Abstract
We studied the possibility of producing powder catalysts Al–Fe/SiO2 and Al–Co/SiO2 by mixing precursor powders of Al, Fe, Co, and SiO2 in a planetary mill in gross weight ratios corresponding to the domain of existence of intermetallic compounds Al3Fe and Al3Co and annealing in vacuum at 900°C for 30 min. Annealing in air at lower temperatures (580–600°С) leads to the formation of corundum Al2O3, mullite Al6Si2O13, and silicides CoSi2, CoSi, Co2Si, FeSi2, and Fe3Si in the composite structure. The synthesized composite powders 13Al/4Co/6.5SiO2 and 13Al/4Fe/6.5SiO2 contain nonequilibrium phases. Powders without sintered masses with good flow and a fractional composition of less than 100 μm are obtained after synthesis in vacuum at 900°C for 30 min. The fractional composition of powder Fe–Al/SiO2 is characterized by a distribution of less than 50 μm (27.1%), 50–63 μm (15.3%), and 63–100 μm (57.4%); the fractional composition of powder Co-Al/SiO2 is 37.5%, 16.2%, and 46.3%, respectively. According to X-ray phase analysis, the powders synthesized at 900°С contain the phases of Fe3Al, Fe0.5Al0.5, Fe14Al86, Co2Al5, and Co27Al73; they do not contain silicides and mullites of the type Al6Si2O13. It is experimentally established that the mass fractions of the precursors Al, Fe, and Co are (Al + Fe) : SiO2 = 31 : 69 and (Al + Co) : SiO2 = 58 : 42 as a result of cladding of powder SiO2. It is concluded that the vacuum annealing mode of 900°C for 30 min does not provide the formation of intermetallic structures of the type Al13Fe4/Al3Fe and Al13Co4/Al3Co during the synthesis. We suggest optimizing the synthesis mode by using precursors of finer fractions and increasing the time of their grinding, mixing, and annealing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The development of effective catalysts for the oil refining and chemical industries is a relevant objective from the point of view of economic and environmental requirements, which is noted in the program of the Presidium of the Russian Academy of Sciences “Carbon Energy: Chemical Aspects” in the direction of “Methods and Catalysts for the Effective Conversion of Technogenic Carbon-Containing Wastes into Raw Materials for Petrochemical and Organic Synthesis” (decisions of the Presidium of the Russian Academy of Sciences no. 98 of May 23, 2017, and no. 132 of July 5, 2017).
Polymetallic catalysts based on the elemental group of Fe, Co, and Ni with the addition of Mn, V, Zr, La, Mo, Al, Si, B, and their compounds are actively studied in world practice. It is of interest to synthesize and study catalysts based on intermetallic compounds of metals such as iron aluminide and cobalt aluminide [1–8] in combination with filler SiO2. Previously, the possibility of obtaining such catalysts by mechanothermal synthesis was estimated in experimental and theoretical ways in [4, 5]. In these studies, a mixture of powders Al–Fe/SiO2 and Al–Co/SiO2 prepared by mechanical alloying [9] was annealed at temperatures of 580–600°С in air for ~4 h. It was noted that intermetallic compounds Al13Co4 and Al13Fe4 break down to form corundum Al2O3, mullite Al6Si2O13, and silicides CoSi2, CoSi, Co2Si, FeSi2, and Fe3Si when SiO2 is used as a substrate, despite the corrosion resistance of intermetallic compounds with respect to air oxygen up to 600°C [10]. Intermetallic compounds Al13Co4 actively interact with the substrate, and the fraction of SiO2 retained in the composite 13Al/4Co/6.5SiO2 in the form of cristobalite significantly decreases during oxygen breakthrough of methane (OBM). The interaction between aluminide Al13Fe4 and SiO2 is not so effective, and not only cristobalite but also quartz crystallizes in the composite 13Al/4Fe/6.5SiO2; the fraction of quartz increases during OBM. It was found that the silicides CoSi2, CoSi, Co2Si, and FeSi2 coexist with the nonstoichiometric phase based on Fe3Si [11]. According to the state diagrams of the systems Co–Si [12] and Fe–Si [11], the resulting composites 13Al/4Co/6.5SiO2 and 13Al/4Fe/6.5SiO2 are nonequilibrium phases.
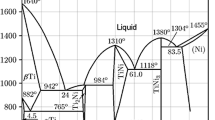
In contrast to annealing at temperatures of 580–600°С for 4 h in air, vacuum annealing at elevated temperatures of 700–900°С makes it possible to obtain intermetallic compounds Al3Fe and Al3Co with little to no oxides in a shorter exposure time. Preliminary experiments using this technology (mechanical alloying of powders Al and Fe followed by vacuum annealing at 900°C for 30 min) [13] indicate the synthesis of intermetallic compounds Al3Fe in a volume of ~75 ± 5% (Fig. 1).
The purpose of this work is to study the formation of a powder intermetallic mixture Al–Fe/SiO2 and Al–Co/SiO2 by a mechanothermal method including sequential technological operations of mechanical alloying of precursor powders Al, Fe, Co, and SiO2 and the synthesis of powder compositions Al–Fe/SiO2 and Al–Co/SiO2 under vacuum annealing at temperature of 900°C for 30 min.
EXPERIMENTAL
Powders with technical grade of Al (≥99%), Fe (≥99.4%), Co (≥99.5%), and SiO2 (≥96%) were used as initial materials. The fractional composition of the powders was ≤160 μm (Al), ≤50 μm (Fe, Co), and ≤100 μm (SiO2).
The powders were mixed and mechanically alloyed in a planetary mill of type PM 400 with steel balls 10 mm in diameter with an average intensity of 300 rpm without surface-active substances (dry grinding) for 20 min. At the first stage of alloying experiment, two mixtures Al–Fe and Al–Co were obtained in the proportions of the components of 40.2 wt % of Fe, Co and 59.8 wt % of Al corresponding to the domain of existence of the intermetallic compounds Al3Fe and Al3Co [5, 6, 13]. At the second stage of alloying, powders Al–Fe and Al–Co were removed from the jars of the planetary mill, and a powder of SiO2 was added to these clad jars. Further dry mixing was continued in the same mode as before. The gross weight ratio of the part of composition Al–Fe and Al–Co that stuck to powder SiO2 (the cladding effect on the jar walls and the surface of the balls [9]) was controlled by intermediate weighing and was 1 : 2 (Al–Fe : SiO2 and Al–Co : SiO2) in the initial state.
Heat treatment was carried out in a shaft-type resistance vacuum furnace SShVE-1.2.5/25 to reduce the likelihood of formation of corundum (Al2O3). Composite powders were annealed in vacuum at a temperature of 900 ± 5°С with a heating rate of 15°С per min and a holding time of 30 ± 1 min [13]. This mode is more efficient than annealing at 580–600°С for 4 h, since the thermal diffusion processes for the synthesis of intermetallic compounds such as Al3Co and Al3Fe proceed more intensely even with a shorter holding time of the samples in the furnace.
After synthesis of the intermetallic structure, the powders were divided using sieves into three fractions (<50 μm, 50–63 μm, and 63–100 μm). There were no fractions with a size of more than 100 μm. X-ray diffraction (XRD) analysis of the samples was performed on a DRON-3 diffractometer in Fe Kα radiation.
RESULTS AND DISCUSSION
The results of experimental and technological studies are presented in Fig. 1 and in Table 1.
An analysis of the results of grinding of powder mixtures Fe–Al/SiO2 and Co-Al/SiO2 (Table 1) indicates that powders of medium fineness with more than 50 wt % with the fraction of 63–100 μm for Fe–Al/SiO2 and more than 40 wt % for Co–Al/SiO2 are formed at a mixing intensity of 300 rpm for 30 min. The mass fraction of finer powders (<63 μm) is less than 50% for Fe–Al/SiO2 and less than 60% for Co–Al/SiO2. After annealing, there were no signs of consolidation (sintering) in the powder mixtures and sintered masses, as noted for Fe–Al and Co–Al compositions without SiO2. X-ray diffraction of powders demonstrates that there are no silicides and mullite of the Al6Si2O13 type, the formation of which was observed upon annealing at 580–600°C in air for 4 h [4]. However, the XRD method allowed us to establish that powder Fe-Al/SiO2 contains a solid solution with the composition of Fe0.5Al0.5 and Fe14Al86 in addition to the aluminide AlFe3 and unreacted iron particles (Fig. 2). The samples of Co–Al/SiO2 contain a significant amount of Co27Al73, Co2Al5, and unreacted cobalt particles.
To experimentally evaluate of weight ratios of precursors, we carried out sequence etching of alloyed mixtures Al-Fe/SiO2 and Al-Co/SiO2 after the second stage of mixing (before annealing) in alkaline (10% NaOH) and acid (5% HCl) aqueous solutions with washing, drying, and weighing. It was found that weight fractions by cladding (sticking) with SiO2 are (Al + Fe) : SiO2 = 31 : 69 ± 5% and (Al + Co) : SiO2 = 58 : 42 ± 5%. As can be seen, powder SiO2 is more intensely (~2 times) clad with a mixture of (Al + Co) than (Al + Fe) under the same conditions of dry grinding in a planetary mill. In this case, after the first stage of mixing (before introducing the SiO2 powder), there were deviations in the ratio of precursors Al : Fe and Al : Co that clad on a working tool (jar, balls) from the initial ratio Al : Fe(Co) ≈ 40 : 60. If it was 35 : 65 ± 5% for Al : Fe, which slightly differs from the initial value, then this ratio for Al : Co (75 : 25 ± 5%) is already significantly different from the initial gross value of ~40 : 60 (Al13Co4, Al3Co). This circumstance allows us to at least partially explain the observed phase transformations (Fig. 2).
The experimental results indicate that, firstly, one should take into account the deviations of the gross compositions of the precursors Al, Fe, and Co from the grinding results at the stage of mechanical alloying, and, secondly, the temperature-time synthesis conditions are insufficient for the formation of intermetallic structures Al13Fe4/Al3Fe and Al13Co4/Al3Co. Obviously, the presence of unreacted residues of Fe and Co indicates the incompleteness of the process of diffusion interaction of the precursors Al, Fe, and Co. One should increase the holding time of powder mixtures Fe–Al/SiO2 and Co–Al/SiO2 at an annealing temperature of 900°C.
The following way for technological optimization of the mechanothermal synthesis can be proposed to obtain a more homogeneous structure with a predominant content of aluminides Al13Fe4/Al3Fe and CoAl3/Co4Al13: it is necessary to adjust the gross composition of precursors Al, Fe, and Co and increase the duration of annealing of the final mixtures Al–Fe/SiO2 and Al–Co/SiO2 at 900°C to 1–1.5 hours. Another way to achieve the specified structural phase composition of Al13Fe4/Al3Fe + SiO2 and Al13Co4/Al3Co + SiO2 is the use of finer precursors or an increase in grinding time in a planetary mill with the same intensity of 300 rpm.
CONCLUSIONS
1. The method of mechanothermal synthesis made it possible to obtain powder samples of Fe–Al/SiO2 and Co-Al/SiO2 of medium fineness with more than 50 wt % with the fraction of 63–100 μm for Fe–Al/SiO2 and more than 40 wt % for Co-Al/SiO2; the rest of the powders have a dispersion of <60 μm.
2. It was experimentally established that mass fractions of precursors Al, Fe, and Co are (Al + Fe) : SiO2 = 31 : 69 ± 5% and (Al + Co) : SiO2 = 58 : 42 ± 5% as a result of cladding of powder SiO2.
3. It was found that annealing of powder samples in vacuum at 900°C for 30 min is insufficient to complete the thermosynthesis and obtain the required composition of composite powders Al13Fe4/Al3Fe + SiO2 and Al13Co4/Al3Co + SiO2. It was also found that not all precursors reacted in the required amount during such annealing in the sample of Co–Al/SiO2. X-ray diffraction of powders demonstrates that there are no silicides and mullite of the Al6Si2O13 type under vacuum annealing.
4. A set of possible measures for the technological optimization of mechanothermal synthesis was proposed. These are correction of the gross composition of precursors Al, Fe, and Co with SiO2, the use of finer precursors, an increase in grinding time in a planetary mill, and an increase in the annealing time at 900°C.
REFERENCES
Arkatova, L.A., Kharlamova, T.S., Galaktionova, L.V., et al., CO2 reforming on the intermetallic catalysts produced by self-propagating high-temperature synthesis, Sovrem. Naukoemkie Tekhnol., 2005, no. 11, pp. 23–24.
Gille, P. and Bauer, B., Single crystal growth of Al13Co4 and Al13Fe4 from Al-rich solution by the Czochralski method, Cryst. Res. Technol., 2008, vol. 43, no. 11, pp. 1161–1167.
Armbrüster, M., Kovnir, K., Friedrich, M., Teschner, D., Wowsnick, G., and Hahne, M., Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation, Nat. Mater., 2012, vol. 11, no. 8, pp. 690–693.
Artukh, V.A., Nipan, G.D., and Yusupov, V.S., Solid-phase synthesis of intermetallic compounds Al13Co4, Al13Fe4, and Al13Co2Fe2, Dokl. Chem., 2016, vol. 471, no. 2, pp. 347–349.
Artukh, V.A., Nipan, G.D., and Yusupov, V.S., Mechanothermal synthesis of cobalt, iron, and titanium aluminides, Inorg. Mater.: Appl. Res., 2017, vol. 8, no. 5, pp. 769–771.
Pugacheva, E.V., Borshch, V.N., Zhuk, S.Ya., Sanin, V.N., Andreev, D.E., and Yukhvid, V.I., Iron-based polymetallic catalysts with a nanostructured surface for deep oxidation processes, Nanotechnol. Russ., 2015, vol. 10, nos. 11–12, pp. 841–849.
Borshch, V.N., Pugacheva, E.V., Zhuk, S.Ya., et al., Polymetallic catalysts for the Fischer–Tropsch synthesis and hydrodesulfurization prepared using self-propagating high-temperature synthesis, Kinet. Catal., 2015, vol. 56, no. 5, pp. 681–688.
Borsch, V.N., Artyukh, V.A., and Zhuk, S.Ya., Intermetallides synthesized from mixture of metals as precursors of catalysts of oxidation and reducing reactions, Mezhdunarodnaya konferentsiya “SVS-50,” 20–21 noyabrya 2017, Tezisy dokladov (Int. Conf “SHS-50,” November 20–21, 2017, Abstracts of Papers), Chernogolovka: Inst. Strukt. Makrokinet. Probl. Materialoved., Ross. Akad. Nauk, 2017, pp. 79–81.
Artyukh, V.A., Yusupov, V.S., Zelensky, V.A., Kholin, M.S., and Fakhurtdinov, R.S., Structural characteristics of a mechanically alloyed Al–Fe powder composite, Inorg. Mater.: Appl. Res., 2017, vol. 8, no. 3, pp. 459–463.
Grushko, B., Wittenberg, R., Bickmann, K., and Freiburg, C., The constitution of aluminum-cobalt alloys between Al5Co2 and Al9Co2, J. Alloys Compd., 1996, vol. 233, no. 1–2, pp. 279–287.
Ishida, K., Nishizawa, T., Schlesinger, M.E., and Schlesinger, M.E., The Co–Si (cobalt–silicon) system, J. Phase Equilib., 1991, vol. 12, no. 5, pp. 578–586.
Lacaze, J. and Sundman, B., An assessment of the Fe–C–Si system, Met. Mater. Trans. A, 1991, vol. 22, no. 10, pp. 2211–2223.
Artyukh, V.A., Borsch, V.N., Yusupov, V.S., Zhuk, S.Ya., Zelensky, V.A., Lazarenko, G.Yu., and Belelyubsky, B.F., Solid-phase synthesis of intermetallic Al–Fe and Al–Co catalysts, Materialy VII mezhdunarodnoi konferentsii “Funktsional’nye nanomaterialy i vysokochistye veshchestva,” Suzdal’, 1–5 oktyabrya 2018 (Proc. VII Int. Conf. “Functional Nanomaterials and High-Purity Substances,” Suzdal, October 1–5, 2018), Moscow: Inst. Metall. Materialoved., Ross. Akad. Nauk, 2018, pp. 269–271.
ACKNOWLEDGMENTS
We thank Dr. G.D. Nipan (Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences) for assistance in conducting a preliminary analysis of phase transformations in the systems Fe–Al/SiO2 and Co–Al/SiO2 at a temperature of 580–600°С in air. We also thank the employees of ISMAN for their active participation in the work.
Funding
This work was supported by the Presidium of the Russian Academy of Sciences (basic research program no. 33 “Carbon Energy: Chemical Aspects”) and by the Russian Academy of Sciences in the development of research topics of the state assignment (project no. 007-00129-18-00, 2018).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by I. Obrezanova
Rights and permissions
About this article
Cite this article
Artyukh, V.A., Borsch, V.N., Yusupov, V.S. et al. Peculiarities of a Solid-Phase Method for the Production of Al–Fe/SiO2 and Al–Co/SiO2 Powder Catalysts. Inorg. Mater. Appl. Res. 11, 709–712 (2020). https://doi.org/10.1134/S2075113320030041
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113320030041