Abstract
The thermochemical processes in a TiN coating deposited on a steel substrate under cyclic irradiation with laser pulses are investigated. It has been established that the combustion of the coating in the irradiated regions occurs spatially inhomogeneously owing to the uneven distribution of microregions in the coating, consisting of titanium nitride compounds with different stoichiometry. It is shown that dissociation reactions of various titanium nitride compounds with the release of free nitrogen occur inside the coating. As a result, the ratio of the mass fractions of titanium nitride compounds with different stoichiometry changes in comparison with the original coating. Diffusion of nitrogen into the steel substrate results in the formation of iron nitride.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Titanium nitride coating is widely used to protect products operating at temperatures not exceeding 400°C, since at higher temperatures, intense oxidation and degradation of the coating occurs. However, in real operating conditions, an abrupt increase in temperature is possible, associated with a change in the normal mode of operation of products, for example, owing to the ingress of foreign particles on the contacting surfaces, the combustion of which causes local overheating of the coating, which leads to its premature degradation and destruction. For this reason, studies of the behavior of coatings under short-term thermal effects are relevant. The study of coating degradation processes under the action of short-term thermal pulses makes it possible to understand the features of these processes and the specifics of thermochemical reactions on the coating surface and at the coating–metal base interface. Such studies are also useful from the point of view of predicting the service life of a product with a TiN coating in emergency s-ituations.
Conducting full-scale experiments to study the resistance of the coating to the effects of cyclic thermal pulses of different power and exposure time is a difficult task. It is advisable to carry out such experiments under model conditions, when a real heating source is modeled, for example, by pulsed laser radiation [1, 2].
In this paper, we consider the processes occurring in a TiN coating deposited on a steel base under the action of cyclic laser thermal pulses.
EXPERIMENTAL
For pulsed thermal heating of the target, an LRS‑150A repetitively pulsed laser with a wavelength of 1.06 μm was used. The laser radiation energy per pulse was 12 and 20 J, the pulse duration was τ = 14 ms, and the irradiation spot diameter was 4 mm. The samples were U8 steel plates 0.8 mm thick coated with titanium nitride 4 μm thick by the ion-plasma method.
The study of the microrelief of the sample surface was carried out on a TESCAN VEGA II scanning microscope. X-ray microanalysis of the coating along the scanning line was carried out on an INCA Energy-250 energy dispersive spectrometer.
The main parameter of laser exposure is the heating temperature of the surface of the coating Th, which can be estimated knowing the power of the heat flux P propagating deep into the metal and the exposure time of the thermal pulse τ [3],
where λ = 41.8 W/(m K) is the thermal conductivity and a = 12.7 m2/s is the thermal diffusivity of the coating material.
This formula is valid for a rectangular pulse. The laser pulse generated under the conditions of our experiment has the shape of a trapezoid, and to approximate it with a rectangular pulse, it is necessary to subtract its initial and final sections from the total exposure time of the laser pulse, which make an insignificant contribution to the total radiation energy. In our case, their total duration Δτ ~2 × 10–3 s is small compared to the total time of laser pulse exposure τ0 ≈ 14 × 10–3 s. Therefore, when calculating Тh according to formula (1), the value of τ was used as τeff = τ0 – Δτ = 12 × 10–3 s.
The power of the heat flux of laser radiation on the surface of the coating is
where Erad is the laser pulse energy; n = S1/S2 = 2.2 is the compression ratio of the laser beam by the focusing lens; S1 = 0.13 cm2 is the cross-sectional area of the focused radiation on the coating surface; S2 = 0.28 cm2 is the area of the end surface of the laser active medium; and A is the absorption coefficient of laser radiation by the coating surface, which at the indicated radiation powers is ~0.25 [3]. Substituting these values into (2), we obtain the heat flux power Р = 4.4 × 103 W/cm2 at Erad =12 J and P = 7.3 × 103 W/cm2 at Erad = 20 J. Estimates of the coating surface temperature by formula (1) give values of 147°C (at Erad = 12 J) and 441°C at (Erad = 20 J).
Although the temperature value Тh = 441°C obtained at the radiation energy Erad = 20 J is not sufficient to initiate oxidative processes, the experiment showed that, already after several pulses with Erad = 20 J, the process of coating combustion begins according to the reaction 2TiN + 2O2 → 2TiO2 + N2 [4, 5]; that is, in fact, the temperature on the surface of the coating is much higher than the calculated one, since the combustion process occurs at temperatures of 700–800°C.
This discrepancy can be explained by the fact that the heating of the coating surface is determined not only by the thermal flux of laser radiation but also by the thermophysical characteristics of titanium nitride. Because of the small thickness of the coating (4 μm), the heat flux and temperature can be considered constant over the thickness of the coating. This assumption is confirmed by the temperature estimate at the coating–steel base interface [3]
where z is the thickness of the coating.
Estimates by formula (3) show that the coating temperature at the interface with the steel base at Erad = 20 J is 439°C, which practically coincides with the temperature on the coating surface. It follows from this that the power of the heat flux when passing through the coating hardly changes, but as the heat flux further passes into the depth of the sample, the surface of the steel base begins to heat up. In this case, the heat flux power at the coating–base boundary can be taken to be the same as on the coating surface, 7.3 × 103 W/cm2. In this case, the estimation of the heating temperature of the surface of the steel base according to formula (1) during the action of the laser pulse τeff at a given power of the heat flux and the values of the thermal conductivity of steel λ = 25.7 W/(m K) and thermal diffusivity a = 5.28 × 10–6 m2/s gives the value T = 784°C. Similarly, for a laser pulse with energy of 12 J, the temperature on the surface of the steel base is T = 476°C.
Since the temperature in the surface layer of the steel base is much higher than the temperature in the coating, there is a reverse heat flow from the interface into the coating. The value of this flux depends on the rate of heat propagation from the interface into the depth of the coating and from the rate of its propagation into the depth of the steel base. Owing to the fact that the coefficient of thermal diffusivity of titanium nitride is significantly higher than that of steel, the rate of heat propagation from the interface into the steel base will be noticeably lower than the rate of its propagation into the coating. Calculations show that, at a coating thickness of 4 μm, the heating temperature of the coating during exposure to a single laser pulse is approximately the same as the temperature of the steel base at the interface. At Erad = 12 J, it will be 476°C, and at Erad = 20 J, it will be 784°C. An increase in the number of laser pulses during irradiation leads to a gradual increase in the heating temperature of the coating.
RESULT AND DISCUSSION
Under the influence of 5 and 20 pulses with energy of Erad = 12 J with an interval between them of 0.3 s, no significant changes in the structure and composition of the coating were observed. X-ray microanalysis of the elemental composition of the irradiated coating showed the presence of titanium and carbon nitride in its composition. The presence of carbon is related to the coating technology. Its inclusions are also registered in the original samples not subjected to irradiation.
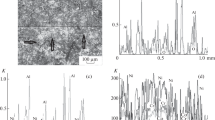
After exposure to 1000 laser pulses with energy Erad = 12 J, two characteristic zones are formed on the coating surface (Fig. 1). The central zone 1 in Fig. 1, in which iron, titanium, nitrogen, and oxygen are present, is an irradiation spot. In the central region of the spot, the temperature is maximal owing to the generation of the central, most energetic modes of the active medium. Zone 2 in Fig. 1, in which titanium nitride and titanium oxide are present, is a heat-affected zone. In this unirradiated zone, located directly behind the irradiation spot, the heating temperature is lower. The presence of titanium oxide in it indicates that partial combustion of titanium nitride occurred in this zone. It should be noted that, in the first zone, the signal amplitude from nitrogen exceeds the signal amplitude from titanium. This can be explained by the fact that titanium compounds with nitrogen can have different TixNy stoichiometry depending on the mass of nitrogen (from Ti10N6 to TiN) [6]. The amplitude of the signal from each element included in the compound depends on its mass. For all possible variants of titanium nitride stoichiometry, the proportion of titanium in the total mass of the compound is much larger than the proportion of nitrogen. Therefore, when registering elements in the heat-affected zone, the amplitude of signals from titanium is greater than the amplitude of signals from nitrogen. The inverse ratio of the intensity of these signals indicates that titanium nitride is absent in the irradiation region under consideration, apparently owing to the fact that, during the irradiation of the coating with a series of laser pulses, its temperature increases from pulse to pulse, and when the critical temperature (700–800°C) is reached, an exothermic titanium oxidation reaction occurs, causing additional heating and evaporation of the material from the coating surface. As a result of the combustion process, thinning of the coating occurs.
It can be assumed that, during laser irradiation, when the coating material is heated, dissociation of titanium nitride compounds occurs, and the nitrogen released in this case diffuses to the coating–substrate interface and reacts with iron, forming iron nitride. At temperatures above 1100°C, nitrogen reacts with titanium to form new titanium nitride compounds with varying stoichiometry different from the initial one [6]. As a result, titanium nitride is gradually depleted of nitrogen. Thus, during irradiation and thinning of the coating, its composition gradually changes. In the later stages of coating combustion, the lower layers of the coating consist predominantly of titanium. Ultimately, the upper layers of the coating in the central irradiation zone burn out, and the titanium reacts with oxygen to form titanium oxide. In areas of the surface where complete combustion of the coating has occurred, iron reacts with oxygen. An increase in the oxygen concentration as these compounds are formed leads to an increased intensity of its signal in the central irradiation zone compared to the peripheral zone and the heat-affected zone (Figs. 1, 2).
When the coating is irradiated with laser pulses with energy of Еrad = 20 J with an interval between pulses of 1 s, nitrides and titanium oxides are recorded in the coating composition after five pulses, which indicates the occurrence of thermochemical combustion processes in it. After exposure to a series of 500 pulses, three zones are formed on the surface of the coating—central zone A, peripheral zone B, and heat-affected zone C (Fig. 2).
In the central zone of irradiation and the heat-affected zone, the same changes in the distribution of elements are observed as when exposed to laser radiation with energy of 12 J. The elemental composition in the peripheral zone corresponds to the composition in the heat-affected zone, but there is more titanium oxide in it, which is associated with a higher coating temperature in the peripheral zone compared to the heat-affected zone and, accordingly, with a higher intensity of the titanium oxidation process. It should be noted that the highest temperature on the surface is reached in the central zone, which is affected by radiation from the central modes of the resonator, which have the maximum energy. In the peripheral zone, the radiation intensity is lower, since it is affected by the side modes of the resonator with a lower radiation energy. The temperature in the heat-affected zone is lower than in the peripheral zone.
Studies of the elemental composition of the original samples, as well as unirradiated coverage areas, showed that the intensity and ratio of the intensities of the titanium and nitrogen signals in neighboring small areas (20–100 μm) differ significantly. The same differences are observed in the heat-affected zone when irradiated with pulses with energy of 12 J, as well as in the peripheral zone and the heat-affected zone when irradiated with pulses with energy of 20 J.
On such small scales, the temperature hardly changes; therefore, the difference in signal intensity can apparently be explained by the technological features of the deposition of the coating in a nitrogen atmosphere and the uneven density of the titanium ion flux to the target surface. As a result, it can be imagined that the coating consists of microregions containing titanium nitride compounds of various stoichiometry and having different combustion temperatures.
To confirm this assumption, in an STA 449-F1 synchronous thermal analyzer in an argon atmosphere with an admixture of oxygen, steel samples 2 × 2 × 2 mm in size, coated on all sides with a layer of titanium nitride 4 μm thick, were heated at a rate of 10°C/min to a temperature of 900°C. Analysis of the elemental composition showed that, in this case too, titanium nitride burns unevenly over the sample surface (Fig. 3). The central dark area in Fig. 3a consists of titanium nitride and titanium oxide. Figure 3b shows the distribution of Ti, N, and Fe. Where complete combustion has occurred (bright areas), mainly iron oxide and iron nitride are observed. The derivatogram shows the presence of peaks at 720, 800, and 850°C. These peaks are associated with the exothermic combustion reaction of titanium nitride compounds with different stoichiometry.
CONCLUSIONS
Oxidation of titanium nitride and evaporation of the coating during cyclic laser irradiation occurs spatially unevenly owing to the uneven distribution of microregions over the surface of the coating consisting of titanium nitride compounds with different stoichiometry.
In the process of cyclic laser irradiation, the reaction of titanium nitride with oxygen burns the surface layers of the coating, and in the depth of the coating, where oxygen is absent, it dissociates into titanium and nitrogen. A change in the nitrogen concentration in the coating affects the ratio of mass fractions of various titanium nitride compounds. Diffusion of nitrogen into the steel base results in the formation of iron nitride.
REFERENCES
Karnavskaya, T.G., Kikin, P.Yu., Perevezentsev, V.N., and Rusin, E.E., Influence of cyclic laser pulses on degradation of a tantalum coating, Inorg. Mater.: Appl. Res., 2017, vol. 8, no. 3, pp. 382–386. https://doi.org/10.1134/S2075113317030133
Karnavskaya, T.G., Kikin, P.Yu., Perevezentsev, V.N., Razov, E.N., and Rusin, E.E., Change in the morphology of the structure of Ta–W coating after exposure to cyclic laser pulses, Inorg. Mater.: Appl. Res., 2019, vol. 10, no. 3, pp. 578–581. https://doi.org/10.1134/S2075113319030146
Grigoryants, A.G., Basics of Laser Material Processing, Boca Raton: CRC Press, 1994.
Samsonov, G.V. and Vinitskii, I.M., Tugoplavkie soedineniya: Spravochnik (Refractory Compounds: Handbook), Moscow: Metallurgiya, 1976.
Voitovich, R.F., Okislenie karbidov i nitridov (Oxidation of Carbides and Nitrides), Kiev: Naukova Dumka, 1981.
Samsonov, G.V., Nitridy (Nitrides), Kiev: Naukova Dumka, 1969.
Funding
This work was carried out within the framework of the state task of the Institute of Applied Physics of the Russian Academy of Sciences for fundamental scientific research for 2013–2020 on topic no. 0035-2014-0401.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Kikin, P.Y., Perevezentsev, V.N., Razov, E.N. et al. Thermochemical Processes Occurring in a Titanium Nitride Coating under the Effect of Thermal Laser Pulses. Inorg. Mater. Appl. Res. 13, 614–618 (2022). https://doi.org/10.1134/S2075113322030169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113322030169