Abstract
The features of the interpretation of the spectral dependence of the luminescent properties of ZnO films on sapphire are presented. ZnO films of various thicknesses obtained in an oxygen atmosphere at various substrate temperatures, including the stage of recrystallization annealing, are considered for a comprehensive analysis. It is shown that, in the cathodoluminescence spectrum of thin ZnO films obtained at a low substrate temperature, only the red (650–1000 nm) band of the sapphire substrate is observed, and the luminescence of the ZnO film is suppressed by excessive defectiveness. Prolonged recrystallization annealing results in improved quality of thin ZnO films and the appearance of a wide (430–740 nm) band in ZnO. With an increase in the substrate temperature and film thickness, only the bands associated with ZnO are revealed in the cathodoluminescence spectra: an edge luminescence band (with maximum at 390 nm) and a red band (at 500–950 nm with a maximum near the region of 710 nm) associated with charged vacancies of zinc. Focusing the beam leads to local heating of the sample and an increase in the concentration of interstitial zinc. Related to this is the shift of the edge luminescence band to the 410 nm region, as well as blue mixing of the band of defect luminescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Interest in zinc oxide (ZnO), owing to the unique combination of optical, electrical, and piezoelectric properties, with its high radiation and thermal resistance and commercial availability, not only does not fade away, but is constantly expanding. One of the new promising directions is the use of ZnO as an ultrafast detector material [1] for ionizing and ultraviolet (UV) radiation. There are two components in the ZnO radiation spectrum: edge luminescence located near the fundamental absorption edge (380–400 nm) with a characteristic emission time of less than 1 ns and green luminescence associated with defects with a maximum in the range of 450–650 nm and slow decay kinetics. In view of this, ultrafast detectors and scintillators require perfect bulk ZnO crystals; however, the main method of hydrothermal synthesis used today for growing large perfect single crystals of ZnO [2] does not provide the required performance. Thin films are an alternative to bulk crystals. The main substrate material for obtaining thin-film structures based on ZnO is sapphire owing to its hardness, chemical resistance, optical transparency in a wide wavelength range, and also the industrial scale of production. There are many works [3–5], as well as review studies [6], devoted to the growth of zinc oxide on sapphire substrates of various orientations and to the study of its electrophysical and optical properties. The technology of forming ZnO films with specified electrophysical properties is sufficiently developed, whereas the nature of many luminescence centers in ZnO has no unambiguous interpretation. The same applies to the centers of luminescence in sapphire. Meanwhile, for the use of ZnO films on sapphire in the technology of detecting ionizing and UV radiation, it is necessary to study the luminescent properties using various excitation methods, for example, X-ray luminescence (XL), photoluminescence (PL), and cathodoluminescence (CL). At the same time, it should be borne in mind that the position and shape of the luminescence emission band depend on the method of excitation, and modification of the material during irradiation is also possible. In addition, it is known that, with such methods as XL and CL, which have a much higher energy than PL, irradiation penetrates not only into the film but also into the substrate and can also lead to local overheating of both. As a result, the interpretation of the obtained luminescence spectra is difficult since the problem of separating the spectral dependences of the luminescent properties of the ZnO film and the sapphire substrate arises.
The purpose of this work is to consider the interpretation of the spectral dependence of the luminescent properties of ZnO films on sapphire using the example of cathodoluminescence spectra under various excitation modes. ZnO films of various thicknesses obtained at different temperatures before and after the stage of recrystallization annealing are used for comprehensive analysis.
EXPERIMENTAL
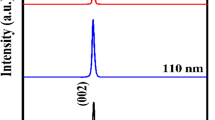
Sapphire wafers with a diameter of 50.8 mm and a thickness of 0.43 mm were used as substrates. The angle of deviation from the R (012) plane was ~0.15°. Both surfaces of the wafers were polished: one by a mechanochemical method to obtain an extremely low roughness, and the second by a mechanical method to obtain an optically transparent surface. The wafers were annealed at a temperature of 1200°C for 1 h and then cut into several parts. A gold sublayer was deposited on the surface of the wafers to improve the crystal quality of ZnO films [7, 8]. ZnO films were deposited on a VATT AMK-MI automated magnetron complex (OOO FerriVatt, Kazan) at the following deposition parameters: type I—substrate temperature Тs was 650°С, discharge current was 100 mA, time was 20 min, oxygen pressure was 10°–2° mm Hg, and film thickness was 2.4 μm; type II—substrate temperature Тs was 850°С, discharge current was 100 mA, time was 4 h, oxygen pressure was 10°–2° mm Hg, and film thickness was 24.4 μm. The thickness was determined by measuring the profile of the groove in the film using probe microscopy. At the next stage, films of type I were additionally subjected to prolonged recrystallization annealing in a muffle furnace at Тs = 850°С under atmospheric conditions. The study by reflection high-energy electron diffraction (RHEED) and the excitation of cathodoluminescence (CL) in the films was carried out by an electron beam of an EG-75 electronograph, the electron energy of which was 40 and 70 kV, with focusing (designation—focus) and defocusing (hereinafter, without designation).
RESULTS AND DISCUSSION
In the process of deposition, samples of type I and type II were obtained. According to the RHEED data, the sample of type I was weakly textured, and the structure was closer to a polycrystalline one. After recrystallization annealing at Тs = 850°С, a significant improvement in the structure occurred, but complete epitaxy was not achieved. According to the RHEED data, the sample of type II was epitaxial and oriented relative to the substrate. In the CL spectrum of the sample of type I (Fig. 1, curve 1) obtained at an accelerating voltage of 40 kV, only a wide band was observed in the 650–1000 nm range with a maximum in the 780 nm region. After annealing, the CL spectrum was radically transformed (Fig. 1, curve 2): a wide band appears in the 430–650 nm range with oscillations, as well as a plateau in the 650–740 nm range and a band in the 740–1000 nm range with two peaks at 760 and 840 nm. Both curves show a weak peak at 694 nm. Also in Fig. 1, curve 2, there are weak satellite peaks in the vicinity of the 694 nm peak. The CL spectra of the sample of type II (Fig. 2, curves 1 and 2) at accelerating voltages of 40 and 70 kV were identical: a narrow band in the region of the edge emission of 380–410 nm with a maximum at 390 nm and a wide band in the range of 500–950 nm with a maximum at 710 nm. The full width at half maximum of the edge luminescence peak was 200 meV. The CL spectrum of the sample of type II at an accelerating voltage of 70 kV with focusing (Fig. 2, curve 3) was characterized by a shift of the peak of the edge emission band by 20 nm and a shift of the maximum of the wide band to the region of 650 nm, as well as a decrease in its intensity. The full width at half maximum of the peak of the edge luminescence was 330 meV.
First of all, attention should be paid to the features that are revealed in ZnO films deposited under conditions of excess oxygen. There is a high probability of the presence of defects such as zinc vacancies VZn and interstitial oxygen Oi. The luminescence peaks in the spectral dependence for them should be at the positions of 520 and 626 nm, according to [9, 10]. The presence of defects of the OZn type is also likely, the peak of which lies in the region of 520 nm [12]. It is difficult to distinguish these defects in the spectral dependences (Figs. 1 and 2), since they lie in a wide band of the so-called defect (green) luminescence of ZnO. However, they can play an important role in the transformation of CL spectra during external impact and recrystallization. It should be noted that there are no defects of the VO type on the initial films (Fig. 1, curve 1; Fig. 2, curves 1–3), which have the lowest energy of formation and are usually present in ZnO films in thermodynamic equilibrium. Growth under conditions of excess oxygen shifts the stoichiometry in the ZnO films toward excess oxygen.
In the initial film of type I (Fig. 1, curve 1), there is no luminescence of the ZnO film itself because of its low crystal quality. This is the well-known effect of quenching luminescence due to the excessive content of defects. At the same time, we have a wide red luminescence band of the sapphire substrate owing to the high energy of electrons. The main peak at 694 nm corresponds to the R line of impurity of chromium, which is almost always present in sapphire crystals. The satellites in the vicinity of the 694 nm peak (Fig. 1, curve 2) can be characterized as anti-Stokes phonon satellites and oscillatory repetitions of the R line [13]. A wide band in the range of 650–1000 nm with a maximum in the region of 780 nm is associated with the presence of trivalent titanium ions in sapphire, as well as with the Jahn–Teller splitting of the ground state [14]. This is especially noticeable in Fig. 1, curve 2, in the form of two humps at 760 and 840 nm. The resolution of these peaks and an increase in the intensity of the red luminescence band of the sapphire substrate also indicate an improvement in the crystal quality of ZnO films of type I after recrystallization annealing. Integral scattering on numerous defects present in the bulk of the initial ZnO film is significantly reduced after its annealing. The presence of a plateau in the range of 650–740 nm (Fig. 1, curve 2) is associated with the luminescence of defects formed under conditions of an excess content of oxygen atoms during annealing and improvement of the film quality. The appearance of a wide band in the region of green luminescence also attracts attention. Apparently, annealing at 850°С for a long time leads to intense desorption of oxygen and a rearrangement of the structure of defects in ZnO films, the appearance of defects such as VO and ZnO, and transitions of the shallow donor–acceptor type associated with oxygen. The presence of oscillations is associated with the rearrangement of the micromorphology of the film as a result of annealing. The initial film with a buffer layer of gold was obtained at a relatively low temperature. Further prolonged recrystallization annealing at high temperatures leads to coagulation of gold atoms at the film–substrate interface and to the oriented growth of ZnO crystallites associated with them. As a result, a close to plane-parallel structure of the ZnO film is formed, operating under sliding irradiation according to the Fabry–Perot principle. It should be noted that the narrow band of edge luminescence intrinsic to high-quality ZnO films did not appear after annealing (Fig. 1, curve 2).
In the CL spectrum (Fig. 2, curves 1–3) of the film of type II, the bands responsible for the sapphire substrate were absent, which is associated with the thickness of the ZnO film. A band of edge luminescence characteristic of epitaxial ZnO films with a full width at half maximum of 200 meV is observed. As expected, its intensity increases with an increase in the accelerating voltage from 40 to 70 kV. At the same time, attention is drawn to the so-called orange-red band in ZnO, which is present in the CL spectrum with a maximum at 710 nm. According to the results of [15], this peak is associated with vacancies of charged zinc and is not associated with an impurity of nitrogen. This is also supported by an increase in the intensity of the peak with an increase in the accelerating voltage from 40 to 70 kV. However, more interesting effects occur when the electron beam is focused, leading to local overheating in the region of the spot. First of all, attention is drawn to the shift of the edge luminescence maximum to the region of 410 nm, as well as its broadening (full width at half maximum is 330 meV). As is known [16], the peak in this region is associated with defects of the Zni type. In this case, the increase of Zni defects is associated with local overheating under the action of a focused electron beam, as evidenced by the broadening of the edge luminescence band. The energy of the formation of oxygen vacancies VO is less than the energy of the formation of Zni, and when heated, the oxygen is intensely desorbed from the surface of the ZnO film. However, excess oxygen atoms over stoichiometry located in interstices fill the formed oxygen vacancies because their diffusion activity increases many times upon heating. Related to this is the absence of peaks in the range of 490–500 nm in the CL spectra (Fig. 2). In addition, local heating of the sample leads to a narrowing of the band gap in ZnO, which can also be a reason for the shift of the edge luminescence maximum to the 410 nm region.
It remains unclear how the wide band of red luminescence of ZnO is suppressed when the beam is focused. Perhaps this occurs owing to the temperature quenching of the CL in this region or to the redistribution of the excitation energy. Nevertheless, the shift of the maximum of the red band to the region of 650 nm can be associated with the so-called “blue” [16] shift of luminescence observed with an increase in the sample temperature.
CONCLUSIONS
The features of the interpretation of the spectral dependence of the luminescence properties of ZnO films on sapphire were presented using the example of cathodoluminescence spectra at different excitation energies. ZnO films of various thicknesses obtained in an oxygen atmosphere at various substrate temperatures including the stage of recrystallization annealing were considered for a comprehensive analysis.
It was shown that, in the cathodoluminescence spectrum of thin ZnO films obtained at a low substrate temperature, only the red (650–1000 nm) band of the sapphire substrate is observed, while the luminescence of the ZnO film is suppressed by excessive defectiveness.
Prolonged recrystallization annealing improves the quality of thin ZnO films and leads to the appearance of a wide (430–740 nm) band in ZnO. With an increase in the film thickness, only bands associated with ZnO appear in the cathodoluminescence spectra: the band of the edge luminescence (with a maximum at 390 nm) and the red band (at 500–950 nm with a maximum in the region of 710 nm) associated with charged vacancies of zinc. Focusing the beam results in local heating of the sample and an increase in the concentration of interstitial zinc. Related to this is the shift of the edge luminescence band to the 410 nm region, as well as the blue mixing of the defect luminescence band.
REFERENCES
Wilkinson, J., Ucer, K.B., and Williams, R.T., The oscillator strength of extended exciton states and possibility for very fast scintillators, Nucl. Instrum. Methods Phys. Res., Sect. A, 2005, vol. 537, nos. 1–2, pp. 66–70.
Pearton, S.J., Norton, D.P., Ip, K., Heo, Y.W., and Steiner, T., Retracted: Recent progress in processing and properties of ZnO, Prog. Mater. Sci., 2005, vol. 50, no. 3, pp. 293–340.
Fons, P., Iwata, K., Niki, S., Yamada, A., and Matsubara, K., Growth of high-quality epitaxial ZnO films on α-Al2O3, J. Cryst. Growth, 1999, vols. 201–202, pp. 627–632.
Chen, Y., Bagnall, D.M., Koh, H.J., Park, K.T., Hiraga, K., Zhu, Z.Q., and Yao, T., Plasma assisted molecular beam epitaxy of ZnO on c-plane sapphire: Growth and characterization, J. Appl. Phys., 1998, vol. 84, art. ID 3912.
Fons, P., Iwata, K., Niki, S., Yamata, A., Matsubara, K., and Watanabe, M., Uniaxial locked growth of high-quality epitaxial ZnO films on (11\(\bar {2}\)0)α-Al2O3, J. Cryst. Growth, 2000, vol. 209, nos. 2–3, pp. 532–536. https://doi.org/10.1016/S0022-0248(99)00614-4
Özgür, Ü., Alivov, Y.I., Liu, C., et al., A comprehensive review of ZnO materials and devices, J. Appl. Phys., 2005, vol. 98, art. ID 041301.
Muslimov, A.E., Butashin, A.V., Kolymagin, A.B., et al., Real structure of the ZnO epitaxial films on (0001) leucosapphire substrates coated by ultrathin gold layers, Crystallogr. Rep., 2016, vol. 61, pp. 63–65.
Vlasov, V.P., Butashin, A.V., Kanevsky, V.M., et al., Influence of ensembles of gold nanoparticles on the growth of ZnO on the sapphire (0001) surface, Crystallogr. Rep., 2014, vol. 59, pp. 736–738.
Zhao, Q.X., Klason, P., Willander, M., Zhong, H.M., Lu, W., and Yang, J.H., Deep-level emissions influenced by O and Zn implantations in ZnO, Appl. Phys. Lett., 2005, vol. 87, art. ID 211912.
Liu, X., Wu, X., Cao, H., and Chang, R.P.H., Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition, J. Appl. Phys., 2004, vol. 95, no. 6, pp. 3141–3147.
Kohan, A.F., Ceder, G., Morgan, D., and Van de Walle, C.G., First-principles study of native point defects in ZnO, Phys. Rev. B, 2000, vol. 61, pp. 15019–15034.
Lin, B., Fu, Z., and Jia, Y., Green luminescent center in undoped zinc oxide films deposited on silicon substrates, Appl. Phys. Lett., 2001, vol. 79, no. 7, pp. 943–945. https://doi.org/10.1063/1.1394173
Nelson, D.F. and Sturge, M.D., Relation between absorption and emission in the region of the R lines of ruby, Phys. Rev. A, 1965, vol. 137, no. 4a, pp. A1117–A1130. https://doi.org/10.1103/PhysRev.137.A1117
Luca, M., Coron, N., Dujardin, C., et al., Scintillating and optical spectroscopy of Al2O3: Ti for dark matter searches, Nucl. Instrum. Methods Phys. Res., Sect. A, 2009, vol. 606, no. 3, pp. 545–551.
Anantachaisilp, S., Smith, S.M., Ton-That, C., et al., Nature of red luminescence in oxygen treated hydrothermally grown zinc oxide nanorods, J. Lumin., 2015, vol. 168, pp. 20–25.
Lin, B., Fu, Z., and Jia, Y., Green luminescent center in undoped zinc oxide films deposited on silicon substrates, Appl. Phys. Lett., 2001, vol. 79, no. 7, pp. 943–945. https://doi.org/10.1063/1.1394173
ACKNOWLEDGMENTS
This work was carried out using the equipment of the Center for Collective Use of the Federal Research Center Crystallography and Photonics.
Funding
As part of the work on the State Task of the Federal Research Center Crystallography and Photonics of the Russian Academy of Sciences, as well as with the support of the Russian Foundation for Basic Research (grant no. 18-29-12099 mk).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by S. Rostovtseva
Rights and permissions
About this article
Cite this article
Ismailov, A.M., Muslimov, A.E. Features of the Interpretation of the Luminescence Spectra of Zinc Oxide Films on Sapphire. Inorg. Mater. Appl. Res. 12, 343–346 (2021). https://doi.org/10.1134/S2075113321020180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113321020180