Abstract
The quality of heat-resistant coatings deposited by flame spraying is largely determined by the adhesion of the coating to the surface of the part. One of the ways to increase adhesion is to deposit intermediate layers of thermosetting powders between the base material and the coating. In this work, two versions of heat-protective coatings are investigated—a two-layer coating consisting of an Al–Ni sublayer (20–80 wt %) and a main ZrO2 layer, and a single-layer coating sprayed from an aluminum-clad zirconium oxide powder (20 ZrO2–80 Al, wt %). The method of differential thermal analysis was used to determine the temperature ranges and values of the exothermic effects of oxidation and redox reactions during heating of Al–Ni and ZrO2 clad powders. A significant exothermic effect was found during oxidation of the aluminum cladding shell in the temperature range of 360°C and a stronger thermal effect due to the redox reaction at a temperature of 920°C. The microstructure and microhardness of the obtained coatings have been studied, and their thermal conductivity and adhesion have been assessed. The resistance of the coatings during thermal cycling tests has been determined. It has been established that thermal protective coatings made of aluminum-clad zirconium oxide powder have the best characteristics under thermal cycling conditions. A higher level of adhesion and thermal cyclic resistance of such coatings are due to an increase in the enthalpy of aluminum-clad ZrO2 powders due to exothermic reactions and the presence of a metal binder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Gas-thermal spraying of heat-protective coatings (HPC) on the surface of the combustion chamber of internal combustion engines (ICE) makes it possible to increase the engine power and its service life. Ceramic oxide materials with low thermal conductivity are used as the main heat-shielding layer, such as Al2O3 (1–1.2 W/(m K)) or ZrO2 (0.6–0.9 W/(m K)) [1–3]. However, the reliability and efficiency of such coatings depend primarily on the adhesion of the deposited layers to the surface of the part to be protected.
When ceramic materials are deposited in molten form on metal surfaces, the level of tensile stresses arising as a result of cooling of molten ceramic particles sharply reduces the adhesion of the coating. Significant differences in thermal expansion coefficients further worsen the situation when operating under thermal cycling conditions.
To increase the adhesion of heat-shielding layers, the application of a sublayer with a thickness of 0.08–0.12 mm from Ni–Cr, Ni–Al, and Me–Cr–Al–Y [3, 4] is used. One of the promising methods is the deposition of a sublayer of high-enthalpy Ni–Al powders.
As a result of exothermic effects, the interaction of nickel with aluminum can release up to 164 kcal/kg [1] (Table 1). An even greater exothermic effect is manifested in oxidative reactions. The amount of heat generated during the oxidation of aluminum is 24 times greater than during the formation of nickel aluminide.
According to the data in Table 1, the interaction of zirconium oxide with aluminum also exhibits an exothermic effect. In [5], on the basis of an analysis of the thermodynamic properties of the components of such a system, it was concluded that it is possible to completely reduce zirconium from its oxide at temperatures below 600°C with a fairly high exothermic effect.
It was found in [1–3] that an increase in the enthalpy of sprayed particles has a positive effect on the adhesion of coatings; therefore, it is recommended to use Al–Ni composite powders as the sublayer material. It was also found there that the weakest link in heat-shielding coatings is the interface between the sublayer and the upper ceramic layer, which is due to a significant difference in the thermal expansion coefficients of these materials.
It was suggested that it is expedient to use zirconium dioxide powder (80 wt %) with a cladding shell of aluminum (20 wt %) obtained according to the technology [6] as a material for heat-shielding coatings. The basis for this assumption was the high heat-insulating properties of zirconium dioxide and the possibility of an exothermic reaction when it interacts with aluminum. In this case, the presence of a developed surface of the aluminum shell on the ceramic core of the powder particle can increase the exothermic effect owing to the partial oxidation of aluminum and, thereby, increase the enthalpy of the sprayed particles. An increase in the enthalpy of the sprayed material leads to an increase in the adhesion of the coating, which can make it possible to completely change the design of heat-shielding coatings and abandon two-layer coatings in favor of metal-ceramic powders.
To confirm this assumption, two types of heat-shielding coatings were studied:
1. two-layer coating consisting of an Al–Ni sublayer (20–80 wt %) 0.1 mm thick and a ZrО2 layer 0.25 mm thick;
2. single-layer coating sprayed from ZrO2 powder clad with Al, 0.35 mm thick.
EXPERIMENTAL
To study the effect of exothermic effects during deposition of Al–Ni powder compositions and oxidative processes when using a ZrO2–Al composition on the heat balance of deposited particles by differential thermal analysis (DTA) on a Derivatograf device, the values of exothermic effects, as well as the temperature of the beginning and end of the reactions, were measured. Samples of powder weighing 5 g were used, and the studies were carried out in the heating mode, while the change in temperature and weight of the powder sample was recorded.
Both types of the studied heat-shielding coatings were obtained by plasma spraying of powders on a substrate of aluminum alloy AK4-1 on a UPU-3 unit with a PP-25 plasma torch. The power of the plasma torch varied from 10 to 26 kW; a mixture of argon with nitrogen or helium was used as the plasma gas; the spraying distance varied from 100 to 200 mm. Optimal deposition modes were determined using regression-correlation analysis of the effect of arc current, voltage, and deposition distance on coating adhesion.
After deposition of the coating according to the adhesive method of normal separation (GOST 9-304-87), the value of adhesion was measured, its thermal conductivity was determined on an IT-λ-400 instrument, and differential thermal analysis, metallographic studies, and thermal tests were also carried out.
RESULT AND DISCUSSION
The adhesion of a two-layer coating is affected by the processes occurring in the Al–Ni sublayer. Figure 1 shows the results of DTA of 20 Al–80 Ni powder (wt %).
An analysis of the rate of exothermic reactions in Al–Ni systems led the authors of [4] to the conclusion that, during the deposition of coatings from these compositions, intermetallic compounds should be formed first of all. It can be seen from Fig. 1 that a sharp increase in sample temperature at 580 and 930°C (curve 1) is accompanied by the simultaneous appearance of peaks on the differential curves of temperature (2) and weight (3), which indicates the unquestionable participation of oxidative reactions in the increase in the enthalpy of the system.
Analysis of the experimental data on the adhesion of coatings obtained under different spraying modes led to an approximating dependence
where I is the plasma torch arc current (A), U is the arc voltage (V), and S is the spraying distance (mm).
The optimal parameters for deposition of the Ni–Al sublayer in a mixture of Ar + N2 plasma-forming gases, at which its adhesion reaches 45 MPa, are I = 350 A, U = 45 V, and S = 150 mm.
The thermal conductivity of the deposited Ni–Al layers was determined indirectly. First, the thermal conductivity and thermal resistance of an AK-4 alloy sample 15 mm in diameter and 4.8 mm high were measured, and then a coating 0.2 mm thick was applied to one end part of the sample. After the measurements, the results were compared with the data obtained on uncoated samples. The values of the thermal conductivity of the deposited Ni–Al layer obtained in this way were 65 W/(m K), so that, despite high adhesion, a material with such a high thermal conductivity is poorly suited for creating heat-shielding coatings.
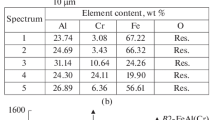
The adhesion of a coating consisting of a sublayer of 20 Al–80 Ni (wt %) 0.1 mm thick and a ZrO2 layer 0.25 mm thick was 27–30 MPa; the destruction of such a coating occurred mainly along the interface of the sublayer of Al–Ni and the main layer of ZrO2 (Fig. 2).
Next, a single-layer heat-shielding coating of zirconium dioxide powder clad with aluminum (80 ZrO2–20 Al, wt %) was studied. Figure 3 presents the results of derivatographic studies of the powder material of 80 ZrO2–20 Al (wt %). It can be assumed that the simultaneous appearance of small peaks on the ΔТ and ΔP curves at a temperature of 360°C is associated with the release of heat during aluminum oxidation. At a temperature of 650°C, the decrease on the ΔТ curve is associated with the melting of the cladding aluminum shell. A significant increase in ΔТ at a temperature of 920°C along with the absence of disturbances on the ΔP curve indicates the passage of redox processes inside the composite material.
Optimum spraying regimes for ZrO2–Al powder were achieved at I = 400 A, U = 60 V, and S = 150 mm. Figure 4 shows a photograph of a section of a single-layer coating deposited in the optimal mode.
As can be seen, the coating consists of a gray ZrO2 phase, the microhardness of which is 12 370–20 070 MPa, occupying 50–70% of the coating volume, and a light Al phase, occupying 5–26 vol %. Inclusions of aluminum oxide (3000–5300 MPa, 2–5 vol %) and intermetallic compounds ZrAl2 and ZrAl3 are also observed, the microhardness of which could not be determined owing to the large dispersion of the phase.
The porosity of the coating was less than 13%. On the AK-4 alloy substrate, the maximum adhesion of the coating reaches 32–36 MPa, which differs little from two-layer coatings.
Measurements of the thermal conductivity of a single-layer coating of clad powder were carried out according to the same scheme as for coatings of Ni–Al powders. As a result, values of 1.5–2.0 W/(m K) were obtained, which is quite comparable with the thermal conductivity of aluminum oxide.
Thermal cycling tests of various coatings were carried out on valves (40Kh10S2M steel) and pistons (AK4 alloy) of internal combustion engines. Each cycle consisted of heating to a temperature of 400°C and cooling in air to 20°C. Two-layer coatings with a sublayer of Al–Ni (20–80%) 0.1 mm thick and a main layer of ZrO2 0.25 mm thick withstood 54 cycles on the AK4 alloy and 61 cycles on steel 40Kh10S2M. A single-layer coating of ZrO2–Al on a steel and aluminum substrate 0.35 mm thick withstood 120 cycles.
REFERENCES
Nikitin, M.D., Kulik, A.Ya., and Zakharov, N.I., Teplozashchitnye i iznosostoikie pokrytiya detalei dizelei (Heat-Protective and Wear-Resistant Coverings of Diesel Parts), Leningrad: Mashinostroenie, 1977.
Antsiferov, V.N., Shmakov, A.M., Ageev, S.S., and Bulanov, V.Ya., Gazotermicheskie pokrytiya (Gas-Thermal Coatings), Yekaterinburg: Nauka, 1994.
Kudinov, V.V. and Bobrov, G.V., Nanesenie pokrytii napyleniem. Teoriya, tekhnologiya i oborudovanie (Sprayed Coating Application. Theory, Technology, Equipment), Moscow: Metallurgiya, 1992.
Nikiforov, G.D., Cidulko, A.G., Kitaev, F.I., and Lekarev, Yu.G., Properties and application of plasma coatings from thermoreactive nickel-aluminum powder, in Trudy 6-go Vsesoyuznogo soveshchaniya po zharostoikim pokrytiyam (Proc. 6th All-Union Meeting on Heat-Resistant Coatings, Leningrad, USSR, March 20–23, 1975, Leningrad: Nauka, 1975, pp. 150–157.
Vedmid’, L.B., Agafonov, S.N., Avraamov, Yu.A., Il’inykh, M.V., Merkushev, A.G., Plotnikov, M.S., and Terlyga, A.F., Thermal analysis for modeling the aluminothermic reduction of zirconium from oxide, Russ. Metall. (Metally), 2017, vol. 2017, no. 8, pp. 652–654. https://doi.org/10.1134/S0036029517080122
Novikov, H.N. and Pustotina, S.R., USSR Inventor’s Certificate no. 1097449, 1984.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
When a composite clad powder of the ZrO2–Al system is sprayed onto the valves of an internal combustion engine, exothermic reactions occur in the coating, which increase the enthalpy of the sprayed material and the adhesion of coatings. The use of a composite clad powder of the ZrO2–Al system during coating deposition gives a greater heat-shielding effect compared to the Ni–Al sublayer and makes it possible to significantly increase the adhesion and thermal cycling resistance of heat-shielding coatings.
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Gusev, V.M., Elagina, O.Y. & Buklakov, A.G. Improving the Properties of Plasma Heat-Resistant Coatings by Means of Spraying Materials That React with Exothermic Effects. Inorg. Mater. Appl. Res. 13, 728–731 (2022). https://doi.org/10.1134/S2075113322030145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113322030145