF-13.13, MF-13.19, MF-13.25, MF-13.27, MF-17-018, MF-19.2, MF-19.5, MF-19.6) from the studied collection could not be assigned to any of the existing actual species. This demonstrates, with a high degree of probability, the presence of undescribed species within the destructivum and dematium species complexes and is evidence of the high species diversity of these complexes.
It has been repeatedly shown that the representatives of Colletotrichum sp. exhibit herbicidal activity against a number of weeds. Previously, based on the strains C. gloeosporioides (Penz.) Penz. et Sacc., the biocontrol agents like BioMal, Collego, and Lubao were developed to manage mallow, aeschynomene, dodder, and sesbania. The potential use of C. dematium has been explored for the control famine weed, C. orbiculare Damm, P.F. Cannon et Crous for the control of spiny cocklebur, C. coccodes for the control of China jute, Colletotrichum truncatum (Schwein.) Andrus et W.D. Moore for the control of hemp sesbania (Chakraborty and Ray, 2021). Phytotoxins colletochlorin A and tyrosol isolated from the liquid culture of C. gloeosporioides led to the development of necroses and wilting of common ragweed (Masi et al., 2018). Colletochlorin F from C. higginsianum Sacc. caused extensive necroses on perennial sow-thistle (Masi et al., 2017).
Among the cultures that we have studied, the isolates that would of the greatest interest for the biocontrol of weeds are MF-13.2, MF-13.25, MF-13.27 (the destructivum species complex) for small-flowered galinsoga and MF-13.14 (C. coccodes) for common ragweed.
REFERENCES
Altschul, S.F., Gish, W., Miller, W., et al., Basic local alignment search tool, J. Mol. Biol., 1990, vol. 215, p. 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Aveskamp, M.M., Verkley, G.J.M., de Gruyter, J., et al., DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties, Mycologia, 2009, vol. 101, no. 3, pp. 363–382. https://doi.org/10.3852/08-199
Belov, G.L., Belosokhov, A.F., Kutuzova, I.A., et al., Colletotrichum coccodes in potato and tomato leaves in Russia, J. Plant Dis. Prot., 2018, vol. 125, no. 3, pp. 311–317. https://doi.org/10.1007/s41348-017-0138-0
Busby, P.E., Ridout, M., and Newcombe, G., Fungal endophytes: Modifiers of plant disease, Plant Mol. Biol., 2016, vol. 90, pp. 645–655. https://doi.org/10.1007/s11103-015-0412-0
Cannon, P.F., Damm, U., Johnston, P.R., et al., Colletotrichum: Current status and future directions, Stud. Mycol., 2012, vol. 73, pp. 181–213. https://doi.org/10.3114/sim0014
Carbone, I. and Kohn, L.M., A method for designing primer sets for speciation studies in filamentous ascomycetes, Mycologia, 1999, vol. 91, no. 3, pp. 553–556. https://doi.org/10.2307/3761358
Chakraborty, A. and Ray, P., Mycoherbicides for the noxious meddlesome: Can Colletotrichum be a budding candidate?, Front. Microbiol., 2021, vol. 12, pp. 1–10. https://doi.org/10.3389/fmicb.2021.754048
Damm, U., Woudenberg, J.H.C., Cannon, P.F., et al., Colletotrichum species with curved conidia from herbaceous hosts, Fungal Diversity, 2009, vol. 39, pp. 45–87.
Damm, U., O’Connell, R.J., Groenewald, J.Z., et al., The Colletotrichum destructivum species complex—Hemibiotrophic pathogens of forage and field crops, Stud. Mycol., 2014, vol. 79, pp. 49–84. https://doi.org/10.1016/j.simyco.2014.09.003
Damm, U., Sato, T., Alizadeh, A., et al., The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes, Stud. Mycol., 2019, vol. 92, pp. 1–46. https://doi.org/10.1016/j.simyco.2018.04.001
Dean, R., Van Kan, J.A., Pretorius, Z.A., et al., The Top 10 fungal pathogens in molecular plant pathology, Mol. Plant Pathol., 2012, vol. 13, no. 4, pp. 414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Doyle, J.J. and Doyle, J.L., A rapid DNA isolation procedure for small quantities of fresh leaf tissue, Phytochem. Bull., 1987, vol. 19, pp. 11–15.
Edgar, R.C., Muscle: A multiple sequence alignment method with reduced time and space complexity, BMC Bioinf., 2004, vol. 5, p. 113. https://doi.org/10.1186/1471-2105-5-113
Farr, D.F. and Rossman, A.Y., Fungal databases, U.S. National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/. Accessed November 3, 2021.
Gannibal, F.B., Gasich, E.L., Berestetskiy, A.O., et al., Materials to the study of micromycetes of weeds and wild herbaceous plants in the south of Russian Far East (Primorye and Khabarovsk krai), Nov. Sist. Nizshikh Rast., 2010, vol. 44, pp. 105–117.
Gardes, M. and Bruns, T.D., ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts, Mol. Ecol., 1993, vol. 2, pp. 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Gasich, E.L., Titova, Yu.A., Berestetskiy, A.O., et al., Mikromitsety sornykh rastenii Evropeiskoi chasti Rossii. Itogi issledovanii 1993–1998 gg. (Micromycetes of Weeds in European Part of Russia. Research Results 1993–1998), St. Petersburg: Vseross. Nauchno-Issled. Inst. Zashchity Rastenii, SPb., 1999.
Gasich, E.L., Gannibal, F.B., Berestetskiy, A.O., et al., Species composition of micromycetes on weeds and wild herbaceous plants in Pskov oblast, Plant Protection News, 2015, vol. 84, no. 2, pp. 28–35.
Gasich, E.L., Gannibal, F.B., Berestetskiy, A.O., et al., Micromycetes of weeds and wild herbaceous plants in the Republic of North Ossetia—Alania, Mikol. Fitopatol., 2016, vol. 50, no. 4, pp. 257–265.
Ginns, J.H., Compendium of plant disease and decay fungi in Canada 1960–1980, Res. Br. Can. Agric. Publ. 1813, 1986.
Jayawardena, R.S., Bhunjun, C.S., Hyde, K.D., et al., Colletotrichum: Lifestyles, biology, morpho-species, species complexes and accepted species, Mycosphere, 2021, vol. 12, no. 1, pp. 519–669. https://doi.org/10.5943/mycosphere/12/1/7
Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., et al., ModelFinder: Fast model selection for accurate phylogenetic estimates, Nat. Methods, 2017, vol. 14, no. 6, pp. 587–589. https://doi.org/10.1038/nmeth.4285
Kimura, M., A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences, J. Mol. Evol., 1980, vol. 16, pp. 111–120. https://doi.org/10.1007/bf01731581
Kimura, M., Estimation of evolutionary distances between homologous nucleotide sequences, Proc. Natl. Acad. Sci. USA, 1981, vol. 78, pp. 454–458. https://doi.org/10.1073/pnas.78.1.454
Kotova, V.V. and Kungurtseva, O.V., Anthracnose of agricultural plants, Vestn. Zashch. Rast., 2014, suppl.
Kumar, S., Stecher, G., Li, M., et al., MegaX: Molecular evolutionary genetics analysis across computing platforms, Mol. Biol. Evol., 2018, vol. 35, pp. 1547–1549. https://doi.org/10.1093/molbev/msy096
Malferrari, G., Monferini, E., DeBlasio, P., et al., High-quality genomic DNA from human whole blood and mononuclear cells, BioTechniques, 2002, vol. 33, no. 6, pp. 1228–1230. https://doi.org/10.2144/02336bm09
Masi, M., Cimmino, A., Boari, A., et al., Colletochlorins E and F, new phytotoxic tetrasubstituted pyran-2-one and dihydrobenzofuran, isolated from Colletotrichum higginsianum with potential herbicidal activity, J. Agric. Food Chem., 2017, vol. 65, pp. 1124–1130. https://doi.org/10.1021/acs.jafc.6b05193
Masi, M., Zonno, M.C., Cimmino, A., et al., On the metabolites produced by Colletotrichum gloeosporioides a fungus proposed for the Ambrosia artemisiifolia biocontrol; spectroscopic data and absolute configuration assignment of colletochlorin A, Nat. Prod. Res., 2018, vol. 32, pp. 1537–1547. https://doi.org/10.1080/14786419.2017.1385020
McPartland, J.M., Clarke, R.C., and Watson, D.P., Hemp Diseases and Pests: Management and Biological Control, Cambridge: CAB International, 2000.
Mel’nik, V.A., Popov, E.S., and Shabunin, D.A., Contributions to the studies of mycobiota in Novgorod and Pskov oblasts. II. Coelomycetes, Mikol. Fitopatol., 2008, vol. 42, no. 1, pp. 43–52.
Minh, B.Q., Nguyen, M.A., and von Haeseler, A., Ultrafast approximation for phylogenetic bootstrap, Mol. Biol. Evol., 2013, vol. 30, pp. 1188–1195. https://doi.org/10.1093/molbev/mst024
Nguyen, L.T., Schmidt, H.A., von Haeseler, A., et al., IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies, Mol. Biol. Evol., 2015, vol. 32, no. 1, pp. 268–274. https://doi.org/10.1093/molbev/msu300
Nitzan, N., Lucas, B.S., Christ, B.J., Colonization of rotation crops and weeds by the potato black dot pathogen Colletotrichum coccodes, Am. J. Pot Res., 2006, vol. 83, pp. 503–507. https://doi.org/10.1007/BF02883511
Pfirter, H. and Defago, G., The potential of Stagonospora sp. as a mycoherbicide for field bindweed, Biocontrol Science and Technology, 1998, vol. 8, pp. 93–101.
Réblová, M., Gams, W., Seifert, K.A., Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales, Stud. Mycol., 2011, vol. 68, pp. 163–191. https://doi.org/10.3114/sim.2011.68.07
Redman, R.S., Dunigan, D.D., Rodriguez, R.J., Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader?, New Phytol., 2001, vol. 151, pp. 705–716. https://doi.org/10.1046/j.0028-646x.2001.00210.x
Sanger, F., Nicklen, S., Coulson, A.R., DNA sequencing with chain-terminating inhibitors, Proc. Natl. Acad. Sci. USA, 1977, vol. 74, no. 12, pp. 5463–5467. https://doi.org/10.1073/pnas.74.12.5463
Tamura, K., Nei, M., Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees, Mol. Biol. Evol., 1993, vol. 10, pp. 512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
Vaidya, G., Lohman, D.J., Meier, R., SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information, Cladistics, 2011, vol. 27, no. 2, pp. 171–180. https://doi.org/10.1111/j.1096-0031.2010.00329.x
Varivoda, O.P. and Maslennikova, E.S., Assessment and selection of source material for creating melon hybrids with integrated resistance to anthracnose and powdery mildew, Ovoshi Rossii, 2019, vol. 5, pp. 20–24. https://doi.org/10.18619/2072-9146-2019-5-20-24
White, T.J., Bruns, T., Lee, S., et al., Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, in PCR Protocols: A Guide to Methods and Applications, Innis, M.A., et al., Eds., San Diego: Academic Press, 1990, pp. 315–322.
Zhang, N., Castlebury, L.A., Miller, A.N., et al., An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny, Mycologia, 2006, vol. 98, pp. 1076–1087.
ACKNOWLEDGMENTS
The authors are sincerely grateful to A.G. Koval for his assistance in collecting the affected plants.
Funding
The study was supported by the Russian Science Foundation (project no. 19-76-30005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Translated by E. Makeeva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazartsev, I.A., Gomzhina, M.M., Gasich, E.L. et al. Biodiversity of Colletotrichum spp. on Several Wild and Cultivated Plants. Biol Bull Rev 13 (Suppl 1), S59–S70 (2023). https://doi.org/10.1134/S2079086423070071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086423070071