Abstract
In this paper, we analyzed the methods of synthesis and sintering of aluminum oxynitride powders. Samples of a ceramic material based on aluminum oxynitride were produced. The effectiveness of two sintering methods in induction and resistance furnaces was evaluated in the temperature range of 1750–1950°C and for the exposure time from 2 to 10 h. Structures of the obtained samples were studied by scanning electron microscopy, while the phase composition was studied using X-ray phase analysis. The effect of the heating parameters, sintering atmosphere, and quality of the initial powders on the formation of the aluminum oxynitride phase was considered. A sample sintered in a vacuum furnace without a nitrogen atmosphere did not have the aluminum oxynitride phase because of the release of nitrogen from the sample volume and had a strong shrinkage. Induction sintering in a nitrogen atmosphere made it possible to achieve ~85% concentration of the target phase, aluminum oxynitride, with density of 85% of theoretical (3.69 g/cm3). These results were obtained in the mode of exposure for 10 h at 1750°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In production of protective structures and dual-use products, there is an urgent need to use new materials that have low weight and fairly high strength while having transparency in the optical, ultraviolet, and infrared ranges [1, 2]. This complex of properties is particularly relevant for the protection of digital electronic equipment, in particular, infrared sensors, lenses, aerometric instruments and systems, etc., used under conditions of high mechanical loads, high temperatures, and aggressive environments. Ceramic material based on aluminum oxynitride meets these requirements.
Aluminum oxynitride (ALON) is a solid solution in the pseudobinary Al2O3–AlN system. The AlON composition corresponds to the formula Al(64 +x)/O32 –xNx, where the composition with x = 5 is the main stable phase in the γ-AlON region. Thus, its stoichiometric formula is Al23O27N5.
For the first time, the possibility of the existence of a spinel-type phase in the Al2O3–AlN system was announced by Japanese scientists Yamaguchi and Yanagida in 1959 [3]. Later, other independent research teams confirmed the existence of this phase. The phase diagram of this system was first introduced in 1973 [4]. This was the beginning of active research and development in the field of obtaining a dense material based on the phase of cubic spinel Al–O–N in the aluminum oxide–aluminum nitride system. Obtainment of the first samples with optical transparency were reported in 1976 [5]. An updated state diagram for the γ phase of aluminum oxynitride was also presented, but it was noted that the technology for producing a transparent ceramic was not completely clear [5].
Although the production methods for ceramic materials, including materials based on aluminum oxynitride, are well known, obtaining this material with the required physical properties and the volume of the desired phase approaching 100% is not easy in practice. Major challenges are the high demands on the equipment and raw materials used. Particularly stringent requirements with respect to purity and dispersion of particles are imposed on the initial powders. According to [6], Al2O3 powder should have a purity of at least the second decimal place and a particle size of less than 1 μm (Table 1), including the almost complete absence of particle size variation.
Special requirements are imposed on molds, sintering furnaces, and the technologies used. Since the initial temperature of the formation of the desired type of ceramic starts from 1700°C (and under certain conditions, it is necessary to heat up to temperatures above 2000°C), sintering should be carried out in vacuum, in an atmosphere of inert gases, or in nitrogen.
Aside from the usual methods of heating, i.e., from the heating element to the sample, microwaves are used for heating. At microwave heating, products initially absorb microwave radiation and then convert the received energy to heat inside the sample volume and so heating occurs very quickly [6]. Hot isostatic pressing (HIP) is also a widely used method: the billet in the mold is simultaneously heated and pressed to reduce porosity. Liquid-phase sintering is one of the most promising methods: the billet of the required composition is heated to a region of heterogeneity, ~2100°C, and then the temperature is reduced, transferring the billet from the region of existence of the liquid solution and solid phase to the region of a homogeneous solid phase [7]. This is required in order for the liquid phase to fill the pores when the temperature drops to the region of homogeneity, thereby increasing the density and strength of the product. All these methods for producing ceramic materials are aimed at obtaining the densest product. When the difference is 3% of the theoretical density, the optical properties of a transparent ceramic sharply decrease [1] along with deterioration of the mechanical properties. An example of the physical and mechanical properties of a ceramic produced by the Surmet company is shown Table 2.
To improve sintering and reduce porosity and sintering temperature, special additives, such as yttrium and lanthanum oxides, are used. Y2O3 and La2O3 are used jointly since they contribute to the formation of a liquid phase, which in turn reduces the number of pores and defects. This is especially important at the grain boundaries. Yttrium ions promote grain growth and mobility of boundaries. On the contrary, lanthanum ions have inverse properties, owing to which it is possible to obtain a minimum of defects at grain boundaries and average out the sizes of all grains [8]. The combination of all these factors will make it possible to achieve greater transparency by adding 0.05–0.15 wt % of such additives.
For good optical transparency properties, the products after sintering are polished and ground to remove microdefects from the surface that hide the transparent ceramic.
The tested sintering modes are hot pressing at 1700°С with holding for 12 min and reaction sintering in a nitrogen atmosphere at 1700–1800°С for 30–120 min [9].
This study is aimed at evaluating the effectiveness of various sintering methods for obtaining samples of a ceramic material based on aluminum oxynitride at 1750–1950°C and an exposure time from 2 to 10 h and at investigating the effect of heating parameters, sintering atmosphere, and quality of the initial powders on the formation of the aluminum oxynitride phase.
MATERIALS AND METHODS
Obtained by plasma chemical synthesis [9–11], aluminum oxide and nitride powders, Al2O3 and AlN, with the characteristics given in Table 3 were used as the initial materials.
Mixtures of the composition of 30 mol % AlN and 70 mol % Al2O3 were mixed in a planetary mill for 1–2 h. Pressing of powder compacts was performed by a force of 50-60 MPa. Sintering was carried out by two different methods: in a vacuum resistance furnace and in a vacuum induction furnace.
In a SShVE-12.5/25-IZ vacuum shaft resistance furnace, the process was carried out at 1800–1850°С in vacuum at a residual pressure of 1.33–0.133 Pa for 10 h. The sample was mounted on a boron nitride substrate. This sintering mode was chosen to estimate the effect of the gaseous (N2) atmosphere on the concentration of the target phase of aluminum oxynitride.
Sintering in an induction vacuum furnace was carried out at 1750–1950°C and an exposure time from 2 to 8 h and the residual pressure was 13.33–6.61 Pa. The sample was placed in a crucible on a boron nitride substrate. To saturate the sample with nitrogen, the crucible was filled with an aluminum nitride powder.
The obtained compacts and initial powders were examined by scanning electron microscopy using a Tescan Vega scanning microscope and by X-ray phase analysis using a Bruker D8 ADVANCE diffractometer.
RESULTS AND DISCUSSION
Samples sintered in the vacuum resistance furnace (sample 1) partially retained their geometric shape of a cylinder, while a strong shrinkage and cracking occurred in the center of the sample (Fig. 1a). In turn, the samples sintered in an induction vacuum furnace (sample 2) lost a significant part of its mass, ~70% of the initial (Fig. 1b). Sample 1 had a dark gray color, while sample 2 had a light gray tint and was optically transparent to light.
The volume of the target phase, aluminum oxynitride (Al23O27N5), was quantified by X-ray phase analysis of the samples. Sample 1 does not have the target phase, only two modifications of alumina and a small admixture of silicon oxide (Fig. 2). These results are possibly related to the lack of nitrogen in the mixture even for the minimal formation of the aluminum oxynitride phase. At heating and holding the billet in the furnace, nitrogen was completely released from the mixture. This explains the strong shrinkage and porosity of the sample, which resulted in its destruction.
Other samples were sintered in an induction furnace at 1750–1950°C with an exposure time of 2 h. Under these parameters, the samples after sintering had three phases of Al2O3, AlN, and a phase of oxynitride (Al5O6N in our case) (Fig. 2b). At the same time, the sample lost about 70% of its weight. The loss of mass of the sample at these temperatures should be studied. Perhaps, this is due to the formation of gaseous AlO2 at temperatures close to 2000°C, as noted in [12].
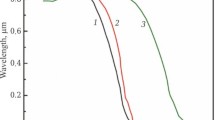
Sintering was also carried out at 1750°C and exposure of 10 h. Under these conditions, about 80% of the target oxynitride phase was obtained, and the rest was aluminum oxide (Fig. 2c). We attribute these results to the fact that, under such conditions, the composition point fell into the homogeneous zone (Fig. 1), while no complete transition of alumina to the oxynitride phase occurred.
The highest density of ceramic samples that was achieved was 84% of theoretical. Unfortunately, no physical and mechanical tests were able to be performed because of the size of the samples.
In further studies, it is planned to pay attention to the parameter of a grain size and densification in order to achieve the required physical, mechanical, and optical properties of the resulting ceramic material [13]. It is also necessary to examine and experimentally test the possibility of adding sintering additives to the composition and, as a consequence, to determine their effect on the structure and properties of aluminum oxynitride ceramic [14].
CONCLUSIONS
We obtained samples of ceramic materials with 80–90 vol % phase of aluminum oxynitride. However, we were unable to achieve a homogeneous composition of the sample, which directly affects the optical, mechanical, and physical properties.
An exposure time of ~10 h at 1750°C in an induction furnace is sufficient for the formation of the aluminum oxynitride phase, but the optimal composition for entering the zone of homogeneity should be chosen according to the diagram of the Al2O3–AlN system.
The sintering gaseous atmosphere, namely, the nitrogen environment, directly affects the diffusion during the oxynitride formation. The nitrogen concentration in the mixture is not sufficient for the diffusion from aluminum nitride to oxynitride since nitrogen is released from the volume of the billet, leading to destruction of the sample.
REFERENCES
Kingery, W.D., Introduction to Ceramics, New York: Wiley, 1960.
Arzhakov, M.S., Zhirnov, A.E., Arzhakov, S.A., Lukovkin, G.M., Kolmakov, A.G., and Zabolotnyi, V.T., Glass ceramic and polymer impact-resistant materials and protective constructions based on them (review), Russ. Metall. (Engl. Transl.), 2015, vol. 2015, no. 10, pp. 800–804.
Yamaguchi, G. and Yanagida, H., Study on the reductive spinel—a new spinel formula AlN–Al2O3 instead of the previous one Al3O4, Bull. Chem. Soc. Jpn., 1959, vol. 32, no. 11, pp. 1264–1265.
McCauley, J.W. and Viechnic, D.J., Reaction studies in Si3N4–Al2O3 system, Am. Ceram. Soc. Bull., 1974, vol. 53, no. 8, pp. 620–620.
McCauley, J.W. and Corbin, N.D., Phase relations and reaction sintering of transparent cubic aluminum oxynitride spinel (ALON), J. Am. Ceram. Soc., 1979, vol. 62, nos. 9–10, pp. 476–479.
Cheng, J., Agrawal, D., Zhang, Y., and Roy, R., Microwave reactive sintering to fully transparent aluminum oxynitride (ALON) ceramics, J. Mater. Sci. Lett., 2001, vol. 20, no. 1, pp. 77–79.
Patel, P.J., Gilde, G., and McCauley, J.W., US Patent 7 045 091, 2006.
Wang, J., Zhang, F., Chen, F., Zhang, J., Zhang, H., Tian, R., Wang, Z., Liu, J., Zhang, Z., Chen, S., and Wang, S., Effect of Y2O3 and La2O3 on the sinterability of γ-AlON transparent ceramics, J. Eur. Ceram. Soc., 2015, vol. 35, no. 1, pp. 23–28.
Prosvirnin, D.V., Larionov, M.D., Kolmakov, A.G., Alikhanyan, A.S., Antipov, V.I., Samokhin, A.V., Lysenkov, A.S., and Titov, D.D., Effect of reaction sintering conditions on properties of ceramics based on alumina oxynitride, Inorg. Mater.: Appl. Res., 2018, vol. 9, no. 4, pp. 599–602.
Alekseev, N.V., Samokhin, A.V., and Tsvetkov, Yu.V., RF Patent 2311225, 2007.
Samokhin, A.V., Sinayskiy, M.A., Alexeev, N.V., Rizakhanov, R.N., Tsvetkov, Yu.V., Litvinova, I.S., and Barmin, A.A., Synthesis of nanoscale zirconium dioxide powders and composites on their basis in thermal DC Plasma, Inorg. Mater.: Appl. Res., 2015, vol. 6, no. 5, pp. 528–535.
Patel, P., Gilde, G., and McCauley, J.W., US Patent 7 045 091, 2006.
Galakhov, A.V., Antipov, V.I., Vinogradov, L.V., Kolmakov, A.G., Alymov, M.I., Barinov, S.M., Solntsev, K.A., Vityaz’, P.A., Kheifets, M.L., Klimenko, S.A., and Kopeikina, M.Yu., The properties and sinterability of nanostructured submicron powders of ZrO2 + 3 mol % Y2O3 composition synthesized by ultrasonic spray pyrolysis, Perspekt. Mater., 2012, no. 4, pp. 70–76.
Kaigorodov, A.S., Ivanov, V.V., Krustov, V.R., and Medvedev, A.I., Production of transparent Nd: Y2O3 ceramics from weakly aggregated nanopowders using pulsed pressing and vacuum sintering, Perspekt. Mater., 2007, no. 2, pp. 36–42.
ACKNOWLEDGMENTS
This work was performed according to the state task no. 007-00129-18-00 and supported by the Russian Foundation for Basic Research (project no. 16-08-00815).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by A. Ivanov
Rights and permissions
About this article
Cite this article
Kolmakov, A.G., Prosvirnin, D.V., Larionov, M.D. et al. Effect of Sintering Parameters on the Phase Composition of Ceramic Based on Aluminum Oxynitride. Inorg. Mater. Appl. Res. 10, 416–419 (2019). https://doi.org/10.1134/S2075113319020242
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113319020242