Abstract—Extended tests of alumina fluid chlorination are performed. The implementation of the chlorine technology is possible when using alumina, which has a high reactivity with chlorine. The improved nepheline technology for obtaining such an oxide is proposed: non-desilicated aluminate liquors undergo carbonization followed by separation of aluminum hydroxide with sodium hydroaluminosilicate and its calcination at 600–800°C. The proposed upgrade, compared to the existing method, is less energy intensive. Two-stage desilication is eliminated, and more favorable conditions are created for the low-temperature digestion of sintered material, reducing the secondary losses of alumina and alkali. The technological parameters of charge chlorination, both powdered and pelleted, are worked out on a scaled-up installation in fluidized bed conditions. The operational and capital cost of a complete aluminum production cycle based on the chlorine technology are evaluated in comparison with the conventional alumina production according to the Bayer process and subsequent electrolysis of cryolite-alumina melts. The comparative cost analysis has shown the advantages of the chlorine technology at the stage of aluminum chloride electrolysis (up to 30%) and the presence of additional costs at the stage of obtaining the intermediate product for chlorination. The chlorine method of aluminum production in modern conditions has the prospects of development not as a mass technology, but as an effective process for obtaining high-purity aluminum (HPA) in small workshops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Nephelines are one of the most promising types of non-bauxite aluminum-containing raw materials in Russia. Owing to the integrated utilization, existing enterprises have achieved high production and economic indicators of this raw material processing by sintering.
Direct chlorination of nepheline, as well as its derivative metallurgical alumina, produced using the existing technology is inefficient because of the large losses of chlorine during the formation of associated alkali metal chlorides of little value and the low alumina reactivity with chlorine. For the implementation of the chlorine technology, alumina should be used, which has a high reactivity with chlorine. Laboratory studies showed that alumina containing sodium aluminosilicate offers such properties [1]. This oxide can be obtained using a modified nepheline technology, in which non-desilicated aluminate liquors are carbonized at 40–50°C with release of aluminum hydroxide and sodium hydroaluminosilicate and subsequent calcination at 600–800°C.
The proposed process upgrade compared to the existing method is less energy-intensive, since two-stage desilication is eliminated and more favorable conditions are created for low-temperature digestion of sintered material, which reduces the secondary loss of alumina and alkali. The alumina to caustic ratio during the digestion can be reduced from 1.42 to 1.28–1.30 without fear of aluminate liquor decomposition.
The purpose of the work is to determine on an expanded-scale basis the technological parameters of the nepheline concentrate treatment process and chlorination of alumina isolated from it during low-temperature carbonization and to perform a comparative technical and economic assessment of the complete aluminum production cycle using the chlorine technology with the conventional Bayer process and subsequent electrolysis of cryolite-alumina melts.
EXPERIMENTAL
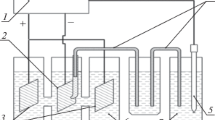
The extended tests were carried out on pilot plants created by IMET together with AO RUSAL VAMI: an active alumina production plant (Fig. 1) and a fluidized bed plant for aluminiс raw material chlorination (Fig. 2).
A technological carbonization unit (Fig. 1) consists of aluminate liquor reactor 1, which is equipped with an external electrical heating and a horseshoe stirrer; carbonizer 2 with water cooling and gas supply; and nutsche filter 3. Two-bladed mixer 5 is designed to prepare a charge of different composition. In the preparation of pelleted charge, disk granulator 6 was used. Heat treatment was carried out in drying furnace 4 and thermostat 7.
To carry out the extended tests, a standardized aluminate liquor was prepared, which corresponded to the liquor obtained by processing nepheline concentrate according to the existing sintering method with limestone and conventional digestion [2]. The aluminate liquor was prepared by dissolving aluminum hydroxide obtained by the Bayer method in a 31% alkali solution heated to 80–90°C with continuous stirring. The prepared solution (70–80 g/L Na2O and 80–90 g/L Al2O3) had an alumina-to-caustic ratio αc = 1.43 and specific gravity of 1.36–1.38 g/cm3. To obtain aluminum hydroxide containing sodium hydro-aluminosilicate, liquid glass containing 26.0 wt % SiO2 was added to the liquor.
The carbonization of the obtained aluminate liquor was carried out at a temperature of 40°C in the carbonizer equipped with a horseshoe stirrer and water-cooled jacket for carbon dioxide-air sparging at a volume ratio of 1 : 7. The CO2 consumption was 0.5 m3/h.
The residual Al2O3 content in the solution was 1%.
After the end of carbonation, the pulp was fed to the nutsche filter. The precipitated aluminum hydroxide was filtered through a belting fabric and washed out with hot water to a residual content of 5–8 g/L of Na2O in the washings. The separated aluminum hydroxide was dried in the drying furnace at 110°C and calcined at 600–800°C. The result was alumina with impurities of sodium and silicon, so-called rough alumina.
The chlorination was carried out with pelleted and powdered charges containing 70 wt % alumina and 30 wt % petroleum coke. In the preparation of the powdered charge, petroleum coke with a particle size of <0.6 mm was used, and for the pelleted charge, the particle size was <0.2 mm. Before pelleting in the blade mixer, a sulfide liquor binder was added to the charge in the amount of 20–25 wt % of the charge weight. The obtained pellets were dried in air for 24 h and then for 5 h in a drying furnace at 130–150°С with occasional stirring.
After that, the pellets were calcined in the thermostat at 700°C for 4 h in a nitrogen atmosphere at a heating rate of 50 K/h. The pellets of 1–4 mm in size were used for chlorination.
It was shown in laboratory conditions that the chlorination of Al2O3 containing sodium aluminosilicate reduces dust losses by 4–5% [13]. The effect of the amount of sodium aluminosilicate on the chlorination process was studied. The chemical composition of the initial materials is shown in Table 1.
The chlorination was carried out at a pilot fluidized bed plant (Fig. 2). The main components of the plant: (1) graphite-lined fluidized bed reactor, (2) vertical furnace, (3) dust chamber, (4) mechanical scrubber for chloride condensation in molten salts, (5) water-cooled condenser for chloride desublimation, (6) wet scrubber for absorption of uncollected chlorides, (7) three-stage alkaline scrubber for flue gas cleaning. The experimental conditions: reactor temperature—900°C, dust chamber temperature—250°C, condenser temperature—40°C; fluidized bed height—500–600 mm, chlorine consumption—3.1–3.6 kg/h, nitrogen consumption—250 L/h.
Before starting the chlorination, reactor 1 was loaded with 3.0 kg of petroleum coke and 2.0 kg of charge. The excess coke allowed reducing fluctuations in the height of the fluidized bed. During the experiments, a vacuum from 5 to 10 mm water gauge was created in the system. Exhaust gases were analyzed for Cl2, CO, CO2, O2, and HCl. The chlorination took place with almost complete chlorine utilization. To do this, with the appearance of traces of chlorine in the exhaust gas, reactor 1 was loaded with the next charge batch. Thus, the periodic batch feeding allowed continuous chlorination, maintaining the given fluidized bed height. Chloride condensation was provided in two versions: in the molten salts in mechanical scrubber 4 and their desublimation in condenser 5. Both condensing systems, depending on the tasks, can work simultaneously or autonomously. After the end of the experiment, the furnace residues and products from dust chamber 3 and condenser 5 were weighed and analyzed for the content of the main components.
RESULTS AND DISCUSSION
According to the test results, the products from the condenser do not contain Al2O3, Na2O, and SiO2. The chlorinity of oxides was calculated from the formula
where po is the amount of the oxide that entered the reactor during the experiment, and ps is the amount of the non-chlorinated oxide, equal to the sum of the oxide mass in the burning residue and the oxide mass in the product from the dust chamber.
The results of a set of experiments on chlorination are presented in Table 2. In experiments 1–4, pellets were chlorinated with different SiO2 content (from 1.11 to 2.45 wt %). The Al2O3 chlorinity was 97.1–98.9 wt %, which is ~10% more than the alumina chlorinity obtained under similar conditions, but not containing sodium aluminosilicate.
Polymorphic transformations were studied during the heat treatment of alumina obtained under various conditions. It was shown that sodium oxide and silicon additives are stabilizers of low-temperature Al2O3 modifications, which are distinguished by a higher reactivity [1]. The improvement in chlorination can also be explained by the formation of a eutectic NaAlCl2 compound in the reaction molten zone, which prevents dust losses. The alumina loss in the products from the dust chamber was only 0.7–2.5%, and when the alumina was chlorinated without sodium aluminosilicate, this value was 10–14%. Thus, the chlorination of Al2O3 obtained by calcination of Al(OH)3, separated by low-temperature carbonization of the aluminate liquor, which was not subject to desilication, makes it possible in great measure to eliminate one of the main drawbacks of fluidization, that is, dust loss.
The silicon oxide chlorinity (Table 2) is lower than that of aluminum and sodium oxides. The furnace residue after the reaction contains from 19 to 34% of non-chlorinated silica. This makes it possible to use furnace residues for the production of alumina-silicon alloys. SiCl4 formed in the chlorination process when solving the problem of its separation from the gas phase can be used as a finished product. The data on the powdered charge chlorination (Table 2, Experiment 5) are of industrial interest, since it is shown that the chlorinity hardly decreases (96.6%) under the same experimental conditions. The insignificant Al2O3 dust loss during the chlorination of the powdered charge is also a consequence of the formation of sodium tetrachloroaluminate. The use of powdered charge as an initial material will make it possible to exclude such technological procedures as pelletizing and pellet firing in an inert environment.
The extended tests of alumina fluid chlorination obtained from non-desilicated aluminate liquors showed the manufacturability of this material in the production of aluminum by the chlorine method. The proposed nepheline processing technique, with allowance for the huge reserves of this raw material, can be considered one of the key priority areas of aluminum production.
The prospect of the development of a complete chlorine-based aluminum production cycle can be considered when comparing technical and economic indicators with the conventional low-temperature Bayer process of alumina production and the subsequent electrolysis of cryolite-alumina melts.
COMPARATIVE ASSESSMENT OF THE COMPLETE CYCLE ALUMINIUM PRODUCTION
The physical and chemical advantages of the electrolysis of aluminum chloride over the alumina electrolytic decomposition are as follows: lower process temperature, lower decomposition potential of AlCl3, inactivity of the carbon anode in relation to chlorine. However, the electrolysis of aluminum chloride is more sensitive to the quality of raw materials, which accordingly leads to additional costs for chlorination. The high purity of the raw materials and the absence of anode ash ensure the purity of commercial aluminum close to HPA.
A raw material for electrolyzers is metallurgical alumina (GOST 305558-98). Consumption per 1 t of aluminum is 2 t of alumina. The intermediate product for aluminum chloride electrolysis should contain at least 99.97% of the main component AlCl3. Consumption per 1 t of aluminum is 5 t of aluminum chloride; per 1 t of aluminum chloride, it is –0.4 t of Al2O3.
Direct chlorination of natural or by-product materials is not effective, since chlorination of impurities of the applied material requires an additional amount of chlorine. Purification of the obtained aluminum chloride from impurities by fractional condensation is an expensive operation, and besides that, it is necessary to solve the problem of disposal of the resulting associated chlorides [4].
Inexpensive raw materials (reactive with chlorine with a minimum content of impurities) for chlorination can be obtained by the hydrochloric acid enrichment of high-silica aluminic raw materials to produce rough alumina [1]. To achieve high chlorinity, it is possible to isolate part of the flow in conventional alumina refineries, to produce rough alumina by low-temperature carbonization without deep desilication at a low calcination temperature (below 800°C). Owing to this simplification, the obtained rough alumina as a raw material for chlorination according to our estimate can be 5–25% cheaper than commercial alumina as per GOST 30558-98. Thus, the cost of raw materials for aluminum chloride electrolysis will be higher than that for alumina electrolysis.
Modern electrolyzers with prebaked anodes carrying a current of 275–350 kA with anodic current density of 0.85–0.88 A/cm2 achieve current efficiency of ~95% and anode consumption of 550/420 kg/t Al (gross/net) (Table 3). Electrolytic decomposition of aluminum chloride when using a bipolar electrode system makes it possible to reduce the specific electrical energy consumption at a current efficiency of 86%, that is, by 35%, and to increase the capacity of a single bath by 4–8 times.
During the electrolysis of cryolite-alumina melts, the non-recoverable loss of AlF3 electrolyte is 15–25 kg/t. Aluminum chloride electrolyzers are sealed and such losses are practically absent. Nonconsumable anodes in the process of aluminum chloride electrolysis also reduce running costs.
Owing to the smaller amount of impurities, aluminum chloride electrolysis provides purer metal quality than alumina electrolysis, and taking into account the additional raw material purification by fractional condensation during chlorination, it is possible to obtain HPA.
The main positive effect of producing aluminum from its chloride will be the end of polyfluorocarbon emission into the atmosphere and the reduction of CO2 emission by 40% due to the airtightness of electrolyzers.
The chlorine method of aluminum production can be implemented on the basis of qualitative technological restructuring and re-equipment of enterprises. The method consists of two main process stages—the production of aluminum chloride and its electrolysis. The advantages of the method include the possibility of using low-quality aluminic raw materials, reducing the specific electric energy consumption by about 30%, eliminating the consumption of high-quality carbon-containing materials, using less scarce and aggressive chlorides instead of fluorides, and reducing costs and harmful emissions into the atmosphere [4, 5]. The Alcoa Corporation came the closest to the commercialization of the chlorine method in the 1970s, but after 6 years of operation, the plant was mothballed until solution to the problem of reducing the AlCl3 cost. Also, a clay-to-aluminum chlorination and purification technology was tested by Toth Aluminum Corporation (TAC) and proposed for industrial implementation [4].
The chlorine method of aluminum production in modern conditions has prospects of development not as a mass technology, but as an effective process for obtaining HPA in small workshops.
CONCLUSIONS
(A) The extended chlorination tests performed on the fluidized bed plant showed that the best process parameters were obtained by chlorination of alumina containing 2 wt % sodium aluminosilicate.
(B) The optimum conditions for chlorination were determined: gas velocity—0.1–0.2 m/s, fluidized bed height—600–700 mm, temperature—800–900°C. In this case, the chlorinator productivity is 0.3–0.35 t/(m2 h) AlCl3, and the alumina chlorinity is 98.9%.
(C) It was shown that the use of powdered alumina charge with 2 wt % sodium aluminosilicate forms in the chlorinator a melt of NaAlCl2, which significantly reduces dust loss.
REFERENCES
Vetchinkina, T.N., Conditions for the production of active aluminum oxide, meeting the requirements of prospective chloride technology, Inorg. Mater.: Appl. Res., 2018, vol. 9, no. 5, pp. 937–946.
Moskvitin, V.I., Nikolaev, I.V., and Fomin, B.A., Metallurgiya legkikh metallov: uchebnik dlya VUZov (Metallurgy of Light Metals: Manual for Higher Educational Institutions), Moscow: Intermet Inzhiniring, 2005, pp. 86–104.
Liner, Yu.A., Vetchinkina, T.N., and Simanovskii, B.A., Specific chlorination of aluminum-containing raw materials in a fluidized bed, Khim. Tekhnol., 2006, no. 8, pp. 17–23.
Borisoglebskii, Yu.V., Galevskii, G.V., Kulagin, N.M., Mintsis, M.Ya., and Sirazutdinov, G.A., Metallurgiya alyuminiya (Aluminum Metallurgy), Novosibirsk: Sib. Otd., Ross. Akad. Nauk, 2000, pp. 32–48.
Akhmedov, S.N., Borisoglebskii, Yu.V., Vetiuko, M.M., Gromov, B.S., Pak, R.V., and Kozlov, V.A., State and development trends of global aluminum production technology, Tsvetn. Met., 2002, no. 3, pp. 47–52.
Funding
This work was carried out according to state order no. 075-00746-19-00.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by A. Kolemesin
Rights and permissions
About this article
Cite this article
Vetchinkina, T.N., Balmaev, B.G. Chlorination of Alumina Obtained from Nepheline Concentrate Processing and Comparative Assessment of the Complete Aluminum Production Cycle. Inorg. Mater. Appl. Res. 10, 1249–1253 (2019). https://doi.org/10.1134/S2075113319050344
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113319050344