Abstract
In this research and development, the extrusion method was used to obtain polyethylene films containing 0.1–1.5 wt % n-SiC and n-Si nanoparticles obtained by the plasma chemical method. Using the spectral analysis method, it was found that the films obtained absorb UV radiation in the range of 200–400 nm, which is harmful to organic matter. The method of X-ray structural analysis was used to determine the average size of particles and the quality of their dispersion in the films. Differential scanning calorimetry and physicomechanical tests have shown that nanoparticles do not affect the formation of the internal structure of the polyethylene (PE) matrix. The degree of crystallinity, melting point, and crystallization point remain unchanged. The properties of the surface of the films, studied by tribological and triboelectric methods and determination of the contact angle, remain constant and do not differ from the properties of PE films with a nanoparticle content from 0.1 to 1%. At 1.5% nSiC content, a change in the surface microrelief is diagnosed, leading to a small increase in the film friction coefficient. The polyethylene films filled with n-SiC and n-Si obtained in this work are recommended for use as UV protective coatings for various purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Important objectives in the development of polymeric composite materials are obtaining new performance properties or combinations thereof, improving the technological properties and processability, waste management and environmental issues, expanding the raw material base and assortment of initial components, and lowering the cost of materials.
One of the promising fillers that change the structural organization and properties of the polymer matrix is the nanosized modification of silicon. Nanocrystalline silicon (n-Si) and silicon carbide (n-SiC) have unique optical and electrophysical characteristics [1], and taking into account the nontoxicity and economy of these substances, the widespread use of materials based on them in various sectors of the national economy is predicted. A particularly important synthetic problem in order to ensure effective practical application and creation of new polymer materials based on n-Si, be it sunscreen films [2] and coatings [3], photoluminescent and electroluminescent composites, and light-fast dyes [4], is the embedding of these nanosized particles n-Si into polymer matrices. In the simplest case, the role of the polymer matrix is to ensure the necessary physical and mechanical properties of the composite (mechanical strength, elasticity, adhesion to the substrate, protection from aggressive media, etc.). In addition, the polymer matrix is also required to prevent agglutination of nanoparticles and to create conditions for uniform distribution of n‑Si over the sample volume or its fixation on the surface. Finally, if the matrix itself has special optical, electrically conductive, and photoelectric properties, then, by adding the functional properties of n-Si particles, we obtain a material with a complex of new properties and characteristics.
It is known that UV radiation of the spectral range 290–330 nm significantly reduces the yield of vegetable crops owing to the cost of part of the plant growth energy to protect against it [5], and in the range 200–420 nm, it reduces the shelf-life of food products [6] and degrades the performance of electronic products [7, 8]. It has also been found that the freshness of a food product sealed in a clear food package in the case of using an oxygen absorbent may unexpectedly be improved by having a UV absorber in the transparent packaging material. A variety of methods are used to protect against UV radiation. As a rule, when chemical substances containing chromophore groups are introduced into polymers, ultraviolet absorption occurs, or it is converted into infrared radiation [9].
The introduction of nanoparticles of metal and nonmetal oxides into polymers, which have the effect of shielding UV radiation, opens up great opportunities for creating technologically advanced nanopolymer composites based on thermoplastics of various chemical structures with a high level of operational characteristics. Such materials in the form of films will be especially used in agriculture, the packaging industry, and medicine [10, 11].
Taking into account the nontoxicity of these nanoparticles to living organisms and the relatively low cost of their production, we can assume their effective use in medical materials and protection from UV radiation [12].
To protect against UV radiation, chemicals are traditionally introduced into polymers containing chromophore groups that absorb ultraviolet light or convert it into infrared radiation [13]. Nanoparticles of metal and nonmetal oxides have the ability to screen UV radiation. This makes it possible to create polymer nanocomposite materials based on thermoplastics of various chemical structures with a high level of performance characteristics, which can be used in agriculture, the packaging industry, and medicine.
The aim of this work was to obtain and study the structure and properties of film materials based on low density polyethylene and nanosized particles of silicon and silicon carbide, which have UV protective properties.
EXPERIMENTAL
The starting materials for the preparation of film nanocomposites were low density polyethylene (LDPE) grade 10 803-020 and ultrafine crystalline silicon and silicon carbide. Silicon and silicon carbide powders were obtained by the method of plasma chemical recondensation of coarse-crystalline silicon into n-Si nanocrystalline powder and then into n-SiC. The synthesis of n-Si was carried out in argon plasma in a closed gas cycle in a plasma evaporator-condenser operating in a low-frequency arc discharge. After synthesis, n-Si particles were subjected to microencapsulation, in which a protective SiO2 shell was created on their surfaces, which protected the powder from atmospheric exposure and made it stable during storage. With further heat treatment, n-Si particles transform into n-SiC. In this work, we used silicon powders with a specific surface area of ~36 m2/g according to the Brunauer–Emmett–Teller (BET) method.
Preliminary mixing of nanosized silicon particles and silicon carbide with polyethylene was carried out using heated laboratory mill of grade VK-6 with a roll diameter of 90 mm and a friction of 1.4. The mixing temperature was 120 ± 5°C, and the mixing time was 3 min. The obtained material was mechanically ground to a particle size from 2 to 5 mm before loading into the extruder.
Molding of films with a thickness of 85 ± 5 μm was carried out on a semi-industrial extruder to obtain a tubular film ARP-20-150 (Russia) at temperatures of 170–190°C in the zones of the extruder and extrusion die and at screw speed of 120 min–1, the collecting speed of tubular sheeting of was 2 m/min, and the blow-up ratio was 2.0. The process parameters of the nanocomposites were selected on the basis of the conditions of thermal stability and the polymer melt intrinsic viscosity recommended for processing.
For physicomechanical tests, films with a thickness of 50 ± 5 μm obtained by melt extrusion through a circular die with subsequent blowing of a tubular sheeting were used. The silicon carbide content in the films varied (0, 0.1, 0.3, 0.5, 1.0, 1.5 wt %), as well as the silicon content (1.0, 1.5 wt %).
The mechanical properties and optical transparency of polymer films, their phase composition and crystallinity, and the association of mechanical and optical properties with the microstructure of polyethy-lene and the granulometric composition of modifying nanoparticle powders were studied.
The physical and mechanical properties of the films under tension (in the direction of extrusion), namely, tensile modulus (MPa), tensile strength (MPa), and elongation at break (%), were measured using a UTS-211 universal tensile tester (Germany). The clamping speed was 100 mm/min; the distance between the clamps was 30 mm. The test temperature was 21°C. The tests were carried out on rectangular specimens 10 mm wide, with a working area of 50 mm. The tests were carried out on a selection of five parallel samples.
The optical transparency of the films was estimated from the absorption spectra. The absorption spectra of the obtained films were measured using an SF-104 spectrophotometer (Russia) in the wavelength range of 200–800 nm. To obtain absorption spectra of polyethylene films and composite films, samples 3 × 3 cm in size, fixed in a special holder to ensure uniform film tension, were examined. To study the phase composition of materials, the degree of crystallinity of the polymer matrix, the size of single-crystal blocks in nanoparticle powders and in the polymer matrix, and the size density function (SDF) of crystallites in the initial nanoparticle powders, X-ray diffraction analysis was used on the basis of the data of wide-angle scattering of monochromatic X-rays.
X-ray diffraction measurements were carried out on a diffractometer with a Guinier scheme (focusing diffraction imaging of the sample in transmission): a G670 (Huber) camera with a curved Ge (111) monochromator of the primary beam, cutting out the characteristic radiation line \({{K}_{{{{\alpha }_{1}}}}}\) (wavelength λ = 1.5405981 Å) of an X-ray tube with a copper anode. The diffraction pattern in the range of diffraction angles 2θ from 3° to 100° was recorded by a plate with optical memory (IP detector) curved around the camera, the image from which was read by a special laser scanner. The set of experimental data was issued in the form of a table of pairs of values of intensity–diffraction angle with a step of 0.005° along the angle 2θ. Diffractometric measurements were carried out with an exposure of about 4 h at each point of the measurement range.
To determine the instrumental function of the device at the same diffractometer setting, the diffraction pattern of the LaB6 reference powder (SRM 660 standard, NIST) was measured.
Qualitative phase analysis was performed by comparing the Bragg peaks of the diffraction pattern with the ICDD PDF-2 X-ray powder diffraction database. The experimental data were processed and analyzed using the STOE WinXPOW software package, version 2.20 (STOE & Cie GmbH, Darmstadt).
Samples of the starting n-Si and n-SiC powders for X-ray diffractometry were prepared by applying a thin layer of the powder onto a 6-μm-thick special film substrate (MYLAR, Chemplex Industries Inc., Cat. No: 250, Lot No: 011671). Film samples of LDPE and its composites were installed in the diffractometer holder without any substrate, but to minimize the texture effect, two film layers were used, oriented with the extrusion directions perpendicular to each other.
The determination of residual charge on the samples on both sides of the film at the first time after electrification (by friction with a synthetic brush) and 15–20 min after vacuum on the metal surface, depending on the concentration of silicon and silicon carbide, was carried out using a STATIRON-M electrostatic voltmeter at a distance from the emitter of the sample of 60 mm. The surface electrical resistance of the samples was measured with a TK-1 teraohmmetra (Russia). The coefficient of friction of the films on steel was measured using a dynamometer by pulling a metal weight along the inclined surface of the film. To determine the surface energy of the films, the contact angle with distilled water was determined using the standard procedure [6].
The study of the samples by differential scanning calorimetry (DSC) was carried out on a Netzsch DSC 204 F1 instrument in an inert atmosphere of argon (Ar) with a heating rate of 10°C/min. The average statistical error in measuring thermal effects was approximately ±3%. The enthalpy of melting was calculated using the NETZSCH Proteus Thermal Analysis 4.8.4 program.
RESULTS AND DISCUSSION
The phase composition of the materials under study and their components were investigated according to the data of wide-angle X-ray scattering. The measured X-ray diffraction patterns of the initial n-Si and n-SiC powders in intensity and the position of the Bragg peaks fully corresponded to the phase of pure crystalline silicon (a cube cell of the diamond type—space group Fd\(\bar {3}\)m, cell parameter aSi = 0.5435 nm), the X-ray the diffraction pattern of which is well studied; therefore, it is not presented here.
At the first stage of the work, we investigated the granulometric composition of the initial n-Si and n‑SiC powders. The density function of the crystallite size distribution in n-Si powder was reconstructed from the X-ray powder diffraction pattern using the PM2K computer program [14], which implements the method [15] for modeling the full diffraction pattern profile based on the theory of physical processes of X-ray diffraction. Modeling was carried out under the assumption of a spherical shape of crystallites and a lognormal distribution of their sizes, taking into account the deformation effects from plane and linear defects of the crystal lattice. The obtained SDFs for the initial n-Si powders are shown graphically in Fig. 1, in the caption to which the statistical parameters of the found distributions are given. These distributions are characterized by such important parameters as Mo(d)—the position of the maximum of the SDF (distribution mode); 〈d〉V—the average crystallite size over the sample volume (arithmetic mean size); and Me(d)—the median of the distribution that determines the size d, indicating that particles with diameters less than this size compound half of the powder volume.
The results shown in Fig. 1 show that the initial n‑Si powders contain particles with a size of less than 10 nm, which are particularly efficient at absorbing UV radiation. The n-Si powder contains quite a few particles with a diameter of more than 100 nm.
The obtained data of wide-angle X-ray scattering provide only qualitative information, since the quantitative phase analysis of polymer films is practically impossible owing to the complexity of the diffraction scattering patterns and the presence of texture [16]. Often, in the phase analysis of polymers, a qualitative comparative analysis is used, which allows one to follow the change in morphology depending on the parameters of the technology for obtaining samples.
The measured diffraction patterns of wide-angle X‑ray scattering by the studied films are shown in Fig. 2. The diffraction patterns are typical for polymers. As a rule, polymers are two-phase systems consisting of an amorphous phase and regions with long-range order, conventionally called crystals. Their diffraction patterns are [16] a superposition of the intensity of scattering by the amorphous phase, which has the form of a wide halo in the low-angle region (in this case, in the 2θ region between 10° and 30°), and the intensity peaks of Bragg scattering by the crystalline phase.
The data in Fig. 2 are presented on a scale of relative intensities (the intensity of the highest peak of the diffraction pattern is taken to be 100%). For convenience, the curves are shown with an offset along the y axis. If the scattergrams are plotted without displacement, then the profiles of the diffraction patterns of the composite films completely coincide with the diffraction pattern of a pure LDPE film, except for the peaks of crystalline silicon, which are absent in the diffraction pattern of the PE. This indicates that additions of n-Si powders hardly changed the crystal structure of the polymer.
On the diffraction patterns of films with silicon, the peaks of crystalline silicon are clearly distinguishable (their reference positions with corresponding Miller indices are indicated by dashes below). The heights of the silicon cognominal peaks (i.e., peaks with the same indices) on the diffraction patterns of the composite films differ by about half, which corresponds to the ratio of mass concentrations of Si set during their manufacture.
Figure 3 shows a diffraction pattern of a sample of synthetic n-SiC powder. Vertical dashes mark the positions and intensities of the diffraction lines of crystalline silicon carbide from the ICDD PDF-2 database.
As can be seen from the figure, all the diffraction peaks of this experimental diffraction pattern completely coincide with the lines of the file diffraction patterns of moissanite (natural mineralogical carborundum)—card numbers in the database are indicated in the figure. The best agreement is obtained with the diffraction patterns of moissanite of the 6H polytype.
Other crystalline phases were not detected in the experimental diffraction pattern within the sensitivity of the method (about 1%). Therefore, the powder under study can be classified as synthetic carborundum, mainly consisting of the 6H polytype.
Owing to the developed polytypism of silicon carbide crystals (more than a hundred polytypes are known), it is difficult to construct a reliable mathematical model of the sample and, therefore, to calculate the crystallite size distribution function on the basis of the experimental diffraction pattern. It is necessary to know exactly what polytypes are present in the sample. This is unknown to date. Therefore, the size of the coherent scattering block averaged over the sample volume was estimated. Calculations were made on the basis of the Scherrer formula and the Wilson formula using the Size/Strain utility from the STOE WinXPOW software package. The calculations show the average block size 〈D〉V = 34 ± 3 nm.
Further, we investigated the effect of dispersed nanoparticles on the structure of films in the bulk. The films were investigated by differential scanning calorimetry, X-ray structural analysis, and physical and mechanical tests.
As shown in Table 1, the nanosized silicon and silicon carbide particles have little effect on the crystallization process of the polyethylene matrix. The melting and crystallization temperatures of PE do not change in the presence of nanoparticles up to 1.5%. The melting heat of PE crystallites melting does not change, and accordingly, the crystallinity of the PE matrix does not change. Nanoparticles appear to act only as nucleating agents of the PE crystal phase. In this case, the amount of the crystalline phase of PE does not change, which is associated with the low degree of crystallinity of the initial low-density polyethylene. In the course of crystallization, most of the nanoparticles are most likely displaced into the amorphous phase, and thus they do not participate in the initiation and growth of crystallites. All of the above also indicates the absence of a strong adsorption interaction between nanoparticles and PE macromolecules. Otherwise, the presence of nanoparticles would affect the crystallization temperature and melting heat of PE.
The behavior of such materials should also depend little on the n-Si and n-SiC content under tension. The results of mechanical tests of films are presented in Table 2, where it is seen that the addition of n-Si and n-SiC particles at concentrations of 1–1.5% hardly affected the strength, elastic modulus, and relative elongation of the films in comparison with the initial PE.
However, at a nanoparticle content of 0.1–0.5%, a slight decrease in the elastic modulus and a simultaneous increase in the elongation at break are observed. It can be assumed that this is due to some softening of the amorphous phase of PE at a low content of nanoparticles.
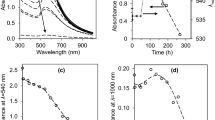
Further in this work, we investigated the spectral characteristics of the films (transmission). The transmission spectra of the films under study in the UV region are shown in Figs. 4 and 5.
It has been shown experimentally that the introduction of n-Si decreases the transparency of the films in the entire investigated wavelength range, but a particularly strong decrease in the transmission (by almost 20 times) is observed in the wavelength range of 220–400 nm, i.e., in the UV region.
The used technology of mixing and preparation of films ensured the preparation of samples with uniform dispersion of nanoparticles over the thickness and perimeter of film materials in the entire concentration range. In this case, the concentration of modifying additives n-Si and n-SiC in the filled films corresponded to the ordered composition, which was confirmed by X-ray phase analysis.
The effect of reduction of UV radiation is especially pronounced in the film of LDPE + 1% n-Si. As seen in Fig. 2, films with n-SiC have a lower absorbance of UV radiation in the range 200–400 nm than films with n-Si with the same content in the materials. This can be explained by the fact that the number of particles with sizes less than 10 nm per unit volume/mass in the n-Si powder is greater than in the n-SiC powder according to the experimentally obtained SDFs. Therefore, PE + n-Si films are the most effective from the point of view of UV protective properties.
The mechanical characteristics obtained in the work correlate with the parameters of the internal structure of the films: melting points, crystallization temperatures, and the degree of crystallinity (see Tables 1 and 2). In this case, silicon and silicon carbide nanoparticles are uniformly distributed over the volume of the amorphous part of the PE matrix and thereby form a continuous protective layer that almost completely absorbs UV radiation in the spectral range which is dangerous to living organisms, plants, and organic substances.
Since the n-Si and n-SiC nanoparticles studied by us hardly interact with the PE matrix in the bulk of the films, it should be expected that the surface properties (morphology) also do not undergo changes. To study the effect of nanoparticles on the surface morphology, we used three methods: tribological, triboelectric, and determination of the contact angle. It is known that superfine fibers [17] and dispersed fillers, including in the nanoscale range, can affect not only the internal structure of polymer matrices but also the surface properties (roughness, polarity, bioresistance, adsorption capacity, etc.) [18]. Therefore, the further goal of this work was to determine the complex of characteristics of the surface of the studied films, sensitive to its morphology: friction coefficient, electrification, and contact angle.
Table 3 shows the data on the electrification of films with n-Si and n-SiC nanoparticles.
It can be seen from the data presented that the studied films are dielectrics which can accumulate charge on the surface. After a long (15–20 min) discharge from the positive electrode (ground), this residual charge remains on the surface of films containing up to 1% n-SiC (see the first column in Table 3). Films with 1.5% n-SiC were completely discharged, which indicates the yield of some of the nanoparticles into the surface layers and directly onto the film surface. In this case, the sorption capacity of the surface of these films for water molecules should increase. The formed adsorbed layer of water (even a very small one) improves the removal of charge from the surface of films. When charging the surface of films with friction on dielectric materials and then discharging to a metal grounded surface, samples with 1.5% n-SiC have a lower surface charge, which confirms our assumptions.
To assess the surface morphology (roughness), we applied a tribological method: measuring the coefficients of friction of films with n-Si and n-SiC on a polyethylene film and a steel surface. The data are presented in Tables 4 and 5.
As follows from Table 4, the coefficient of friction for films with n-SiC is slightly lower than that for PE films. Apparently, this is due to the electrification of the surface of films during friction against each other. For films with n-Si, the coefficients of friction vary over a wide range and are approximately the same as for PE films.
The friction coefficients of films on a polished steel surface were also investigated. The data are presented in Table 5.
As can be seen from the data presented, the friction coefficient of films on steel is at the level of values for PE or slightly lower. The exception is films containing 1.5% n-SiC. Their friction coefficient significantly exceeds the values for all samples. Most likely, this may be due to the presence of n-SiC particles on the surface of the films, as well as the associated slight hydrophilization of the surface, as we have already discussed above.
To check the fact that silicon carbide nanoparticles are on the surface of the films and there is hydrophilization of their surface, we used the method for determining the contact angle. It was determined that the contact angle for all films, regardless of composition, has a constant value: 90°. This means that the surface of the films is completely hydrophobic and is not wetted with water. In this regard, our assumption about the presence of nanoparticles on the surface of PE films and their partial hydrophilization has not been confirmed. Apparently, in PE films with 1.5% nanoparticles, the size of which according to X-ray diffraction data is 34 nm for n-SiC and 76 nm for n-Si (and their individual agglomerates can reach from 100 to 300 nm), the surface microrelief changes (roughness) as a result of the presence of these particles near the surface of the films. The changed surface microrelief leads to an increase in the friction coefficient.
CONCLUSIONS
On the basis of the results of research and development, it was found that, when using the stage of premixing with the help of heated mixing mills, it is possible to satisfactorily disperse in the LDPE melt low concentrations of n-SiC and n-Si nanoparticles obtained by the plasma chemical method. Using a typical extrusion machine for the production of film with parison swell, it was possible to form films with a uniform volume distribution of n-SiC and n-Si nanoparticles in the studied concentration range. Spectral analysis revealed that the films obtained absorb UV radiation in the range 200–400 nm, which is harmful to organic matter. At the same time, on the basis of the X-ray diffraction data, the average particle size and the quality of their dispersion in the films were determined. It was established by DSC and physical and mechanical tests that nanoparticles do not affect the formation of the internal structure of the PE matrix. The crystallinity and melting and crystallization temperatures remain unchanged. Also, the properties of the surface of the films, studied by tribological and triboelectric methods and determination of the contact angle, remain constant and do not differ from the properties of PE films with a nanoparticle content of 0.1 to 1%. At 1.5% n-SiC content, a change in the surface microrelief is diagnosed, leading to a slight increase in the film friction coefficient. The polyethylene films obtained in this work filled with n-SiC and n-Si nanoparticles are recommended for use as UV protective coatings for various purposes.
REFERENCES
Kumar, V., Nanosilicon, Kumar, V., Ed., Amsterdam: Elsevier, 2008.
Pickrell, J.A., Erikson, L.E., Dhakal, K., and Klabunde, K.J., Toxicity of inhaled nanomaterials, in Nanoscale Materials in Chemistry, Klabunde, K.J. and Richards, R.M., Eds., Chichester: Wiley, 2009, chap. 22, pp. 730–769.
Ishchenko, A.A., Ol’khov, A.A., and Gol’dshtrakh, M.A., RF Patent 2429189, Byull. Izobret., 2011, no. 26.
Sanders, R.W., Cooke, S.L., Fischer, J.M., Fey, S.B., Heinze, A.W., Jeffrey, W.H., Macaluso, A.L., Moeller, R.E., Morris, D.P., Neale, P.J., Olson, M.H., Pakulski, D.J., Porter, J.A., Schoener, D.M., and Williamson, C.E., Shifts in microbial food web structure and productivity after additions of naturally occurring dissolved organic matter: results from large-scale lacustrine mesocosms, Limnol. Oceanogr., 2015, vol. 60, no. 6, pp. 2130–2144. https://doi.org/10.1002/lno.10159
Torres-Nuñez, E., Sobrino, C., Neale, P.J., Ceinos, R.M., Du, S.J., and Rotllant, J., Molecular response to ultraviolet radiation exposure in fish embryos: implications for survival and morphological development, Photochem. Photobiol., 2012, vol. 88, no. 3, pp. 701–707. https://doi.org/10.1111/j.1751-1097.2012.01088.x
Neale, P.J., Sobrino, C., Segovia, M., Mercado, J. M., Leon, P., Cortés, M.D., Tuite, P., Picazo, A., Salles, S., Cabrerizo, M.J., Prasil, O., Montecino, V., Reul, A., and Fuentes-Lema, A., Effect of CO2, nutrients and light on coastal plankton. I. Abiotic conditions and biological responses, Aquat. Biol., 2014, vol. 22, pp. 25–41. https://doi.org/10.3354/ab00587
Fetisov, G.V., X-ray phase analysis, in Analiticheskaya khimiya i fiziko-khimicheskie metody analiza (Analytical Chemistry and Physicochemical Analysis), Ishchenko, A.A., Ed., Moscow: Akademiya, 2010, vol. 2, chap. 11, pp. 153–184.
Sahi, S., Magill, S., Ma, L., Xie, J., Chen, W., Jones, B., and Nygren, D., Wavelength-shifting properties of luminescence nanoparticles for high energy particle detection and specific physics process observation, Sci. Rep., 2018, vol. 8, art. ID 10515.
WINXPOW Version 1.06, Darmstadt: STOE & CIE, 1999.
Nanostructured Materials: Processing, Properties and Applications, Koch, C.C., Ed., Amsterdam: Elsevier, 2009.
Ischenko, A.A., Ol’khov, A.A., Gol’dshtrakh, M.A., Es’kova, E.V., Kononov, N.N., and Dorofeev, S.G., RF Patent 2441046, Byull. Izobret., 2012, no. 3
Verdaguer, D., Jansen, M.A.K., Llorens, L., Morales, L.O., and Neugart, S., UV-A radiation effects on higher plants: exploring the known unknown, Plant Sci., 2017, vol. 255, pp. 72–81. https://doi.org/10.1016/j.plantsci.2016.11.014
Olkhov, A.A., Liaw, D.J., Fetisov, G.V., Goldschtrakh, M.A., Kononov, N.N., Krutikova, A.A., Storozhenko, P.A., Zaikov, G.E., and Ischenko, A.A., Polymer nanocomposites films on polyethylene with UV-protective properties, Polym. Res. J., 2013, vol. 7, no. 1, pp. 79–90.
Mittelman, A.M., Fortner, J.D., and Pennell, K.D., Effects of ultraviolet light on silver nanoparticle mobility and dissolution, Environ. Sci.: Nano, 2015, vol. 2, pp. 683–691.
Scardi, P., Recent advancements in whole powder pattern modeling, Z. Kristallogr. Suppl., 2008, vol. 27, pp. 101–111.
Strbeck, N., X-Ray Scattering of Soft Matter, Berlin: Springer-Verlag, 2007.
Olkhov, A.A., Krutikova, A.A., Goldshtrakh, M.A., Orlov, N.A., Ischenko, A.A., Staroverova, O.V., and Iordanskii, A.L., New fibrous materials based on poly-3-hydroxybutyrate for biomedical purposes, made via electrospinning technique, Inorg. Mater.: Appl. Res., 2018, vol. 9, no. 1, pp. 100–107.
Abdalla, S., Al-Marzouki, F., Obaid, A., and Gamal, S., Action of colloidal silica films on different nano-composites, Results Phys., 2016, vol. 6, pp. 209–214.
ACKNOWLEDGMENTS
We used equipment of the Common Use Center New Materials and Technologies of the Institute of Biochemical Physics of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Olkhov, A.A., Stanishevskaya, I.E., Semenova, M.N. et al. Film Materials Based on Polyethylene with Silicon Nanoparticles and Silicon Carbide. Inorg. Mater. Appl. Res. 12, 17–24 (2021). https://doi.org/10.1134/S2075113321010299
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113321010299