Abstract—
In three rodent species with different types of nutrition, a targeted “predator–prey” type interaction with mobile prey was revealed for the first time and described in detail. The granivorous striped field mouse, herbivorous narrow-skulled vole, and omnivorous Campbell’s dwarf hamster have an equally efficient, stereotypical hunting behavior that is in many ways similar to the behavior of the common shrew (specialized insectivorous species). At the same time, the hunting rate in rodents is lower than in insectivores. Unlike insectivorous species, rodents have a stereotypical hunting behavior that is manifested facultatively (completely, but not in all individuals). The portion of “hunters” in narrow-skulled voles is two times lower than that in striped field mice. The tactics of prey killing vary in different species: striped field mice, narrow-skulled voles, and shrews immobilize an insect with a series of quick bites; Campbell’s dwarf hamsters bite off limbs of the prey, which is apparently a manifestation of a more specialized hunting behavior. The nature of hunting attacks is different: first capturing the prey by teeth, rodents move to a capture with paws, while shrews use only teeth, which indicates a relative primitiveness of their predatory behavior. Campbell’s dwarf hamsters can start the attack with a capture using both teeth and paws, which characterizes their hunting behavior as the most evolutionarily advanced among the studied species. The stereotypes of hunting behavior in all three rodent species are manifested according to the principle “all at once” and are not affected by experience. The hunting behavior of rodents can be considered an evolutionarily stable strategy that supports the ability of populations to hunt moving insects in order to expand the spectrum of food resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rodents are a diverse and prosperous group. They account for ~40% of mammals by the species number and possess (in addition to morphological and physiological peculiarities) a number of behavioral adaptations. The abilities of rodents to choose the optimal diet and to switch to new food sources in a changing environment have so far been studied mainly for herbivorous species relative to different plant species (Soininen et al., 2013), as well as for omnivorous species with wide preferences. Thus, manifestations of neophobia in relation to new food odors are minimal in gray rats Rattus norvegicus Berkenhout living in a highly volatile environment (Modlinska and Stryjek, 2016). Almost all rodent species are omnivorous to some extent (Landry, 1970), and the inclusion of invertebrates and even small vertebrates in the diet was noted for many of them (Levenets et al., 2016). However, the information on animal consumption by rodents was obtained from an analysis of stomachs and feces (Levenets et al., 2016). The observations of prey capture were performed on rodents caught in natural conditions and placed in a laboratory (Rowe, A. and Rowe, M., 2006); individual observations of hunting for invertebrates (grasshoppers and butterflies) in nature are known only for Campbell’s dwarf hamster (Levenets et al., 2019). Until recently, the process of hunting for mobile prey was studied only on the example of several species: the predatory grasshopper mouse Onychomys torridus Coues, the omnivorous white-footed and deer mice Peromyscus leucopus Rafinesque and P. maniculatus Wagner (Kreiter and Timberlake, 1988; Timberlake and Washburne, 1989), the golden hamster Mesocricetus auratus Waterhouse (Langley, 1986; Polsky, 1977), and the gray rat R. norvegicus (Haug and Johnson, 1991). The role of individual skills was revealed by hamsters: from the first meeting with a potential prey, specialized grasshopper mice exhibit innate hunting behavior in every detail, while efficient interaction with the prey in white-footed and deer mice requires an accumulation of experience (Kreiter and Timberlake, 1988). This means that the coordination of motor acts in hunting behavior in different rodent species is controlled by genetic programs to varying degrees. These works laid the foundation for the study of different adaptations in rodents during the hunt for mobile prey. It was found that the members of the grasshopper mouse Onychomys genus are specialized hunters who kill invertebrates and small vertebrates both in vivo and in laboratory conditions and have morphological and physiological adaptations to interaction with mobile prey, including dangerous prey such as scorpions (Sarko et al., 2011). The hunting behavior of laboratory lines of house mouse in relation to crickets has been used in recent years as a model for studying perceptual (Hoy et al., 2016) and neurological (Han et al., 2017) mechanisms of the interaction between rodent and prey. Laboratory experiments recently found that the hunting behavior in relation to insects in the field mice Apodemus agrarius Pallas (Panteleeva et al., 2013) and bank voles Myodes glareolus Schreber (Konczal et al., 2016), which (in addition to house mice) have no morphological or physiological adaptations to hunting, indicate the presence of behavioral adaptations in rodents with different types of nutrition for the capture of mobile invertebrates. A question arises about the identification of specific behavioral stereotypes in rodents, the use of which can expand the adaptive potential of the species due to the possibility of a switch to mobile prey. Under stereotype, we mean a behavioral sequence consisting of persistently repeating elements (Panteleeva et al., 2010).
In our studies, a constant interaction of small mammals with red wood ants was detected for the first time in vivo (Panteleeva et al., 2016). This gave rise to laboratory experiments was detected for the first time the active hunting of mobile insects (including numerous, aggressive, and dangerous insects, such as ants) by A. agrarius field mice (Panteleeva et al., 2013; Reznikova et al., 2017). It was demonstrated that the hunting behavior of the field mice is organized into a stereotype, which includes the detection, pursuit, attack, and processing of the prey. As part of the hunting stereotype, the attack includes the throwing and grasping of the prey and can be considered a fixed complex of actions (FCA) (Dewsbury, 1981; Zorina et al., 2013), i.e., is a species-specific, innate, and template complex of motor acts. In ethology, the behavioral act is considered innate if it is manifested with sufficient completeness from the first event (Dewsbury, 1981). Our experiments (Reznikova et al., 2017) demonstrated that a holistic hunting stereotype is completely manifested in young, “naive” animals (i.e., grown without contact with the appropriate stimuli) without the preliminary experimentation, i.e., on the principle of “all at once” (Reznikova, 2005), and is comparable in efficiency to the appropriate stereotypes of specialized carnivorous species. It is important to note that the holistic innate stereotype of hunting behavior in the field mouse is facultative, i.e., it is manifested completely, but not in all animals.
We hypothesized that the presence of carriers of the hunting stereotype in the populations of nonpredatory rodent species is an evolutionarily stable strategy (Maynard Smith and Price, 1973) that allows populations to expand the food spectrum with mobile insects. The theory of evolutionarily stable strategies (ESSs) was proposed by Maynard Smith and Price (1973) to explain the coexistence of the groups of individuals with alternative strategies of conflict resolution in populations (“dove” and “hawk” strategies are classical examples). An ESS is a strategy that, when accepted by a sufficiently large number of individuals in a population, cannot be displaced by another strategy; i.e., no other strategy can provide higher adaptability. With changing external conditions, the ratio of representatives of alternative strategies in the population can vary, but the strategies remain evolutionarily stable. A strategy is understood as the set of rules determining which of the alternative behavioral patterns will be accepted by the individual in any situation throughout life. Each individual can accept only one strategy, and different strategies suggest corresponding differences in the genotypes. Unlike a strategy, tactics are the use of one of the existing phenotypic variants of behavioral patterns. According to the authors, ESS theory was based in part on the ideas of game theory and in part on the works of MacArthur (1965) and Hamilton (1967) on the evolution of the sex ratio. This predetermined a relatively wide use of the ESS model, particularly, to describe the strategies of mating (Dominey, 1984) and foraging behavior in conditions of intraspecific competition (Sirot, 2000). Ideas similar to ESS that are based on game theory underlie the studies on predator–prey interaction on the example of the Eurasian sparrowhawk and common redshank (Quinn and Cresswell, 2004). The labile ratio in populations of the groups of individuals with different nutrition strategies from the position of ESS has not yet been discussed.
To verify the hypothesis about the presence of hunters in rodent populations with different types of nutrition as a manifestation of an ESS, as a first step, we studied the stereotypes of hunting behavior in three rodent species as compared with insectivorous species (the common shrew) as a “predator standard.” We were interested in indices of the stability of the stereotypical hunting behavior, its species specificity, and the proportion of hunters in the studied rodent groups reflecting the situation in natural populations. The stability and specificity of the stereotype indicate that it is a manifestation of individual strategy, but not tactics.
MATERIALS AND METHODS
Laboratory studies were carried out in 2014–2018 on three rodent species with different types of nutrition and population structure: the granivorous striped field mouse A. agrarius (n = 26; 13 males and 13 females), the herbivorous narrow-skulled vole Lasiopodomys gregalis Pallas (n = 46; 23 males and 23 females), and the euryphage Campbell’s dwarf hamster Phodopus campbelli Thomas (n = 19; 8 males and 11 females). Animals of each species were not related to each other. According to the classification by V.S. Gromov (2008), field mice are related to species with a system of aggregation of individual habitats, narrow-skulled voles live in poorly consolidated family groups or colonies, and Campbell’s dwarf hamsters occupy separate individual plots. Of the 26 field mice, 17 individuals were caught in vivo in the territory of the forest–steppe Priobsk province 30 km from Novosibirsk, and nine were descendants of the second generation of previously captured animals. All narrow-skulled voles were caught in the territory of Karasuk Field Station of the Institute of Systematics and Ecology of Animals (Siberian Branch, Russian Academy of Sciences) (Novosibirsk oblast). The outbred Campbell’s dwarf hamsters (n = 19) were provided by the vivarium of the Institute of Cytology and Genetics (Siberian Branch, Russian Academy of Sciences), where they were kept for 20 generations. All Campbell’s dwarf hamster individuals and the nine field mice born in the vivarium were not previously in contact with insects, i.e., they were naive relative to the potential prey. The behavior of the common shrew Sorex araneus Linnaeus taken from natural conditions (n = 11) was studied for comparison. The speckled cockroaches Nauphoeta cinerea Olivier (an average body length of 27.93 ± 0.4 mm) was used as a mobile, safe prey. Additional experiments with Campbell’s dwarf hamsters (n = 10) were carried out in 2018. In different series of tests, the hamsters were offered both safe and dangerous insects (red wood ants Formica aquilonia Yarr.) presented in arenas in groups of ten individuals (similar to Panteleeva et al., 2013). All animals were kept in individual cages and had a constant access to water and food without limitations. The diet included cereal mixtures, fruits, and vegetables, as well as protein components (cottage cheese, boiled eggs).
To study the hunting behavior, animals in transparent arenas 30 × 30 × 35 cm in size were offered the prey, and a video of their reactions was recorded (a total of ~100 h observations). Those individuals who hunted upon the first presentation of an insect were consistently offered up to two more prey units within a single test. Testing continued until the completion of prey consumption, or it was stopped after 10 min if the animal showed no interest in the prey. Thus, depending on the reactions of the animal, the test could end on the first, second, or third unit of the prey. The first reaction to the prey could be observed for each animal only once: upon the first presentation in the first test. To obtain a comparable number of behavioral sequences, the field mice and common shrews were tested two times; the narrow-skulled voles were tested three times; and the Campbell’s dwarf hamsters were tested seven times. The sequences of behavioral elements obtained in all tests were detected and analyzed with the Noldus Observer XT 10.1 program (for details on the analysis method, see Reznikova et al., 2017). This allowed a comparison of the nature and tactics of hunting behavior in different species. The hunting effectiveness was estimated as the ratio of the number of successful attacks ending with the prey capture and the number of unsuccessful attacks, in which the prey was lost and the animal stopped actively searching. The hunting rate was estimated as the number of behavior elements committed per second. Only successful hunting acts that ended with prey capture and consumption were analyzed in detail, but all cases of unsuccessful attacks were also registered. In each test, the animal could demonstrate up to three successful attacks and unlimited unsuccessful attacks. The proportions of hunting and nonhunting individuals in different species, as well as successful and unsuccessful attacks for different individuals, were compared with the Fisher’s exact test and Bonferroni correction. The number of unsuccessful attacks that preceded successful attacks was compared for different species with the Mann–Whitney criterion. To construct a scheme of the stereotypical hunting behaviors, the matrices of the probabilities of transition from one behavioral element to another (first-order Markov process) were calculated (Casarrubea et al., 2008). The hunting rate and number of elements in the stereotypes were compared with the Kruskal–Wallis H-criterion with Bonferroni correction. In the comparison, the median, first, and third quartiles are presented: Me (Q1–Q3).
RESULTS
All of the studied species demonstrated targeted interaction with mobile prey according to the predator–prey type (Caro, 1980), which includes detection, rapprochement (pursuit), attack, prey processing, immobilization, and consumption. Unlike insectivorous species with obligate manifestation of hunting behavior (all 11 shrews hunted), the stereotype in rodents was facultative (it did not occur in all animals). Upon first presentation of the prey, 65.4% (17 of the 26) field mice, 36.8% (7 of 19) Campbell’s dwarf hamsters, and 18.5% (9 of 46) narrow-skulled voles demonstrated active pursuit and attack. We note that, among the hunting field mice, six animals (two males and females females) were born in the vivarium, i.e., six of nine naive animals showed a complete reaction to prey upon the first presentation, while three did not. For the animals captured in natural conditions, the ratio was approximately the same: 11 of 17.
After all of the repeated tests, the number of individuals exhibiting hunting behavior slightly increased: up to 80.8% in field mice; up to 63.2% in Campbell’s dwarf hamsters; and up to 39.1% in narrow-skulled voles. The growth in the number of hunters is not significant: p = 0.1939 for hamsters, p = 0.06595 for voles; p = 0.3487 for field mice (Fisher’s exact test).
The hunting performance in different species was assessed only for hunting individuals. According to the results of the first test (with the sequential presentation of up to three insects), field mice and common shrews were the most successful hunters: accordingly, 69.2% (45 of 65) and 62.3% (33 of 53) attacks ended with capture and consumption of the prey. This was significantly higher than that in Campbell’s dwarf hamsters (20%; 7 of 20 attacks) and narrow-skulled voles (25.4%; 16 of 63 attacks) (Fisher’s exact test, p < 0.0017 for both cases). In the first test, all hunting mice and shrews caught and ate the prey, sometimes after several unsuccessful attacks. Only unsuccessful attacks were observed in 22% (2 of 9 individuals) hunting, narrow-skulled voles and in 57% (4 of 7 individuals) of Campbell’s dwarf hamsters; i.e., hunting behavior was manifested, but the prey was never caught. All attacks were successful in nine field mice and two narrow-skulled voles; i.e., they never missed a prey. In Campbell’s dwarf hamsters, at least one unsuccessful attack preceded the successful attacks in all hunts. Voles had more unsuccessful attacks (5.0 (2.8–6.3)) preceding successful attacks than field mice (1.0 (1.0–2.0)), Campbell’s dwarf hamsters (1.0 (1.0–1.3)), and common shrews (2.0 (1.0–2.8)) (H = 11.1, p < 0.05).
According to the results of all repeated tests, the hunting performance (the ratio of successful and unsuccessful attacks by field mice (83 and 39), Campbell’s dwarf hamsters (43 and 42), and common shrews (61 and 34)) was not significantly different either between species or with the results of the first testing for each of species. The hunting performance of narrow-skulled voles remained the lowest (34 and 92) (p < 0.0024 for all cases). The number of unsuccessful attacks preceding successful prey capture after repeated tests (excluding the first) for field mice (1.0 (1.0–1.0)), Campbell’s dwarf hamsters (1.0 (1.0–1.0)), and common shrews (1.0 (1.0–1.0)) changed insignificantly, while it significantly decreased to 2.0 (1.0–3.0) in narrow-skulled voles (H = 11.2, p < 0.05) and became similar to the same index in the field mice and common shrews. A change in this index in voles did not affect hunting performance.
Common shrews hunted the fastest, committing 2.9 (1.8–4.2) behavior elements per second. Their hunting rate was significantly higher than that for rodents: field mice, 2.1 (1.5–2.8) (H = 11.2, p < 0.001); narrow-skulled voles, 1.6 (1.2–2.2) (H = 20.5, p < 0.001; and Campbell’s dwarf hamsters, 1.4 (1.0–1.9) (H = 35.2, p < 0.001). The hunting rate for field mice was higher than that for Campbell’s dwarf hamsters (H = 20.0, p < 0.001), while narrow-skulled voles did not differ significantly by this index from field mice or hamsters.
Nineteen elements were highlighted in the hunting behavior of the studied species, two of which were observed only in shrews or only in rodents. The behavioral elements were divided into three types:
(1) key elements, without which the realization of a stereotype is impossible: pursuit of prey by running (Q) or calm step (S), bite (W), prey capture with the paws (E) (only by rodents);
(2) additional elements (hunt “preparations” and prey “processing”) were present not in all stereotypes: sniffing (D), transfer of the prey in teeth (G), prey interception with paws (R) (only by rodents), biting off the prey limbs (H), holding the prey with one (N) or two paws (M) (only by shrews);
(3) “noise” elements not affecting the realization of a stereotype: fading (C), 90° body rotation (V), 180° body rotation (B), head rotation (F), vertical stand (I), arena-supported stand (Y), backward movements (U), jump (J), and cleaning (X).
Table 1 presents the obtained values of the amount of individual behavioral elements per hunting stereotype in different species.
To get an idea of how different studied species attack the prey and how they manipulate it, we compared the number of behavioral elements associated with the attack and processing of prey per one stereotype (Table 1). The number of bites in narrow-skulled voles and Campbell’s dwarf hamsters is lower than that for field mice (H = 87.5, p < 0.001; H = 17.7, p < 0.001, respectively) and common shrews (H = 7.8, p < 0.005; H = 11.7, p < 0.005). Narrow-skulled voles made captures with paws and prey interception very rarely, much more rarely than Campbell’s dwarf hamsters (H = 14.6, p < 0.0016; H = 14.9, p < 0.001, respectively) and field mice (H = 9.9, p < 0.0083; H = 14.9, p < 0.001). Campbell’s dwarf hamsters bit off the prey limbs more often than individuals of all other species, which reflects the pattern specifics: field mice (H = 33.8, p < 0.001), narrow-skulled voles (H = 14.4, p < 0.001), and common shrews (H = 21.2, p < 0.001).
To determine how a hunter damages the prey, the repeatability of a “bite” behavioral element was studied. The totality of these elements was divided into three groups: single, double (two successively committed bites), and multiple (three and more successive bites).The results are presented in Table 1. The number of single bites in hunting stereotypes of common shrews was significantly lower than that in the stereotypes of field mice (H = 36.4, p < 0.001) and Campbell’s dwarf hamsters (H = 32.2, p < 0.001). The number of single bites did not differ in narrow-skulled voles and common shrews. The rodent stereotypes did not differ by the number of single and double bites. The field mice and common shrews made multiple bites more often than narrow-skulled voles (H = 16.6, p < 0.001; H = 12.5, p < 0.001) and Campbell’s dwarf hamsters (H = 43.3, p < 0.001; H = 37.6, p < 0.001). Despite the smaller number of multiple bites in the stereotypes of narrow-skulled voles, we can say that they are similar to field mice and shrews by the nature of the attack, i.e., they catch insects with their teeth and damage them with a series of bites. Thus, field mice, narrow-skulled voles, and common shrews complete the attack on an insect in a similar way (bite to death).
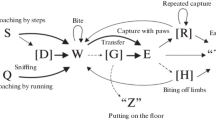
The schemes of hunting stereotypes were constructed based on matrices of probabilities of transitions between behavioral elements (Fig. 1). In all four species, the hunting stereotype started with rapprochement of the prey with running (Q) or a calm step (S), which could be followed by sniffing (D). Further, field mice and narrow-skulled voles captured prey with their teeth (bite; single, but more often several consecutive) (W). In Campbell’s dwarf hamsters, rapprochement with the prey was more likely followed by the capture with the teeth (bite) (W) and less likely by prey capture with the paws (E). We note that the probability of primary prey capture with the paws in hamsters is more than 25%, while such actions in mice and voles were observed in single cases and ended unsuccessfully. While hamsters are able to hold the prey after its initial capture with the paws and move on to damaging and eating it, mice and voles immediately lose the prey after capturing it with the paws, and successful attacks are possible for them only at the beginning of the attack by capture with teeth. After prey capture, the schemes of hunting-behavior stereotypes for rodents and common shrews diverge. After the bite (W), rodents capture the prey with both paws (E) for eating. This behavioral element is absent in shrews; they press the prey to the bottom of the arena (usually with two forelimbs (М), less often with one (N)). continuing to bite, and begin to eat over time. After the capture, all animals were able to carry the prey in their teeth (G). If the attack started with a bite (W), rodents further captured the prey with their paws (E); then, interceptions (R) that included manipulations with the prey held in the paws (rotations, revolutions) could be observed. The behavioral element “interception” was naturally absent in shrews. Subsequently, all animals could bite off the prey limbs (H); such behavior was most often noted in Campbell’s dwarf hamsters. In behavioral sequence, the last element of the behavior preceding eating acts as the end of a successful hunting stereotype. These final elements can include a bite (W), capture with paws (E), interception (R), or the biting off of prey limbs (H) for rodents or a bite (W), holding of the prey with one paw (N), biting off of prey limbs (H), sniffing (D), and the transfer of prey in the teeth (G), which occurred singly in shrews.
Schemes of hunting stereotypes of the field mouse (a), narrow-skulled vole (b), Campbell’s dwarf hamster (c), and common shrew (d). See designations of behavioral elements in the Results section. The additional elements are indicated in square brackets. Some unstable connections between elements are designated by a thin dotted line (p < 0.2). Stable connections are indicated by a simple line (0.2 ≤ p < 0.5). Highly stable connections between elements are designated by a bold line (p ≥ 0.5).
It should be reminded that all of the studied hamsters and some of the field mice were born in the laboratory and were naive in relation to potential prey. The stereotypical hunting behavior in these animals was either completely manifested at the first or one of the subsequent meetings with the prey (according to the all-at-once principle) or was not manifested at all. Comparison of the hunting stereotypes for field mice born in the laboratory (n = 6) and field mice caught in natural conditions (n = 11) revealed no differences in their hunting behavior. In addition to interspecific comparisons, the number of behavioral elements per stereotype was analyzed: bite (H = 0.8, NS), capture with paws (H = 0.2, NS), interception (H = 1.2, NS), biting off of prey limbs (H = 0.3, NS), and the number of single (H = 0.2, NS), double (H = 2.9, NS), and multiple bites (H = 0.3, NS). The schemes of the stereotype in wild and laboratory field mice also did not differ and were completely identical to the scheme presented in Fig. 1.
The obtained data made it possible to form an idea of the hunting tactics of different species, i.e., a set of methods for the damaging or killing of prey. The nature of hunting attacks for rodents and insectivores is different: rodents carry out a capture with paws after capturing the prey by teeth, while shrews use only the teeth. The hunting tactics were similar in field mice, narrow-skulled voles, and common shrews: rapid damage to the prey with a prolonged series of bites. The hunting tactic of Campbell’s dwarf hamsters is very specific: damage to and immobilization of the prey always occurs by the biting off of limbs. This is expressed in the stability of associations between behavioral elements capture with paws and biting off of the limbs (0.2 ≤ p < 0.5). This method was stably used by all individuals (n = 19). Since such hunting tactics were for detected the first time in rodents, we decided to determine whether it is universal for this species and whether Campbell’s dwarf hamsters use the same method for immobilization of not only safe prey but also dangerous insects. For this, red wood ants were offered to a separate group of ten hamsters. However, while ants have a high hedonistic value for field mice and are eaten without residue (Panteleeva et al., 2013), Campbell’s dwarf hamsters, as it turned out, kill them but do not eat them. While the hunting behavior of field mice in relation to cockroaches and ants is generally similar (as an edible object), Campbell’s dwarf hamsters have a completely different method of damaging dangerous insects. They capture only by teeth (never by paws) and then take the insect in the paws. Turning the ant abdomen towards themselves, they immediately (or after several interceptions), bite it and then throw the damaged insect to the bottom of the arena. We note that, while the hunting behavior relative to cockroaches was not manifested in all hamsters and was not always successful, damage to aggressive ants was observed in all individuals with equally high efficiency. The behavior of hamsters in relation to ants can be attributed not to hunting but to defensive behavior, and it is manifested not facultatively (as in the case of hunting) but obligately.
DISCUSSION
A targeted, predator–prey type of interaction with mobile prey was found for the first time in our experiments. It was described in detail for three rodent species with different types of nutrition. The granivorous striped field mouse, herbivorous narrow-skulled vole, and omnivorous Campbell’s dwarf hamster have an equally efficient, stereotypical hunting behavior that is in many ways similar to the behavior of the common shrew (a specialized, insectivorous species). At the same time, the hunting rate for rodents is lower than that in insectivores. The structure of the hunting stereotype was universal in all studied rodent species, regardless of their food specialization. With the same set of hunting behavior elements and similar action procedures and probabilities of transition between them in different species, it is worth noting that such behavioral elements as capture with paws (after catching with teeth) and interception (search for a more comfortable position) are significantly repeated less in narrow-skulled voles as compared with other species; they also have fewer multiple bites in the killing of an insect than other species. This gives them a kind of uncertainty in prey processing prey and is probably the reason for their slightly lower hunting performance as compared with other species. Different species have different tactics for prey damage: field mice, narrow-skulled voles, and common shrews immobilize the insect with a series of quick bites (bite to death), while Campbell’s dwarf hamsters bite off limbs of the prey while holding it in their paws. This behavioral element was found in rodents for the first time, and it is apparently a manifestation of more specialized hunting behavior. It is interesting to note that the grasshoppers found in the holes of Campbell’s dwarf hamsters in vivo were deprived of heads and limbs, and it can be assumed that the same method was applied to them (Levenets et al., 2019).
To form ideas about the possible evolution of hunting behavior in the studied species, we should refer to the existing picture of the evolution of hunting behavior in rodents. The evolutionary origins of the hunting behavior of terrestrial mammals supposedly originate from a common omnivorous—insectivorous ancestor (Ewer, 1973), and an evolutionary transition from a herbivorous lifestyle to a predatory lifestyle is less likely than that from omnivorous one (Shipman and Walker, 1989). Synthesis of the anatomical, genetic, and paleontological data established that a small, insectivorous, tree animal was a common ancestor of placental mammals (Meredith et al., 2011; O’Leary et al., 2013). The origin of hunting behavior in rodents remains unclear. Rodents could have inherited the hunting stereotype for small, mobile prey already available in insectivorous ancestors, or such behavior may have formed repeatedly. Some ideas about the evolutionary pathway of hunting behavior in rodents can be obtained with a comparative ethological approach. As a product of selection, behavioral traits can be used for phylogenetic constructions. Thus, based on the fact that instinctive movements have a systematic significance, Lorenz was the first to use the traits of behavior and vocalization to consider the duck system phylogeny (Lorenz, 1941). Subsequently, his student Leyhausen used a similar approach to reconstruct the evolutionary tree of hunting behavior in terrestrial mammals (Leyhausen, 1965; Eisenberg and Leyhausen, 1972). Together with Eisenberg, they recorded the sequence of actions performed by a predator during hunting, presenting them as ethograms. The researchers covered a vast number of species (from insectivores to primates), but rodents were not included in this list. Upon the generalization of comparative ethological data, they came to a number of conclusions. The attack on the prey with a series of bites (prey capture and killing only with jaws) is a primitive form of hunting behavior. The emergence of prey capture with the forepaws in the repertoire is a more progressive trait in evolutionary terms. A displacement of the prey capture function to the forelimbs became the basis for the further differentiation of bite types: either a precisely directed, single, deadly bite or a series of bites. The deadly bite is a “recent achievement,” while series of bites are characterized as a more archaic trait. In evolutionary terms, such a method, in which the forelimbs are used to capture and hold the prey and the jaws are closed in a deadly bite, increases predator efficiency. Langley (1987, 1994) studied in detail the stereotypes of hunting of invertebrates in specialized predatory rodents and euryphage rodents. Based on the picture of the evolution of hunting behavior (Eisenberg and Leyhausen, 1972), he was the first to classify the hunting behavior of rodents and demonstrated that nonspecialized euryphage rodents in most cases start the prey attack by capture with teeth (bite); then, holding it in their teeth, they capture it in the front paws (Langley, 1987, 1994). Specialized predatory grasshopper mice act in a fundamentally different way; most often, they capture the prey in the front paws at the beginning of the attack and only then kill it with a single or several bites. Different members of the insectivore order (particularly, shrews (Soricidae), hedgehogs (Erinaceidae), tenrecs (Tenrecidae)) attack the prey only with a series of bites, demonstrating the most primitive type of the attack (Eisenberg and Leyhausen, 1972). Thus, progressive traits (prey capture with paws) are observed in the hunting stereotype of rodents as compared with insectivores, while the most advanced hunting stereotype is observed in specialized, predatory grasshopper mice (Langley, 1994).
In order to determine the place of the species that we studied in this picture, it is important to analyze the beginning of the prey attack. Among three studied species, the nature of hunting attacks by rodent and insectivores is different: rodents carry out prey capture with the paws after capture with teeth, while shrews use only the teeth. Since capture with the paws by field mice and narrow-skulled voles follows capture with the teeth, their hunting stereotype can be considered more primitive than that in specialized, predatory grasshopper mice. Campbell’s dwarf hamsters can begin the attack by capture with both teeth and paws (in more than 25% of cases), which characterizes their hunting behavior as the most advanced among the studied species.
The species scenario of the formation of the hunting-behavior stereotype in the ontogenesis of all of the studied rodent species was similar to those in specialized, predatory grasshopper mice: the stereotype was manifested in naive animals according to the all-at-once principle, which is holistic and does not changing with repeated presentations of prey. Some animals apparently required a cumulative effect of stimuli from the prey to “wake up” a holistic stereotype. The role of stimulus accumulation in the launch of “innate releasing mechanisms” (Dewsbury, 1981) was demonstrated in studies on the hunting behavior of the common toad (Ewert, 1987); later, it was demonstrated repeatedly that animals must accumulate sensory signals in the cerebral cortex to make decisions (Watson and Platt, 2008). The proportion of individuals with the postponement of the manifestation of the hunting stereotype in our samples is 15–26% in three rodent species. We note that there was no improvement in hunting methods in our experiments with experience, as previously demonstrated in nonspecialized hamsters of the Peromyscus genus (Kreiter and Timberlake, 1988). Of the species that we studied, only narrow-skulled voles showed a slight increase in the number of successful hunts upon repeated meetings with the prey. The hunting rate, the nature and efficiency of attacks, and, most importantly, the order and ratio of behavioral elements in the stereotypes for all three rodent species (as well as in shrews) were not influenced by experience.
Our laboratory experiments apparently reflect the ratio of hunters and individuals indifferent to insects in natural populations of the studied species. It is noteworthy that, although there were half as many hunters among herbivorous voles in our experiments as among field mice, all rodent species were similar by the nature of the stereotype, the hunting rate, and the efficiency. This suggests that some proportion of individuals carrying the hunting-behavior stereotype is constantly present in populations of rodents with different types of nutrition (at least in some species), while the stereotype itself can be considered a behavioral adaptation that expands the spectrum of food resources via active insect hunting. The hunting-behavior stereotypes in the studied rodent species are a universal scenario with manifestation of species-specific traits that are expressed in the nature of attacks and the methods of prey immobilization, as well as the frequency of the manifestation of individual behavioral elements. The marked, species-specific characteristics do not vary in the members of each species. They are innate and are either manifested in a certain proportion of individuals or are not manifested at all. These key characteristics indicate that the presence of hunting stereotypes in rodents is a strategy within ESS theory.
In general, the hunting behavior of rodents can be apparently considered an ESS, in which the proportion of carriers of a certain type of behavior can increase under certain conditions without completely taking over the population (Maynard Smith and Price, 1973). A similar potential of rodent hunting behavior was indicated by experiments with a targeted selection of hunters in granivorous–herbivorous species (the bank vole M. glareolus) (Konczal et al., 2016). After 13 generations of such selection, the proportion of voles demonstrating hunting behavior in relation to crickets was five times higher in individual lines of animals than in the control groups.
Such experiments were previously carried out on different genetic lines of house mice (Butler, 1973) and Syrian hamsters (Polsky, 1978). In hamsters, the lines of “catchers” and “brakes” were obtained for eight generations: the delay before the prey attack differed in these groups by five times. Studies on the genetic mechanisms responsible for the manifestation of hunting behavior in inbred mouse lines demonstrated that a high level of predatory aggression (as physiologists and genetics previously called hunting behavior) is a dominant trait (Nikulina and Popova, 1983).
The nature of hunting behavior in rodents was not studied in detail prior to our work, and special experiments are required to study the comparative “competitiveness” of differing stereotypes in conditions of targeted selection. Based on our preliminary data, it is possible to make a cautious assumption that the number of carriers of the hunting-behavior stereotype can vary between populations in different years and is apparently associated with fluctuations in environmental conditions. It should be noted that, although the hunting-behavior stereotype is manifested in a certain proportion of individuals of the studied species according to the all-at-once principle, animals indifferent to insects can be carriers of “dormant” fragments of a holistic stereotype (dormant behavioral patterns; Reznikova and Panteleeva, 2008). We recently demonstrated the phenomenon of fragmentation of the hunting-behavior stereotype on the example of some rodent species (Reznikova et al., 2017). It can be assumed that distributed social learning can be a behavioral mechanism that makes it possible, if necessary, to increase quickly the number of hunters in populations (Reznikova and Panteleeva, 2015): if the population has carriers of the complete hunting-behavior stereotype, the potential carriers of stereotype fragments can quickly “complete” them due to simple forms of social learning. These issues require further studies.
CONCLUSIONS
Detailed experimental studies on the interaction of small mammals with mobile insects demonstrated that the granivorous striped field mouse, herbivorous narrow-skulled vole, and omnivorous Campbell’s dwarf hamster possess a hunting behavior as efficient as that of the insectivorous species (the common shrew). The structure of the hunting-behavior stereotype in the studied rodent species is universal and does not depend on food specialization. Different species have different tactics for prey damage: field mice and narrow-skulled voles immobilize the insect with a series of quick bites, just like shrews do; Campbell’s dwarf hamsters bite off the limbs of the prey, which is apparently a manifestation of a more specialized hunting behavior. The nature of hunting attacks in rodents and insectivores is different: first capturing the prey with their teeth, rodents move on to prey capture with the paws, while shrews use only the teeth, which indicates a relative primitiveness of their predatory behavior. Campbell’s dwarf hamsters can begin the attack by capture with both teeth and paws, which characterizes their hunting behavior as the most evolutionarily advanced among the studied species. The nature and ratio of behavioral elements in all three rodent species are manifested according to the principle of all at once and are not influenced by the experience. The hunting behavior of rodents can be considered an ESS that supports the ability of populations to hunt for moving insects in order to expand the range of food resources.
REFERENCES
Butler, K., Predatory behavior in laboratory mice: strain and sex comparisons, J. Comp. Physiol. Psychol., 1973, vol. 85, no. 2, pp. 243–249.
Caro, T.M., The effects of experience on the predatory patterns of cats, Behav. Neural Biol., 1980, vol. 29, no. 1, pp. 1–28.
Casarrubea, M., Sorbera, F., and Crescimanno, G., Multivariate analysis of the modifications induced by an environmental acoustic cue on rat exploratory behavior, Physiol. Behav., 2008, vol. 93, no. 4, pp. 687–696.
Dominey, W.J., Alternative mating tactics and evolutionarily stable strategies, Am. Zool., 1984, vol. 24, no. 2, pp. 385–396.
Dewsbury, D.A., Comparative Animal Behavior, New York: McGraw-Hill, 1978.
Eisenberg, J.F. and Leyhausen, P., The phylogenesis of predatory behavior in mammals, Z. Tierpsychol., 1972, vol. 30, no. 1, pp. 59–93.
Ewer, R.F., The Carnivores, Ithaca: Cornell Univ. Press, 1973.
Ewert, J.P., Neuroethology of releasing mechanisms: prey-catching in toads, Behav. Brain Sci., 1987, vol. 10, no. 3, pp. 337–368.
Gromov, V.S., Prostranstvenno-etologicheskaya struktura populyatsii gryzunov (Spatio-Ethological Structure of Rodent Population), Moscow: KMK, 2008.
Hamilton, W.D., Extraordinary sex ratios, Science, 1967, vol. 156, no. 3774, pp. 477–488.
Han, W., Tellez, L.A., Rangel, M.J., Motta, S.C., et al., Integrated control of predatory hunting by the central nucleus of the amygdala, Cell, 2017, vol. 168, no. 1, pp. 311–324.
Haug, M. and Johnson, F., Environmental and biological incidences over predatory aggression in rodents, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1991, vol. 99, no. 3, pp. 205–293.
Hoy, J.L., Yavorska, I., Wehr, M., and Niell, C.M., Vision drives accurate approach behavior during prey capture in laboratory mice, Curr. Biol., 2016, vol. 26, no. 22, pp. 3046–3052.
Konczal, M., Koteja, P., Orlowska-Feuer, P., Radwan, J., Sadowska, E.T., and Babik, W., Genomic response to selection for predatory behavior in a mammalian model of adaptive radiation, Mol. Biol. Evol., 2016, vol. 33, no. 9, pp. 2429–2440.
Kreiter, N. and Timberlake, W., Form and development of predation on crickets in adults of Peromyscus maniculatus bairdi and P. leucopus noveboracensis,J. Comp. Psychol., 1988, vol. 102, no. 3, pp. 269–278.
Landry, S.O., Jr., The Rodentia as omnivores, Q. Rev. Biol., 1970, vol. 45, no. 4, pp. 351–372.
Langley, W.M., Development of predatory behavioral in the southern grasshopper mouse (Onychomys torridus), Behaviour, 1986, vol. 99, no. 3, pp. 275–295.
Langley, W.M., Specializations in the predatory behavior of grasshopper mice (Onychomys leucogaster and O. torridus): a comparison with the golden hamster (Mesocricetus auratus), J. Comp. Physiol. Psychol., 1987, vol. 101, no. 4, pp. 322–327.
Langley, W.M., Comparison of predatory behaviors of deer mice (Peromyscus maniculatus) and grasshopper mice (Onychomys leucogaster), J. Comp. Physiol. Psychol., 1994, vol. 108, no. 4, pp. 394–400.
Levenets, J.V., Panteleeva, S.N., and Reznikova, Zh.I., Experimental study of feeding by insects in rodents, Euroasian Entomol. J., 2016, vol. 6, no. 6, pp. 550–554.
Levenets, J.V., Panteleeva, S.N., Reznikova, Zh.I., Gureeva, A.V., Feoktistova, N.Yu., and Surov, A.V., Experimental comparative analysis of the hunting behavior of four species of subfamily hamsters Cricetinae, Zool. Zh., 2019, vol. 98, no. 6, pp. 1–11.
Leyhausen, P., Über die Funktion der Relativen Stimmungshierarchie (Dargestellt am Beispiel der phylogenetischen und ontogenetischen Entwicklung des Beutefangs von Raubtieren), Z. Tierpsychol., 1965, vol. 22, no. 4, pp. 412–494.
Lorenz, K., Vergleichende Bewegungsstudien an Anatinen, J. Ornithol., 1941, vol. 89, pp. 19–29.
MacArthur, R.H., Patterns of species diversity, Biol. Rev., 1965, vol. 40, no. 4, pp. 510–533.
Maynard Smith, J. and Price, G.R., The logic of animal conflict, Nature, 1973, vol. 246, no. 5427, pp. 15–18.
Meredith, R.W., Janečka, J.E., Gatesy, J., Ryder, O.A., Fisher, C.A., et al., Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification, Science, 2011, vol. 334, no. 6055, pp. 521–524.
Modlinska, K. and Stryjek, R., Food neophobia in wild rats (Rattus norvegicus) inhabiting a changeable environment—a field study, PLoS One, 2016, vol. 11, no. 6, p. e0156741.
Nikulina, E.M. and Popova, N.K., Genetic analysis of predator aggression in mice, Genetika, 1983, vol. 16, no. 7, pp. 105–110.
O’Leary, M.A., Bloch, J.I., Flynn, J.J., Gaudin, T.J., Giallombardo, A., et al., The placental mammal ancestor and the post–K-Pg radiation of placentals, Science, 2013, vol. 339, no. 6120, pp. 662–667.
Panteleeva, S.N., Danzanov, Zh.A., and Reznikova, Zh.I., The complexity of behavioral stereotypes in ants by the example of an analysis of hunting behavior of Myrmica rubra (Hymenoptera, Formicidae), Zool. Zh., 2010, vol. 89, no. 12, pp. 500–509.
Panteleeva, S., Reznikova, Z., and Vygonyailova, O., Quantity judgments in the context of risk/reward decision making in striped field mice: first “count,” then hunt, Front. Psychol., 2013, vol. 4, pp. 45–53.
Panteleeva, S.N., Reznikova, Zh.I., and Sin’kova, O.B., Spatio-ethological aspects of interactions between small mammals and wood ants, Zh. Obshch. Biol., 2016, vol. 77, no. 5, pp. 329–341.
Polsky, R.H., The ontogeny of predatory behavior in the golden hamster (Mesocricetus a. auratus): I. The influence of age and experience, Behaviour, 1977, vol. 61, nos. 1–2, pp. 26–57.
Polsky, R.H., The ontogeny of predatory behavior in the golden hamster (Mesocricetus a. auratus): IV. Effects of prolonged exposure, ITI, size of prey and selective breeding, Behaviour, 1978, vol. 65, nos. 1–2, pp. 27–39.
Quinn, J.L. and Cresswell, W., Predator hunting behavior and prey vulnerability, J. Anim. Ecol., 2004, vol. 73, no. 1, pp. 143–154.
Reznikova, Zh.I. and Panteleeva, S.N., The ontogeny of hunting behavior in ants: experimental study, Dokl. Biol. Sci., 2005, vol. 401, nos. 1–6, pp. 110–111.
Reznikova, Zh. and Panteleeva, S., An ant’s eye view of culture: Propagation of new traditions through triggering dormant behavioral patterns, Acta Ethol., 2008, vol. 11, no. 2, pp. 73–80.
Reznikova, Zh.I. and Panteleeva, S.N., Possible evolutionary mechanisms of ‘culture’ in animals: the hypothesis of distributed social learning, Zh. Obshch. Biol., 2015, vol. 76, no. 4, pp. 309–323.
Reznikova, Zh.I., Panteleeva, S.N., and Novikovskaya, A.A., Fragmentation of behavioral stereotypes as a possible basis for distributed social learning in populations and communities, Materialy III Mezhdunarodnoi konferentsii k 130-letiyu so dnya rozhdeniya N.I. Vavilova i 110-letiyu so dnya osnovaniya Gosudarstvennogo Darvinovskogo muzeya “Sovremennye problemy biologicheskoi evolyutsii” (Proc. III Int. Conf. dedicated to the 130 Anniversary of N.I. Vavilov and 110 Anniversary of Foundation of the State Darwin Museum “Modern Problems in Biological Evolution”), Moscow: Gos. Darvinovskii Muz., 2017a, pp. 57–60.
Reznikova, Z., Levenets, J., Panteleeva, S., and Ryabko, B., Studying hunting behavior in the striped field mouse using data compression, Acta Ethol., 2017b, vol. 20, no. 2, pp. 165–173.
Rowe, A.H. and Rowe, M.P., Risk assessment by grasshopper mice (Onychomys spp.) feeding on neurotoxic prey (Centruroides spp.), Anim. Behav., 2006, vol. 71, no. 3, pp. 725–734.
Sarko, D.K., Leitch, D.B., Girard, I., Sikes, R.S., and Catania, K.C., Organization of somatosensory cortex in the Northern grasshopper mouse (Onychomys leucogaster), a predatory rodent, J. Comp. Neurol., 2011, vol. 519, no. 1, pp. 64–74.
Shipman, P. and Walker, A., The costs of becoming a predator, J. Hum. Evol., 1989, vol. 18, no. 4, pp. 373–392.
Sirot, E., An evolutionarily stable strategy for aggressiveness in feeding groups, Behav. Ecol., 2000, vol. 11, no. 4, pp. 351–356.
Soininen, E.M., Ravolainen, V.T., Bråthen, K.A., Yoccoz, N.G., Gielly, L., and Ims, R.A., Arctic small rodents have diverse diets and flexible food selection, PLoS One, 2013, vol. 8, no. 6, p. e68128.
Timberlake, W. and Washburne, D.L., Feeding ecology and laboratory predatory behavior toward live and artificial moving prey in seven rodent species, Anim. Learn. Behav., 1989, vol. 17, no. 1, pp. 2–11.
Watson, K.K. and Platt, M.L., Neuroethology of reward and decision making, Philos. Trans. R. Soc. B, 2008, vol. 363, no. 1511, pp. 3825–3835.
Zorina, Z.A., Poletaeva, I.I., and Reznikova, Zh.I., Osnovy etologii i genetiki povedenya: Uchebnik (Fundamentals of Ethology and Genetics of Behavior: Manual), Moscow: Mosk. Gos. Univ., 2013, 3rd ed.
Funding
The studies were supported by the Russian Foundation for Basic Research (project nos. 17-04-00702 and 18-34-00119) and the fundamental research program of state academies of sciences for 2013–2020 (project no. VI.51.1.10., AAAA-A16-116121410120-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement on animal welfare. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Panteleeva, S.N., Levenets, J.V. & Reznikova, Z.I. Facultative Hunting Behavior in Rodents as a Possible Evolutionarily Stable Strategy. Biol Bull Rev 10, 407–416 (2020). https://doi.org/10.1134/S2079086420050060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086420050060