Abstract
The paper describes the results of a complex study of phytoplankton carried out at the end of August to the first half of September 2020 in the southeastern part of the Barents Sea and the southwestern part of the Kara Sea simultaneously with the determination of hydrological and hydrochemical characteristics. The taxonomic list of microalgae found in the studied area included 35 representatives identified to species. Of these, 14 (40%) were found in both basins. In the Pechora Sea, the average number of phytoplankton in the water column varied from 10 650 to 41 840 cells/L, and the biomass varied from 71.04 to 300.55 μg/L; in the southwestern part of the Kara Sea, the values of these indicators were 3510–28 420 cells/L and 16.31–66.96 μg/L, respectively. Both communities were at the autumn stage of seasonal succession; in terms of biomass, forms of arcto-boreal origin, predominantly oceanic, prevailed, and in the list of dominants, large-celled centric diatoms and dinoflagellates accounted for equal proportions. Species not exhibiting high abundance values were distinguished by a great degree of patchiness in spatial distribution: they were present at a small number of stations and not in all seawater layers. Thus, the results of the comparative analysis allow us to assert that the pelagic algocenoses of the compared regions, despite the difference in the hydrological parameters, were characterized by a significant degree of similarity. In general, the situation described confirms the hypothesis of the floristic unity of the southeastern part of the Barents Sea and the southwestern part of the Kara Sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Biogeographic studies of marine phytoplankton have a history of more than a century, but so far there is no single methodological concept in this area of hydrobiology. And although the assertion that the water masses of the World Ocean is a unity of the environment and the biota living in it is recognized as obvious, often each area within the boundaries of marine water bodies is considered as a separate structure with a pelagic algoflora inherent only to it. This approach, called “biotopic” (Moiseev, 1986), assumes the identification of some abiotic characteristics of relatively homogeneous areas of the water area by similarity, which are considered phytogeographic regions on this basis.
There is also a directly opposite view on the conduct of floristic research: similar areas are identified by juxtaposition of all available taxonomic lists of microalgae in the literature (Okolodkov, 2000; Ilyash and Zhitina, 2009). It looks more adequate to the task, but in fact it has the same main drawback as the biotope described above—the artificial separation of the environment and biota. As a result, the overall high species diversity and vast ranges of most organisms lead to not entirely correct results. This is clearly manifested in attempts to identify biogeographic regions in the Arctic basin. Practically all communities phytoplankton inhabiting the gyres of the Greenland, Norwegian, and Barents seas and the southwestern part of the Kara Sea largely originate from the same source, namely, from the large-scale Subarctic circulation located in the North Atlantic south of Greenland (Vinogradova and Gruzov, 1990). The bringing of rich cosmopolitan flora from the North Atlantic to the Arctic creates a high level of taxonomic similarity. And then, on the basis of only the species composition, we can make a paradoxical conclusion that in all the listed water areas we are dealing with the same algocenosis. A similar point of view is found in the literature (Guillard and Kilham, 1977; Heimdal, 1989); moreover, there is even a concept that considers the entire World Ocean as a biotope, and the entire phytoplankton as a community (Williams et al., 1981)—however, the latter term is rejected by the authors themselves, but the essence of this does not change.
In the current situation, the most promising is the comparison of the algoflora of nearby areas geographically belonging to different water bodies. A good example of these are the southeastern part of the Barents Sea (SEBS) and the southwestern part of the Kara Sea (SWKS). The question of the unity of their biogeographic status was already considered earlier (Druzhkov and Makarevich, 1999). Both approaches described above gave grounds for this assumption: these areas are characterized by a high similarity of hydrological and ice regimes, as well as microalgae species lists (Grönlund et al., 1994, 1995, 1997; Matishov et al., 1996; Druzhkov et al., 1997). The low degree of interannual variability of both abiotic environmental parameters and biological indicators is also important (Loeng, 1989; Izmenchivost’…, 2004; Larionov, 2016; Sukhanova et al., 2017).
However, in order to avoid the above disadvantages, at least two conditions must be met when making such a comparison. Firstly is the use of methodological procedures of terrestrial phytocenology. In studies of continental natural communities, they are identified on the basis of a comparison not of the entire species composition of organisms, but of groups of dominants, primarily edificatory species, which are almost always flowering plants. In pelagic ecosystems, these are namely representatives of the dominant groups of phytoplankton. Being the main primary producers of organic matter, they determine the overall productivity of the water area, cause “blooming” of seawater, and also, because of different metabolism, affect most hydrochemical parameters. Ultimately, they turn out to be an environment-forming factor for the remaining components of the biocenosis, forming a system of consortive relationships and, to a large extent, the entire structure of the ecosystem (Khailov et al., 2005).
Secondly, the correct choice of the season for conducting research is necessary. The described taxonomic similarity of Arctic algocenoses is a characteristic feature of the winter stage of development with a predominance of cryoflora representatives and the spring stage, when the maximum biomass is actually created by one set of diatom species of the ice-neritic complex (Makarevich and Druzhkova, 2007). Therefore, for a reliable comparison of the composition of microalgae, observations should be made in the final phase of the annual succession cycle, in the late summer and autumn seasons (Wang et al., 2018).
The aim of our work was to test the possible unity of the phytogeographic status of two geographically different water areas (the southeastern part of the Barents Sea and the southwestern part of the Kara Sea) on the basis of a comparative analysis of phytoplankton communities in these areas at the end of the growing season.
MATERIALS AND METHODS
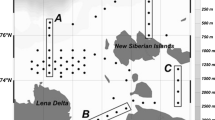
Observations were made in the southeastern part of the Barents Sea and the southwestern part of the Kara Sea (Fig. 1) at the end of August to the first half of September 2020 during the voyage of the R/V Dalnie Zelentsy (Reisovyi otchet…, 2021).
The temperature and salinity of water masses were determined using an SBE 19plus STD probe. Water samples for phytoplankton and chlorophyll a content and biogenic elements were sampled from the same 10-L Niskin bottles of the ROSETTE HydroBios MWS-12 complex immediately after hydrological sounding. Sampling was performed at six stations in the Pechora Sea and eight stations in the Kara Sea from the surface, intermediate, and near-bottom horizons.
The obtained material was processed according to standard hydrobiological methods: phytoplankton samples with a volume of 1–2 L concentrated by back filtration to a final volume of 4–5 mL and fixed in 40% formaldehyde solution (final concentration was 2–4%) for subsequent microscopy (Sukhanov, 1983). Taxonomic identification of organisms and cell counts were performed under an Amplival light microscope (with magnification ×400) in a Nageotte counting chamber with a volume of 0.05 mL according to the standard methodical scheme (Fedorov, 1979). The names of species and systematic groups are given according to the nomenclature adopted in electronic sources: AlgaeBase (https://www.algaebase.org/), WoRMS (http://www.marinespecies.org). The biomass was calculated using tables of average cell volumes of microalgae (Makarevich et al., 1993).
Seawater samples were filtered for the content of pigments (volume 3–5 L) in the ship’s laboratory using a vacuum filtering unit (pump manufactured by GAST, United States). We used membrane filters with a working surface diameter of 47 mm and a pore size of 0.6 μm. The concentration of chlorophyll a was determined by the spectrophotometric method (GOST, 2001). The extract was studied on a Nicolett Evolution 500 UV-Visible spectrophotometer (Spectronic Unicam, United Kingdom).
In parallel, hydrochemical studies were carried out: the content of dissolved oxygen in water and mineral forms of the main biogenic elements were assessed. The oxygen concentration was determined by the Winkler method; nitrites, nitrates, phosphates, and silicates were measured on a PE-5300VI photoco-lorimeter. Inorganic dissolved phosphorus (P-\({\text{PO}}_{4}^{{3 - }}\)) was determined by the Morphy-Riley method, dissolved silicon (Si-\({\text{SiO}}_{3}^{{2 - }}\)) was determined by the Korolev method, and nitrite (N-\({\text{NO}}_{2}^{ - }\)) and nitrate nitrogen (N-\({\text{NO}}_{3}^{ - }\)) was determined by the method of Bendschneider and Robinson (Rukovodstvo…, 1993).
RESULTS
Hydrological and Hydrochemical Parameters
The distribution of the main hydrological indicators in the plane of the sections of the studied areas is shown in Figs. 2 and 3. The southwestern part of the Kara Sea was characterized by a distinct pycnocline located at a depth of 10–15 m in the mouth area of Baydaratskaya Bay (stations 4 and 6) and at a depth of 25–30 m in the rest of the water area. The total range of temperature fluctuations was from –1.51 to +11.39°C, and the salinity varied from 29.08 to 34.38 psu; in the upper mixed layer, the minimum values reached +8.85°C, and the salinity was 32.87 psu. At all stations, except for the fourth and sixth, a layer of water with below-zero temperature and a slightly lower salinity was found in the depth range from 35 to 60 m. It is formed by the Kara Sea proper water mass, which forms in winter as a result of convective mixing (Fomin and Petrov, 1985; Zatsepin et al., 2010). Below are warmer and more saline waters, most likely of Barents Sea origin (Zatsepin et al., 2010).
In the Pechora Sea, owing to shallow depths, the distribution of temperature and salinity was almost uniform, and the range of variability of their values was 8.81–10.94°C and 27.52–31.56 psu, respectively. At the same time, the minimum temperature values and the maximum salinity values were recorded only in the near-bottom horizon at station 21, located on the eastern edge of the section, where the influence of transformed Atlantic waters is very weak (Byshev et al., 2003).
A characteristic feature of the distribution of hydrochemical parameters in the waters of the southwestern part of the Kara Sea was the presence of a significant vertical gradient of values with an intermediate minimum in the pycnocline layer and a maximum near the bottom (Table 1). The concentration of silicon in the surface layer varied from 0.70 to 1.91 µg-at/L; its highest content was noted at station 8, the deepest one in this area. The remaining biogenic elements studied were characterized by low values in the zero horizon and their drop in the density jump layer to analytical zero, which may be a consequence of only the photosynthetic activity of autotrophic biota. Nitrate nitrogen was a limiting factor for phytoplankton: at a zero level of nitrates, there was some excess of phosphates and silicates, and the N/Si ratio did not exceed 1.0 on average. The minimum value of the last characteristic (0.04) was recorded in the surface layer at station 10 (at a low level of silicates: 0.89 µg-at/L).
Also, in the distribution of biogens in this part of the water area, some mosaicism was revealed: in the zero horizon at stations 7 and 8, an area of low concentrations of both phosphates and nitrates was distinguished, and at station 6, on the contrary, their content was the highest. At the same time, the Si/P ratio at the first two stations averaged 51.8, and at the last one, 9.5: this situation clearly indicates the localization of various water masses in these areas (Khmelnitskaya, 2011).
In the Pechora Sea, the hydrochemical parameters were characterized by low values and homogeneity of spatial distribution (Table 1).
The maximum concentrations were found in the east of the region (stations 22, 23), in the zone of fresh runoff. The most significant correlations were found in the salinity–phosphate pairs (r = –0.79, R2 = 0.62) and “salinity–silicates” (r = –0.71, R2 = 0.52). The N/P ratio is low on average (0.6), which indicates a deficiency in nutrients (Ilyin et al., 1985). At stations 25 and 26, zero values of nitrites and nitrates were found at the surface and near the bottom, with the lowest silicon content for the horizon (1.73 µg-at/L at the bottom, 0.65 µg-at/L at 0 m).
The concentration of chlorophyll a in the surface layer of the Kara Sea water area did not exceed 0.45 mg/m3 (average 0.22 ± 0.15 mg/m3); in the pycnocline zone, the range of values of this indicator was from 0.15 to 0.41 mg/m3, while at some stations they turned out to be lower than the sensitivity of the applied method; in the near-bottom horizon, a similar situation was observed at most stations (Table 2). In the Pechora Sea, owing to shallow depths, almost complete uniformity was observed in the distribution of pigment throughout the water column. In the surface layer, the content of chlorophyll a averaged 0.92 ± 0.29 mg/m3; in the density jump layer, it averaged 1.15 ± 0.46 mg/m3; in the near-bottom layer, it averaged 0.99 ± 0.22 mg/m3. Low average pigment content in the photic layer (in SWKS, 0.24 ± 0.13 mg/m3; in SEBS, 1.02 ± 0.33 mg/m3) and the proportion of pheophytin >50% in both water bodies characterize the late summer and autumn stages of the seasonal succession of pelagic algocenoses.
Qualitative and Quantitative Indicators of Phytoplankton Development
A complete taxonomic list of microalgae found in the studied water area is given in Table 3. It includes 35 organisms identified to the species level, as well as unidentified representatives of several genera and large taxa of various ranks. According to the systematic position, 15 species belong to the class Bacillariophyceae, 16 belong to the class Dinophyceae; the other 4 go to the class Ebriophyceae (Ebria tripartita), Dictyochophyceae (Octactis speculum), Prasinophyceae (Polyasterias problematica), and Pyramimonadophyceae (Halosphaera viridis). In terms of phytogeographic affiliation, 10 species are of arcto-boreal origin, 9 are of boreal origin, and 16 are cosmopolitans. In ecological terms, 21 species were characterized as neritic, 7 as oceanic, and 7 as panthalassic.
A logical question may be the absence of coccolithophorids (small flagellates from the class Prymnesiophyceae) in this list. This group has especially attracted the attention of researchers over the past twenty years, since it regularly forms powerful blooms in the Barents Sea basin in summer, which is usually explained by increased inflow of Atlantic waters (Sergeeva et al., 2020; Pautova, 2021). We did not find these organisms, most likely for methodological reasons. When fixed with formalin, coccolithophores very quickly lose their calcareous plates, and their reliable identification becomes impossible. However, the cells themselves are not destroyed, and therefore they are counted with other flagellates as “unidentified flagellates.” And here it should be noted that this group in none of the water bodies at any station composed a significant share in the total number of microalgae, and therefore, coccolithophorids, at least in the autumn season, do not play a significant role in the algocenoses of the studied areas. This fact can be fully explained by the fact that both in the SWKS and in the SEBS, the influence of Atlantic waters is extremely weak (Nikiforov et al., 2003; Zatsepin et al., 2010), and even over the past decades no tangible changes have been observed either in the ice regime, or in the hydrological environment, or in the taxonomic composition of phytoplankton (Pautova, 2003, 2021; Izmenchivost’…, 2004; Sukhanova et al., 2011; Larionov, 2016).
At the same time, a comparison of two sections of the study area revealed the following features. Among the microalgae found throughout the water area (almost every one of them had very similar quantitative indicators in both areas under consideration); five species of diatoms, eight dinophytes, and one representative Ebri classophyceae (Ebria tripartita) were detected. Five forms had Arkto-boreal origin, one had boreal origin, and eight had cosmopolitan origin. According to the ecological characteristics, nine neritic species, three oceanic, and two panthalassic species were noted.
Among the organisms recorded only in the southwestern part of the Kara Sea, diatoms and dinoflagellates composed equal shares (six species each), and representatives of other systematic groups were also present: Octactis speculum, Polyasterias problematica, Halosphaera viridis. The phytogeographic structure of this algocenosis included four arcto-boreal species, three boreal species, and eight cosmopolitans; the ecological structure included seven neritic forms and four each of oceanic and panthalassic forms. In the Pechora Sea, among the species found only in this reservoir, there were four representatives of diatoms and two representatives of dinophytes; five forms were of boreal origin and one was of arcto-boreal origin; five species belonged to the neritic form and one belonged to the panthalassic form.
The described indicators indicate that, in general, the communities of pelagic microalgae in the studied part of the Arctic basin were characterized by the complete predominance of representatives of two classes—Bacillariophyceae and Dinophyceae—approximately at the same ratio. At the same time, organisms of all three phytogeographic and three main ecological groups characteristic of this region were recorded, with the exception of freshwater and reliably identified forms of microphytobenthos. The largest share in algocenoses was cosmopolitans, which, however, were absent among the species found only in the Pechora Sea. Representatives of the neritic algal flora predominated throughout the studied area, while panthalassic and oceanic forms played a much smaller role (organisms of the latter group were not recorded at all in the Pechora Sea area). All of the above features can generally be considered typical of the Arctic pelagic ecosystems of the northern seas. Neither in the taxonomic, nor in the phytogeographic, nor in the ecological structures were significant differences found between the algocoenoses of the Barents and Kara Sea pelagic zone. The list of species found only in the Pechora Sea is poorer, but this may be due to the fact that observations were made only in one rather narrow coastal area of the reservoir (Larionov, 2016).
We see a somewhat different picture when considering the spatial distribution of the dominant forms of microalgae. Among them, small, unidentified representatives of diatoms, dinophytes, and euglena algae stand out in terms of abundance, reaching high concentrations throughout the entire studied area. In addition to them, diatoms Thalassiosira decipiens, Thalassionema nitzschioides, and Paralia sulcata make up a significant proportion in the Barents Sea area. In the Kara Sea waters, the level of their abundance is much lower, and the first species is absent altogether. In terms of biomass, the leading position is occupied by the following organisms: in the entire studied area, Chaetoceros borealis and Tripos longipes; occurring in both areas, but prevailing in this indicator only in the Kara Sea, Dinophysis norvegica and Scrippsiella trochoidea; only in the Pechora Sea, Paralia sulcata, Thalassionema nitzschioides, and Tripos fusus. Also among the dominants registered only in the Kara Sea waters are Leptocylindrus danicus, Tripos arcticus, Gonyaulax sp., Gyrodinium lachryma, Protoperidinium brevipes, and Halosphaera viridis; only in the Barents Sea waters are Thalassiosira decipiens and Tripos horridus. Thus, quite pronounced differences between the considered algocenoses can be traced: the main part of the biomass of microalgae is formed by their various representatives, including those developing in any one of the water bodies. In addition, if in the community of the Kara Sea there are no forms that are noticeably predominant in abundance, and among the species found only in this area, there are a large number of leaders in biomass, then in the Pechora Sea the situation is reversed.
At the same time, attention is drawn to a clearly manifested feature of the spatial distribution, which is characteristic of all identified phytoplankton organisms without exception: none of them was recorded at all stations of the studied basin. Even forms that unquestionably dominated in terms of abundance or biomass reached significant average values of these parameters owing to very high concentrations only in small areas of water bodies, often at two or three stations, while in the rest of the water area their abundance levels were minimal or zero. However, the assemblages of species found only in one of the two compared areas differ in this respect. In the southwestern part of the Kara Sea, there are also no representatives of the algocoenosis that would be present at each station, while in the Pechora Sea this feature is not absolute: all dominants were recorded throughout the entire studied area of the reservoir.
As for the general quantitative indicators of phytoplankton development, they also reveal differences between the communities of the studied areas of the Pechora and Kara seas (Table 4, Figs. 4, 5). In general, the range of fluctuations in the values of both characteristics over the water area was small, with the exception of algocenoses at stations 4 and 6, located in the mouth area of Baidaratskaya Bay. Moreover, if diatoms Skeletonema costatum completely predominate in abundance there and to a lesser extent Leptocylindrus danicus and L. minimus (only at station 4, at station 6 both species are absent), then the biomass is dominated by dinoflagellates Tripos longipes, and dinophytes Gonyaulax sp. and Dinophysis norvegica and diatoms Chaetoceros borealis, L. danicus (at station 4), and Rhizosolenia hebetata (encountered only at these two stations and only in the zero horizon) play a secondary role. Thus, there is another manifestation of the mosaic nature of the spatial distribution of microalgae.
Comparison of the total values of quantitative indicators of phytoplankton development in the two considered sites revealed (without taking into account the community at station 4) a higher level of both in the Pechora Sea, on average, by about a factor of 2. For the same region, a greater scatter between observation points was also noted.
In the vertical structure of pelagic algocenoses, there are no noticeable differences between stations and water bodies (again, with the exception of station 4). In the Barents Sea region, it can be considered homogeneous in the entire water column. The Kara Sea area was characterized by very similar values of the abundance of organisms in the surface layer and at the depth of the density jump, and significantly lower values in the near-bottom horizon. The latter fact can be fully explained by differences in relief: the studied area of the southwestern part of the Kara Sea was characterized by depths of the order of 100 m or more, while in the shallow Pechora Sea, their values were in the range of 12–22 m.
DISCUSSION
It is known that the inflow of Atlantic waters has a significant impact on the hydrometeorological regimes of the southeastern part of the Barents Sea. (Grönlund et al., 1995, 1997; Matishov et al., 1996) and the southwestern section of the Kara Sea (Stepanov, 1979). The region under consideration is characterized by a complex dynamics of currents formed by three main circulations. The first is the cyclonic (counterclockwise) circulation of the Pechora Sea, which is especially pronounced in the spring–summer period. (Grönlund et al., 1995, 1997; Arkhipov and Popov, 1996; Matishov et al., 1996); the second is a clockwise circulation, enveloping the coast of the Novaya Zemlya archipelago (Batskikh and Denisov, 1995); the third is the cyclonic gyre, which occupies the entire southwestern part of the Kara Sea (Stepanov, 1985). The result is a stable and diverse network of water exchange between the Pechora and Kara seas, which is carried out through the straits of the Kara Gate and Yugorsky Shar. There is also intense ice exchange between these two basins, but it is seasonal (Loeng, 1991). There is every reason to assume that there is also a drift of organisms, as a result of which (given that the transfer of water from west to east is predominant) it is the phytoplankton of the Pechora Sea that plays a decisive role in the formation of the pelagic algoflora of the entire Novaya Zemlya phytogeographic province (Druzhkov and Makarevich, 1999).
An analysis of the results of past studies conducted in these areas generally confirms this hypothesis. It shows that the communities of planktonic microalgae in the southeastern part of the Barents Sea and the southwestern part of the Kara Sea are formed mainly by arcto-boreal neritic species with a high proportion of cosmopolitans. At the same time, the predominance of arcto-boreal forms in the pelagic zone is a characteristic feature not only of the Barents and Kara seas but also of other marginal basins of the Arctic. Moreover, in all these water areas there is a great similarity in the composition of the dominant complex of organisms (Guillard and Kilham, 1977; Heimdal, 1989; Druzhkov and Makarevich, 1996). The remaining ecological and phytogeographic groups do not occupy a leading position in the ecosystems of these areas. The presence of boreal (warm-water) elements in them can be explained by the influence of transformed Atlantic waters. The contribution of the cryophilic flora (mainly pennate diatoms) to the communities of the SEBS and SWKS is insignificant, and in the open water summer period, it is minimal, since the ice cover here is seasonal (Stepanov, 1985). The main differences in the taxonomic composition of pelagic algocenoses in these areas are found among microphytobenthic and freshwater forms, but these are allochthonous and uncharacteristic components, and therefore their consideration can be neglected.
Our observations covering the winter and spring periods since the 1980s (Makarevich, 1998; Makarevich and Druzhkova, 2010) also indicate the similarity of communities in these water bodies. The timing of the start of vegetation and the set of dominant forms in the Pechora Sea and the southwestern part of the Kara Sea are very close; the differences are only in the presence of single specific taxa for individual areas and in small deviations in the calendar dates of the onset of the maximum phytoplankton bloom.
However, all the considered materials have one significant drawback: they hardly affect the summer and autumn phases of the annual succession cycle of algocenosis development. In fact, the conclusion about the uniformity of their composition in these areas is based mainly on a comparison of species complexes that form the peak of spring flowering. Such an approach can be justified by the fact that it is these representatives that absolutely dominate in the pelagic zone in terms of abundance and/or biomass. And it ultimately leads to a logical conclusion about the uniformity of the unicellular algoflora of all northern seas, called panarctic (Guillard and Kilham, 1977) or ice-neritic (Vinogradova and Gruzov, 1990). Moreover, even in those studies that include a complete list of organisms, the same technique is used: the forms characteristic of the summer–autumn period are ignored, since they do not form abundance levels comparable to spring ones, and are characterized by a mosaic spatial distribution, occurring not on the entire area of the water body and only in some areas reaching high concentrations. However, one should not forget that such a pattern is generally typical of marine phytoplankton communities that are in the phase of mixed synthesis of the annual production cycle (Makarevich et al., 2012). Species leading in terms of quantitative indicators at this stage, even if for a short time and in a limited water area, should nevertheless be recognized as dominants and, as such, be included in a comparative floristic analysis.
In this regard, the results of the work presented in this article are of particular value, since they make it possible to fill this gap and make the necessary adjustments to determine the biogeographic status of the study areas. The previous section clearly shows significant differences between the waters of the SEBS and SWKS in terms of hydrological and hydrochemical parameters. The reasons for this are obvious: the difference in depths and dynamics of currents. The consequence, in turn, is the differences in the absolute values of the abundance and biomass of microalgae. Against this background, the revealed similarities of the compared communities will undoubtedly indicate their closeness.
One of these, of course, should be considered a characteristic of the seasonal state of phytoplankton. Pelagic algocenoses of both water bodies, according to all signs, were at the end of the summer to the beginning of the autumn stage of succession. The thermohaline structure of the water column fully corresponded to this phase of the annual hydrological cycle, which was quite well studied both in the Pechora Sea (Nikiforov et al., 2003) and in the area of the Kara Sea area under consideration (Zatsepin et al., 2010). The average and limit values of nutrient concentrations were typical of the specified season and did not show noticeable differences from the studies described in this period of time in different years for the Barents Sea basin (Pozdnyakova and Vinogradov, 1966; Makkaveev et al., 2003; Sergeeva et al., 2018) and for the Kara Sea (Shirokolobov, 1982; Makkaveev and Stunzhas, 1994; Makkaveev et al., 2010; Morozova et al., 2013). The levels of chlorophyll a and the structure of its spatial distribution also almost coincide with those published in the literature based on the materials of observations of different years carried out in September in the SEBS (Vedernikov et al., 2001, 2003) and the SWKS (Vedernikov et al., 1994; Mosharov, 2010).
A similar result is obtained by comparing the qualitative and quantitative characteristics of phytoplankton with those given earlier for the same season in the Pechora Sea (Makarevich, 1996; Pautova, 2003; Larionov, 2016) and the southwestern part of the Kara Sea (Druzhkov and Makarevich, 1996; Sukhanova et al., 2017). Both the general taxonomic lists and the sets of dominant species and the average absolute values of abundance and biomass are consistent. From this, by the way, we can conclude that the interannual variability of these indicators is weak. The reason for this is a long-established fact: the range of long-term fluctuations in climatic factors, primarily the timing of the formation and melting of ice cover, is extremely small in these areas of the basin (Loeng, 1989; Zubakin, 1987; Izmenchivost’ prirodnykh uslovii…, 2004).
On the basis of the foregoing, according to the results of a comparison of the two communities under consideration, it seems necessary to highlight the following important features.
(1) In both studied algocenoses, microalgae belonging to the spring species complex are completely absent, even in single quantities.
(2) Of the total number of identified organisms, 40% were recorded in the pelagic zone of both areas under consideration.
(3) Among the phytoplankters recorded both in the Southwestern Kara Sea and the Southeastern Barents Sea, only forms of arcto-boreal origin, predominantly oceanic, were in the lead in terms of biomass. Among those found only in the Pechora Sea are boreal neritic; only in the Kara Sea are cosmopolitans and arcto-boreal species from various ecological groups.
(4) In the list of dominants, both common to both water bodies and found only in one of them, large-celled centric diatoms and dinoflagellates accounted for almost equal shares.
(5) Microalgae, which did not reach high abundance values, were distinguished by a greater “mosaic” degree of spatial distribution: they are present at a smaller number of stations and not at all horizons.
Thus, the analysis allows us to state that the pelagic algocenoses of the compared regions, despite the difference in hydrological parameters, were at the same stage of seasonal succession and were characterized by a significant similarity in their qualitative composition, including ecological and phytogeographic affiliation. An important feature of the summer and even more so autumn phases of their annual succession cycle is the absence of species that far outnumber the others in quantitative indicators over a vast area of the water area. It is this picture that we observe in the presented material: against the background of the general taxonomic diversity, a relatively large number of forms dominate in small areas of the water area. At the same time, the differences in the composition of microalgae within the same water body are not less but even more than those between them.
CONCLUSIONS
The described situation as a whole confirms the hypothesis about the floristic unity of the Pechora Sea and the southwestern part of the Kara Sea pelagic zone. At the same time, it should be especially emphasized that such confirmation was obtained on the basis of material that practically excludes alternative explanations. The hydrological and hydrochemical parameters of the considered water bodies in the autumn of 2020 were characterized by significant differences, which caused different levels of abundance of organisms, but at the same time did not affect the qualitative composition of the algoflora. The selected season of observations determined the presence of communities at the stage of succession with the maximum species diversity and high mosaic distribution of most species; despite this, they clearly showed similarities in the set of dominants and the ratio of forms of different origin.
As a result, we can conclude that the main mechanism of floristic integration of the southeastern part of the Barents Sea and the southwestern part of the Kara Sea is a stable intense water exchange between these areas. Nevertheless, further research and comparison with the description of the summer–autumn state of pelagic algocenoses in other water bodies of the Arctic Basin is necessary for the final verification of the hypothesis under discussion: at the moment, these data are few or completely absent.
REFERENCES
Arkhipov, B.V. and Popov, S.K., Modeling of density and wind currents in the southeastern part of the Barents Sea, Okeanologiya, 1996, vol. 36, pp. 805–813.
Batskikh, Yu.M. and Denisov, V.V., Ice and icebergs, in Sreda obitaniya i ekosistemy Novoi Zemli: Arkhipelag i shel’f (Habitat and Ecosystems of Novaya Zemlya: Archipelago and Shelf), Apatity: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 1995, pp. 29–35.
Byshev, V.I., Galerkin, L.I., Galerkina, N.L., and Shcherbinin, A.D., Dynamics and structure of waters, in Pechorskoe more: Sistemnye issledovaniya (Pechora Sea: System Research), Moscow: MORE, 2003, pp. 93–116.
Druzhkov, N.V. and Makarevich, P.R., Spatio-temporal organization of pelagic phytocenosis in open shelf waters of the Western Arctic (Kara Sea), in Ekosistemy pelagiali morei Zapadnoi Arktiki (Ecosystems of the Pelagial of the Western Arctic Seas), Apatity: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 1996, pp. 37–72.
Druzhkov, N.V. and Makarevich, P.R., Comparison of the phytoplankton assemblages of the southeastern Barents Sea and southwestern Kara Sea: Phytogeographic status of the regions, Bot. Mar., 1999, vol. 42, pp. 103–115.
Druzhkov, N.V., Grönlund, L., and Kuznetsov, L.L., The phytoplankton of the Pechora Sea, the Pechora Bay and the Cheshskaya Bay, in Pechora Sea Ecological Studies in 1992–1995: Final Report, Helsinki: Finnish-Russian Offshore Technology Working Group. Rep. B13, 1997, pp. 41–52.
Fedorov, V.D., O metodakh izucheniya fitoplanktona i ego aktivnosti (Methods of Studying Phytoplankton and Its Activity), Moscow: Mosk. Gos. Univ., 1979.
Fomin, O.K. and Petrov, V.S., The role of natural factors in the distribution of plankton biomass in the Kara Sea, Priroda Khoz. Severa, 1985, vol. 13, pp. 34–45.
Grönlund, L., Kuznetsov, L.L., Fomin, O.K., Leppanen, J.-M., and Makarevich, P.R., Hydrography, chemistry, chlorophyll-a, phytoplankton and zooplankton species composition and biomass in the pechora sea in 1993, in Pechora Sea Ecological Studies in 1993, Helsinki: Finnish-Russian Offshore Technology Working Group. Rep. B5, 1994, pp. 3–34.
Grönlund, L., Kuznetsov, L.L., Fomin, O.K., Leppanen, J.-M., and Larionov, V.V., Plankton production, nutrient concentration and hydrography at the Pechora Sea in 1994, in Pechora Sea Ecological Studies in 1993, Helsinki: Finnish-Russian Offshore Technology Working Group. Rep. B6, 1995, pp. 3–30.
Grönlund, L., Kuznetsov, L.L., and Druzhkov, N.V., Hydrology of the Pechora Sea, the Pechora Bay and the Cheshskaya Bay, in Pechora Sea Ecological Studies in 1992–1995: Final Report, Helsinki: Finnish-Russian Offshore Technology Working Group. Rep. B13, 1997, pp. 15–28.
Guillard, R.R.L. and Kilham, P., The ecology of marine planktonic diatoms, in The Biology of Diatoms, Oxford: Blackwell, 1977, pp. 372–469.
Heimdal, B.R., Arctic ocean phytoplankton, in The Arctic Seas: Climatology, Oceanography, Geology and Biology, New York: Van Nostrand Reinhold, 1989, pp. 123–146.
Il’in, G.V., Nesvetova, G.I., Petrov, V.S., and Tsekhotskaya, L.K., Biogenic elements and oxygen regime, in Zhizn’ i usloviya ee sushchestvovaniya v pelagiali Barentseva morya (Life and Its Conditions in the Pelagial of the Barents Sea), Apatity: Kol’sk. Filial Akad. Nauk SSSR, 1985, pp. 46–63.
Il’yash, L.V. and Zhitina, L.S., Comparative analysis of sea-ice diatom species composition in the seas of Russian Arctic, Zh. Obshch. Biol., 2009, vol. 70, pp. 143–154.
Izmenchivost’ prirodnykh uslovii v shel’fovoi zone Barentseva i Karskogo morei (Variability of Natural Conditions in the Shelf Zone of the Barents and Kara Seas), Danilov, A.I., Ed., St. Petersburg: Arkt. Antarkt. Nauchno-Issled. Inst., 2004.
Khailov, K.M., Yurchenko, Yu.Yu., and Snegirev, S.M., Ot rasteniya k noosfere: Antiuchebnik (From Plant to Noosphere: Anti-Textbook), Odessa: Druk, 2005.
Khmel’nitskaya, O.K., Principal hydrochemical parameters of intermediate and bottom water masses of the North Atlantic, Vestn. Mosk. Univ., Ser. 5: Geogr., 2011, no. 6, pp. 60–66.
Larionov, V.V., The annual development cycle of phytoplankton communities in various areas of the Pechora Sea, in Sovremennye ekologicheskie, biologicheskie i khimicheskie issledovaniya, tekhnologii i produktsionnye tekhnologii. Mat-ly Mezhdunar. nauch.-prakt. konf. (Modern Ecological, Biological and Chemical Research, Technologies and Production Technologies. Proc. Int. Sci.-Pract. Conf.), Murmansk: Murmansk. Gos. Techn. Univ., 2016, pp. 196–202.
Loeng, H., Ecological features of the Barents Sea, Proc. 6th Conf. Com. Arctique Int., 13–15 May 1985, Leiden: E.J. Brill, 1989, pp. 327–365.
Loeng, H., Features of the physical oceanographic conditions of the Barents Sea, Polar Res., 1991, vol. 10, no. 1, pp. 5–18.
Makarevich, P.R., Phytoplankton communities, in Ekosistemy, bioresursy i antropogennoe zagryaznenie Pechorskogo morya (Ecosystems, Bioresources and Anthropogenic Pollution of the Pechora Sea), Apatity: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 1996, pp. 50–54.
Makarevich, P.R., Spring state of the microphytoplankton community in the southeastern Barents and southwestern Kara Seas in ice-covered areas, in Biologiya i okeanografiya Karskogo i Barentseva morei (po trasse Sevmorputi) (Biology and Oceanography of the Kara and Barents Seas (along the Northern Sea Route)), Apatity: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 1998, pp. 138–150.
Makarevich, P.R. and Druzhkova, E.I., Functioning of pelagic and cryopelagic ecosystems in the ice-covered areas of the Barents and Kara Seas, in Biologiya i okeanografiya Severnogo morskogo puti: Barentsevo i Karskoe morya (Biology and Oceanography of the Northern Sea Route: Barents and Kara Seas), Moscow: Nauka, 2007, pp. 50–63.
Makarevich, P.R. and Druzhkova, E.I., Sezonnye tsiklicheskie protsessy v pribrezhnykh planktonnykh al’gotsenozakh severnykh morei (Seasonal Cyclical Processes in Coastal Planktonic Algocenoses of the Northern Seas), Rostov-na-Donu: Yuzhn. Nauchn. Tsentr Ross. Akad. Nauk, 2010.
Makarevich, P.R., Larionov, V.V., and Druzhkov, N.V., Mean weights of dominant phytoplankton of the barents sea, Algology, 1993, vol. 13, pp. 103–106.
Makarevich, P.R., Druzhkova, E.I., and Larionov, V.V., Primary producers of the Barents Sea, in Diversity of Ecosystems, Rijeka: InTech, 2012, pp. 367–393. http://www.intechopen.com/books/diversity-of-ecosystems/primary-producers-of-the-barents-sea.
Makkaveev, P.N. and Stunzhas, P.A., Hydrochemical characteristics of the Kara Sea waters, Okeanologiya, 1994, vol. 34, pp. 662–667.
Makkaveev, P.N., Stunzhas, P.A., and Makkaveev, A.P., Hydrochemistry, in Pechorskoe more: Sistemnye issledovaniya (Pechora Sea: System Research), Moscow: MORE, 2003, pp. 134–170.
Makkaveev, P.N., Stunzhas, P.A., Mel’nikova, Z.G., Khlebopashev, P.V., and Yakubov, Sh.Kh., Hydrochemical characteristics of the waters of the western part of the Kara Sea, Oceanology, 2010, vol. 50, pp. 699–697.
Matishov, G.G., Il’in, G.V., and Matishov, D.G., General regularities of the oceanological regime and oceanological conditions of the ice-free period, in Ekosistemy, bioresursy i antropogennoe zagryaznenie Pechorskogo morya (Ecosystems, Bioresources and Anthropogenic Pollution of the Pechora Sea), Apatity: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 1996, pp. 25–39.
Moiseev, P.A., Biotopic approach to the study of biological resources of the World Ocean, in Biotopicheskaya osnova raspredeleniya morskikh organizmov (Biotopic Basis for the Distribution of Marine Organisms), Moscow: Nauka, 1986, pp. 3–6.
Morozova, O.A., Vesman, A.V., Dobrotina, E.D., Tarasenko, A.D., Shumskaya, N.K., et al., Hydrochemical structure of the Kara Sea waters in summer 2012, Probl. Arkt. Antarkt., 2013, no. 1 (95), pp. 61–71.
Mosharov, S.A., Distribution of the primary production and chlorophyll a in the Kara Sea in September of 2007, Oceanology, 2010, vol. 50, no. 6, pp. 884–892.
Nikiforov, S.L., Dunaev, N.N., Ogorodov, S.A., and Artem’ev, A.V., Physical and geographical characteristics, in Pechorskoe more: Sistemnye issledovaniya (Pechora Sea: System Research), Moscow: MORE, 2003, pp. 27–92.
Okolodkov, Yu.B., Dinoflagellates (Dinophyceae) of the Eurasian Arctic Seas, Doctoral (Biol.) Dissertation, St. Petersburg: Bot. Inst. Russ. Acad. Sci., 2000.
Pautova, L.A., Phytoplankton of the Pechora Sea, in Pechorskoe more: Sistemnye issledovaniya (Pechora Sea: System Research), Moscow: MORE, 2003, pp. 171–194.
Pautova, L.A., Phytoplankton of the Barents Sea, in Sistema Barentseva morya (System of the Barents Sea), Moscow: GEOS, 2021, pp. 217–230.
Pozdnyakova, L.E. and Vinogradov, V.I., Some features of the distribution of hydrochemical elements in the southeastern part of the Barents Sea in August–September 1959, in Sostav i raspredelenie planktona i bentosa v yuzhnoi chasti Barentseva morya (Composition and Distribution of Plankton and Benthos in the Southern Part of the Barents Sea), Moscow: Akad. Nauk SSSR, 1966, pp. 140–156.
Reisovyi otchet kompleksnoi ekspeditsii na NIS “Dal’nie Zelentsy” v Barentsevo more (10.03–14.04.2021) (Voyage Report of the Integrated Expedition to the R/V Dalnie Zelentsy in the Barents Sea), Makarevich, P.R., Ed., Murmansk: MMBI, 2021.
Rukovodstvo po khimicheskomu analizu morskikh vod (Guidelines for the Chemical Analysis of Sea Waters), Leningrad: Gidrometeoizdat, 1993.
Sergeeva, V.M., Zhitina, L.S., Mosharov, S.A., et al., Phytoplankton community structure in the polar front of the Eastern Barents Sea at the end of the growth season, Oceanology, 2018, vol. 58, no. 5, pp. 700–709.
Sergeeva, V.M., Sukhanova, I.N., Flint, M.V., et al., Phytoplankton of the St. Anna trough: Influence of abiotic factors, Oceanology, 2020, vol. 60, no. 4, pp. 458–472.
Shirokolobov, V.N., Hydrological and hydrochemical conditions of the southwestern part of the Kara Sea, in Kompleksnye issledovaniya prirody severnykh morei (Comprehensive Studies of the Northern Seas Nature), Apatity: Kol’skii Filial Akad. Nauk SSSR, 1982, pp. 7–17.
Stepanov, S.I., Spatial-temporal variability of salinity fields in the Kara Sea during the navigation period, Tr. Arkt. Antarkt. Nauchno-Issled. Inst., 1979, vol. 361, pp. 59–62.
Stepanov, S.I., Kara Sea water circulation during the navigation period, Tr. Arkt. Antarkt. Nauchno-Issled. Inst., 1985, vol. 389, pp. 43–45.
Sukhanova, I.N., Concentration of phytoplankton in the sample, in Sovremennye metody kolichestvennoi otsenki raspredeleniya morskogo planktona (Modern Methods of Quantitative Assessment of the Distribution of Marine Plankton), Moscow: Nauka, 1983, pp. 97–105.
Sukhanova, I.N., Flint, M.V., Sergeeva, V.M., and Kremenetskiy, V.V., Phytoplankton of the south-western part of the Kara Sea, Oceanology, 2011, vol. 51, no. 6, pp. 978–992.
Sukhanova, I.N., Flint, M.V., Georgieva, E.J., et al., The structure of phytoplankton communities in the eastern part of the Laptev Sea, Oceanology, 2017, vol. 57, no. 1, pp. 75–90.
Vedernikov, V.I., Demidov, A.B., and Sud’bin, A.I., Primary production and chlorophyll in the Kara Sea in September 1993, Okeanologiya, 1994, vol. 34, pp. 693–703.
Vedernikov, V.I., Gagarin, V.I., and Burenkov, V.I., Distribution of primary production and chlorophyll in the Pechora Sea in August–September 1998, Okeanologiya, 2001, vol. 41, pp. 69–79.
Vedernikov, V.I., Gagarin, V.I., and Vetrov, A.A., Primary production and chlorophyll, in Pechorskoe more: Sistemnye issledovaniya (Pechora Sea: System Research), Moscow: MORE, 2003, pp. 195–206.
Vinogradova, L.A. and Gruzov, L.N., On the biocenological zoning of the epipelagic of the North Atlantic and adjacent areas, Tr. Gos. Okeanogr. Inst., 1990, vol. 182, pp. 112–133.
GOST (State Standard) 17.1.4.02-90: Water. Spectrophotometric Determination of Chlorophyll a, Moscow, 2001.
Wang, Y., Xiang, P., Kang, J., Ye, Y., Lin, G., et al., Microphytoplankton community structure in the western Arctic Ocean: Surface layer variability of geographic and temporal considerations in summer, Hydrobiologia, 2018, vol. 811, pp. 295–312.
Williams, W.T., Bunt, J.S., John, R.D., and Abel, D.J., The community concept and the phytoplankton, Mar. Ecol. Progr. Ser., 1981. vol. 6, pp. 115–121.
Zatsepin, A.G., Zavialov, P.O., Kremenetskiy, V.V., Poyarkov, S.G., and Soloviev, D.M., The upper desalinated layer in the Kara Sea, Okeanology, 2010, vol. 50, no. 5, pp. 657–667.
Zubakin, G.K., Krupnomasshtabnaya izmenchivost’ sostoyaniya ledyanogo pokrova Severo-Evropeiskogo basseina (Large-Scale Variability in the State of the Ice Cover of the North European Basin), Leningrad: Gidrometeoizdat, 1987.
ACKNOWLEDGMENTS
We sincerely thank the staff of the Plankton Laboratory of the Murmansk Marine Biological Institute, Russian Academy of Sciences, for their help in collecting and processing the material.
Funding
This work was supported financially by the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of the welfare of animals. The article does not contain any studies involving animals in experiments performed by any of the authors.
Rights and permissions
About this article
Cite this article
Makarevich, P.R., Larionov, V.V., Vodopyanova, V.V. et al. Phytoplankton Communities in the Southeastern Barents Sea and the Southwestern Kara Sea as Indicators of the Phytogeographic Status of These Regions. Biol Bull Rev 13, 469–481 (2023). https://doi.org/10.1134/S2079086423050122
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086423050122