Abstract
It remains unclear whether the effect exercised by alien dominants on the species richness in plant communities is on average stronger than the effect exercised by native dominants. To clear up this point, 20 synanthropic plant communities dominated by species of different biogeographical origins were surveyed in the Belaya River valley, Western Caucasus (190–680 m above sea level). For each of the studied communities, aboveground biomass samples were collected on 25–30 plots 0.25 m2 in size with different shares of dominants; the collected samples were subsequently sorted by species and weighed. Analysis of the field data made it possible to draw the following conclusions: (1) samples with high shares of alien and native dominants differ statistically insignificantly in the average number of species; (2) on average, the relationship between the degree of dominance of alien species and the species richness is as strong as the relationship between the degree of dominance of native species and the species richness; (3) in most cases, the relationship between these parameters can be satisfactorily explained by the “energy–diversity” hypothesis; and (4) the proportion of synanthropic plant species in communities with high shares of alien and native dominants is not higher than that in communities with low shares of these dominants. Overall, the results indicate that alien and native dominants exercise similar and mostly nonselective effects on accompanying species in plant communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
According to the “energy–diversity” hypothesis (Wright, 1983) and the “larger number of individuals” hypothesis (Srivastava and Lawton, 1998) that explains it, the higher the productivity of habitats, the higher is the total biomass of individuals in areas occupied by communities (and, accordingly, their density), and the higher is the probability that these individuals belong to many species. Field data generally confirm the validity of these assumptions in relation to plant communities, except for situations when communities differ in species pool size and significantly differ in the average height (size) of individuals (Grime, 1973; Garsia et al., 1993; Oksanen, 1996; Zobel and Partel, 2008; Símová et al., 2013).
It also follows from the above hypotheses that the higher is the share of dominants (i.e., the degree of dominance) in a plant community, the less resources remain available for other (associated) species, and the lower are the total biomass and number of such species. This makes it possible to explain the strong relationship between the degree of dominance and the species richness that is often observed in small fragments of phytocoenoses, including synanthropic ones, especially if they belong to the competitive type (CRS organization model) (Mirkin, 1994; Mirkin et al., 2007; Akatov et al., 2018, 2019). This mechanism implies that an increase in the share of dominants leads to a nonselective (i.e., random) elimination of other (associated) species from such sites. Therefore, the impact exercised by dominants may result in a significant decrease in the occurrence frequency of associated species; however, with a certain probability, any of the associated species can be discovered in any fragment of the phytocoenosis with any share of the dominant. This means that species pools of these fragments are approximately the same in size. If so, a certain amount of biomass of associated plant species sampled in fragments of a plant community featuring high shares of the dominant should include roughly the same number of species as a similar amount of biomass sampled from fragments of the same community with low degrees of dominance of this dominant.
However, dominants can also affect associated species in other ways, e.g., by transforming ecotopes (litter accumulation, changes in the light and hydrological regimes, changes in physicochemical soil properties, etc.) or through allelopathy (Rabotnov, 1983; Levine et al., 2003; Callaway and Ridenour, 2004; Reinhart et al., 2005; Csergő et al., 2013; Lanta et al., 2013; Bartha et al., 2014; Gioria and Osborne, 2014; Blackburn et al., 2019). The environment-forming activity of dominants can prevent the growth of some (unstable) species in communities, but have no effect on other (stable) species (selective impact). As a result, the species pool in fragments of phytocoenoses with high degrees of dominance is smaller than on sites with low degrees of dominance. In such cases, a certain amount of biomass of associated plant species sampled in fragments of a plant community featuring high shares of the dominant should include a smaller number of species than a similar amount of biomass sampled in fragments of the same community with low degrees of dominance of this dominant. It can also be assumed that the relationship between the degree of dominance and the species richness in communities in this case should be stronger in comparison with a situation when this relationship is determined only by the amount of resources available to associated species.
One of the consequences caused by the saturation of regions with alien species is that such species become predominant in some phytocoenoses (Rejmánek et al., 2013). This usually happens after severe disruptions in communities (Reinhart et al., 2005; Smith et al., 2009), but apparently other variants cannot be ruled out. For a number of reasons, this process can pose threats to the phytodiversity of recipient regions. First, alien species may be stronger competitors for resources than native species that normally predominate in such habitats and hence reach higher abundance levels. The higher the degree of dominance of alien species, the stronger is their impact on other species (Meiners et al., 2001; Silliman and Bertness, 2004; Hejda et al., 2009; Seabloom et al., 2015). Second, the available data indicate that local plant species can be more resistant to environment-forming effects of native dominants because of their long periods of coexistence (conjugate evolution) compared to alien dominants (Rabotnov, 1983; Meiners et al., 2001; Rejmánek and Simberloff, 2017; Hejda et al., 2017; Blackburn et al., 2019). Finally, some studies indicate that the replacement of native dominants with alien ones often increases the share of synanthropic plants among associated species because of their greater resistance to this factor (Hejda and Pyšek, 2006; overviews: Gusev, 2018; Veselkin and Dubrovin, 2019). As a result, the species composition of communities with low and high shares of an alien dominant can differ significantly (low species similarity) (Hejda et al., 2009).
But does the impact of dominants on the species richness in plant communities really depend on their origin (i.e., whether they are alien or native)? Many studies support this supposition (Richardson et al., 1989; Standish et al., 2001; Akatov et al., 2012; Lanta et al., 2013; Gusev, 2016, 2017, 2018; Hejda et al., 2017; Rijal et al., 2017; Vítková et al., 2017; Blackburn et al., 2019; Veselkin et al., 2020; etc.). However, there is also an alternative opinion: the available data are rarely based on comparisons of representative community samples with dominants of different origins; therefore, despite the importance of this question for conservation practice, there are still insufficient grounds for a positive answer to it (Houlahan and Findlay, 2004; Sagoff, 2005; Davis et al., 2011; Blackburn et al., 2019; Hejda et al., 2021). In addition, information on the nature of effects exercised by dominants, both alien and native, on large areas of vegetation remains scant and ambiguous (Richardson et al., 1989; Powell et al., 2013; Stohlgren and Rejmánek, 2014; Chase et al., 2015; Rejmánek and Stohlgren, 2015).
This study examines the above issue through the example of 20 sites occupied by synanthropic plant communities in the low-mountain belt of the Western Caucasus with the well-pronounced predominance of alien and native species. We attempted to answer the following questions:
1. Is the relationship between the degree of dominance and the species richness in communities predominated by alien species really stronger (on average) than that in communities predominated by native species?
2. Is the effect of alien dominants on associated species really more selective (on average)? And, accordingly, does it have more significant consequences for large fragments of plant communities (in terms of species pool size) than the effect of native dominants?
3. Are communities with high degrees of dominance of alien species really distinguished by a higher proportion of synanthropic species in their composition than communities with low shares of alien dominants or communities with high degrees of dominance of native species?
MATERIALS AND METHODS
Field Data Collection
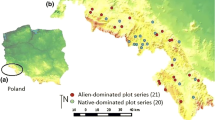
The area under investigation is located in the Belaya River basin, Western Caucasus; it stretches from the city of Maykop (190–220 m above sea level) to the village of Guzeripl’ (660–680 m above sea level). Twenty sites 40–60 m2 in size occupied by synanthropic plant communities were surveyed. On ten sites, the predominance of the following alien species was clearly manifested: Asclepias syriaca (2 sites); Solidago сanadensis (2 sites); and Ambrosia artemisiifolia, Helianthus tuberosus, Impatiens glandulifera, Paspalum thunbergii, Silphium perfoliatum, and Xanthium albinum (1 site each). The other ten sites were dominated by native species: Botriochloa ischaemum, Calamagrostis epigeios, Echium vulgare, Equisetum telmateia, Melilotus officinalis, Rubus caesius, Setaria viridis, Sisymbrium loeselii, Trifolium pratense, and T. arvense (1 site each). The names of vascular plant species are provided in accordance with Zernov (2006). The natural ranges of many alien species (Helianthus tuberosus, Asclepias syriaca, Solidago сanadensis, Ambrosia artemisiifolia, and Silphium perfoliatum) are located in North America; the center of origin of Xanthium albinum is South and Central America; for Impatiens glandulifera, it is the Himalayas; and for Paspalum thunbergii, it is the Far East. The majority of these species (Impatiens glandulifera, Solidago сanadensis, Ambrosia artemisiifolia, Helianthus tuberosus, and Xanthium albinum) are considered invasive (Vinogradova et al., 2009). On the basis of our data, Asclepias syriaca can also be attributed to this group of plants within the area under investigation since it occurs in forest glade communities on river terraces, reaching high abundance levels in some locations.
The test sites are located in habitats belonging to different types: (1) abandoned fields and vegetable gardens in the vicinity of the city of Maykop (two sites predominated by Asclepias syriaca and sites dominated by Solidago сanadensis, Calamagrostis epigeios, Melilotus officinalis, Echium vulgare, Trifolium pratense, and T. arvense) at 200 m above sea level, in the vicinity of the village of Kamennomostsky (one site with Silphium perfoliatum) at 370 m above sea level), and in the village of Guzeripl’ (two sites predominated by Helianthus tuberosus and Impatiens glandulifera) at 660 m above sea level; (2) along dirt roads in the vicinity of the city of Maykop (three sites predominated by Ambrosia artemisiifolia, Botriochloa ischaemum, and Equisetum telmateia) at 200–220 m above sea level and near the township of Krasnooktyabrsky (one site with Sisymbrium loeselii) at 280 m above sea level; (3) along edges of disturbed forest areas on the Belaya River terrace in the vicinity of the city of Maykop (two sites dominated by Solidago сanadensis and Rubus caesius) at 200 m above sea level; (4) on a forest glade used for horse grazing in the vicinity of the village of Guzeripl’ (predominated by Paspalum thunbergii) at 680 m above sea level; and (5) on the meander bar of the Belaya River in the vicinity of the city of Maykop (two sites dominated by Xanthium albinum and Setaria viridis) at 190 m above sea level.
Within each of the selected sites occupied by plant communities, 25–30 sampling plots 0.5 × 0.5 m in size were established. Some of them were established using the regular method in the form of one or two transects (10 plots per transect), while others were in series of 5–15 plots per test site. In the second case, fragments of communities with high and low projective covers of dominants (estimated visually) were selected. On plots with low shares of dominant species, none of the associated species had a clearly manifested advantage over other species. Aboveground biomass samples were collected on each plot. The following parameters were determined for each sample: (1) total weight of fresh aboveground biomass (W), biomass of the dominant species (Wd), and biomass of associated species (Ws); (2) total number of species (S) and number of associated species (Ss); and (3) degree of dominance (D = Wd/W). In addition, the total number of species (N) and the total number of associated species (Ns) were determined for each series of samples.
Field Data Analysis
Analysis of the field data involved the following procedures:
1. Using Spearman’s rank correlation coefficient, the relationship character (sign) and strength were estimated for the following pairs of parameters: (1) D and Ss, (2) D and Ws, (3) Ws and Ss, and (4) D and Sr, where Sr is the proportion of variation in the Ss variable not explained by the regression equation (either linear or polynomial) produced for Ss and Ws. In other words, Sr is the deviation of actual Ss values from those computed using this equation.
2. On the basis of the produced regression models and computed Pearson correlation coefficient, the character and strength of relationships between D and Ss, D and Ws, Ws and Ss, and D and Sr were estimated for communities dominated by alien species in general and by native species in general. In addition, the average numbers of associated plant species in biomass samples with similar degrees of dominance (less than 0.20, 0.20–0.39, 0.40–0.59, 0.60–0.79, and ≥0.80) of alien and native species were compared. Statistical significance of the difference between mean values of this parameter was assessed using the F-test (ANOVA).
3. For each site (series of samples), groups of five samples with minimum shares of the dominant were formed, and for each of these groups, the total biomass of associated species was determined (1); groups of samples with maximum shares of the dominant where the total biomass of associated species was approximately equal to that in groups of samples with minimum shares of the dominant were formed (2); and the total numbers of species in groups of samples with high and low shares of dominants (\(N_{{\text{s}}}^{'}\)) and concurrently with approximately equal biomass of associated species were compared (3). The ratio between the detected (\(N_{{\text{s}}}^{'}\)) and not detected (Ns − \(N_{{\text{s}}}^{'}\)) numbers of species in samples with low shares of the dominant was considered the expected value for samples with high shares of the dominant. Statistical significance of differences between these ratios was assessed using the χ2 test. In addition, the species composition similarity was estimated for groups of biomass samples with high and low shares of dominants. For this purpose, the Sørensen similarity index was used: Ks = 2C/(A + B), where A and B are the numbers of species in the compared groups of samples and C is the number of shared species identified in both compared groups of samples. The statistical significance of the difference between mean values of this parameter (Ks) computed for sites dominated by alien and native species was assessed using the Mann–Whitney U test;
4. Additionally, biomass samples collected in different parts of the studied communities were combined into four groups, and their species richness and similarity were compared. Two of these groups included samples with the lowest shares of alien and native dominants (50 samples each); the other two groups included samples with the highest shares of alien and native dominants (145 and 121 samples, respectively). The total biomass of associated species was similar in the combined groups of samples with high and low shares of alien dominants, as well as in the combined groups of samples with high and low shares of native dominants.
5. Summary lists of species were produced for the combined groups of samples with high and low shares of native and alien dominants, and for each of them, the proportion of synanthropic (both obligate and facultative) species was determined, as well as proportions of species belonging to other florocoenoelements. The species were subsumed under certain florocoenoelements in accordance with Ivanov (2019). Also, the occurrence frequencies of synanthropic and nonsynanthropic species in groups of samples with different shares of native and alien dominants were compared.
If the relationship between the share of the dominant and the species richness cannot be fully explained by the “energy–diversity” and “larger number of individuals” hypotheses (the number of associated species is a positive function of their biomass, while their biomass is a negative function of dominant’s relative abundance), then the relationship between D and Sr can be expected to be significantly negative, and the group of samples with high shares of a certain dominant can be expected to include a significantly smaller number of species than the group of samples with a similar total biomass of associated species and low shares of this dominant. These effects, in turn, can be considered indicators of selective effects exercised by dominants on associated species. Higher proportions of synanthropic species in communities with significant shares of the dominant compared to communities with low shares of the dominant can also indicate this (Hejda and Pyšek, 2006; Hejda et al., 2009; Veselkin and Dubrovin, 2019), as well as a low species similarity between sites with high and low degrees of dominance (Hejda et al., 2009).
Therefore, if alien dominants pose a greater threat to the species richness of plant communities than native ones, then the following patterns can be expected: (1) the relationships between D and Ss and between D and Sr should be stronger on sites predominated by alien species than on sites with native dominants; (2) the situation when a group of samples with high degrees of dominance includes a significantly smaller number of species than a group of samples with a similar total biomass of associated species and low degrees of dominance should be observed more often on sites dominated by alien species than on sites with native dominants; (3) the proportion of synanthropic species in the composition of phytocoenoses with high and low shares of alien dominants should differ to a greater extent compared to communities with high and low degrees of dominance of native species; and (4) on average, the species composition similarity between groups of samples with high and low shares of alien dominants should be lower than the similarity between groups of samples with high and low degrees of dominance of native species.
RESULTS
Data on the shares of dominants and numbers of associated plant species in biomass samples collected on 20 sites occupied by synanthropic vegetation are presented in Table 1. As can be seen, the biomass of alien dominants in the studied parts of communities is on average higher in comparison with native dominants. Silphium perfoliatum, Helianthus tuberosus, and Impatiens glandulifera (i.e., alien species) feature the highest (on average) biomass. The biomass of some native species (e.g., Echium vulgare, Equisetum telmateia, and Sisymbrium loeselii) is also significant, but still lower in comparison with the above alien plants. Table 1 also indicates that the shares of dominants are very high in all the studied communities (at least on some of sampling plots 0.25 m2 in size): in biomass samples, their shares reach more than 90%. On average, the share of alien dominants in biomass samples is slightly higher in comparison with native dominants. The average numbers of species identified in individual biomass samples collected in communities dominated by alien and native species are approximately the same, as well as the numbers of species in series of samples.
The relationships between D and Ss, D and Ws, Ws and Ss, and D and Sr are provided in Table 2. The following conclusions can be drawn from it: (1) in most communities (dominated both by alien and native species), a moderately or strongly negative relationship between D and Ss is observed; (2) the relationship between D and Ws is strongly or moderately negative; (3) the relationship between Ws and Ss, is strongly, moderately, or weakly positive; and (4) a statistically significant negative relationship between D and Sr is observed only on two sites dominated by native species Melilotus officinalis and Rubus caesius. On average, the strength of relationships (i.e., values of Spearman’s rank correlation coefficient) between all the examined parameters (D and Ss, D and Ws, Ws and Ss, and D and Sr) on sites predominated by alien and native species differs statistically insignificantly (the null hypothesis was tested using the nonparametric Mann–Whitney U test).
The relationships between D and Ss, D and Ws, Ws and Ss, and D and Sr in communities predominated by alien species in general and predominated by native species in general are provided in Table 3. As can be seen, the character of these relationships is similar in communities predominated by species of different origin: in both cases, the first three relationships represent relatively weak, but statistically significant correlations; the relationship between D and Sr is nonexistent in both cases. Figure 1 shows relationships between D and Ss and D and Sr. It can be seen that the regression lines in the field of graphs are either located close to each other (Fig. 1a) or coincide (Fig. 1b). As follows from Table 4, the average species richness of samples with similar degrees of dominance of alien and native species differs in most cases statistically insignificantly.
Ss(D) and Sr(D) ratios in communities predominated by alien species in general and predominated by native species in general. Black circles and dashed regression lines represent biomass samples with the predomination of alien species; white circles and solid regression lines represent biomass samples with the predomination of native species.
Data on total numbers of associated plant species (\(N_{{\text{s}}}^{'}\)) in groups of biomass samples with the highest and lowest shares of dominants (D) are provided in Table 5. Groups with low shares of dominants consist of five samples; groups with high shares consist of 8–21 samples. Importantly, the total biomass of associated species (Ws) is similar in the compared groups of samples. As can be seen in this table, only in five groups of samples with high shares of dominants (alien: Asclepias syriaca and Solidago сanadensis; native: Trifolium arvense, Equisetum telmateia, and Rubus caesius) are the total numbers of species significantly smaller than in groups of samples with low shares of dominants (the difference is significant at P < 0.05). Combined groups of biomass samples with relatively low shares of dominants consist of 50 samples (collected on plots whose total area is 12.5 m2); with relatively high shares, they consist of 145 samples predominated by alien species (collected on 36.25 m2) and 121 samples with native dominants (collected on 30.25 m2). As can be seen in this table, the total numbers of plant species in the compared groups of samples (\(N_{{\text{s}}}^{'}\)) differ statistically insignificantly.
On average for ten plots, the species composition similarity (Ks) between groups of samples with high and low shares of certain alien dominants is 0.69 ± 0.04; between groups of samples with high and low shares of certain native dominants, it is 0.63 ± 0.03. The difference between these values is not statistically significant (Mann–Whitney U test, U = 37). The lowest species composition similarity was identified between groups of samples with high and low shares of native dominants Melilotus officinalis, Trifolium arvense, and Rubus caesius (0.50–0.55). The species composition similarity (Ks) between combined groups of samples with high and low shares of alien dominants is 0.80; between combined groups of samples with high and low shares of native dominants, it is 0.76.
Figure 2 shows that the occurrence frequency of associated plant species in combined groups of samples with high shares of alien and native dominants is mostly lower than in groups of samples with their low shares. In addition, it was determined that the proportion of synanthropic species (both obligate and facultative ones) that feature a higher occurrence frequency in the combined group of samples with low shares of native dominants than in the group of samples with their high shares is 0.81; the share of nonsynanthropic species is 0.77 (the difference is not statistically significant: t = 0.57, P < 0.05). For groups of samples predominated by alien species, the values of these parameters are 0.86 and 0.88, respectively (the difference is also statistically insignificant: t = 0.32, P < 0.05). Table 6 shows the distribution of species identified in groups of biomass samples with low and high shares of native and alien dominants by florocoenoelements. It follows from this table that, in communities dominated by alien species, the proportion of synanthropic (ruderal) plant species is somewhat lower, while the proportion of meadow species is somewhat higher than in communities with native dominants (on sites with both their low and high shares).
Occurrence frequency of associated plant species (F) in combined groups of samples with high and low shares of native (a) and alien (b) dominants. The x axis shows occurrence frequency (F) ranks of species in biomass samples with low shares of dominants. Solid lines show F values of species in samples with low shares of dominants; crosses show F values of the same species in samples with high shares of dominants.
DISCUSSION
Overall, alien dominants feature significantly higher aboveground biomass values and somewhat higher degrees of dominance in the studied synanthropic communities than native dominants. Having said that: (1) the average numbers of species on sites 0.25 m2 in size with high shares of alien and native dominants are roughly the same; (2) on average, the relationship between the degree of dominance of alien species and the species richness is approximately as strong as the relationship between the degree of dominance of native species and the species richness; and (3) the “energy–diversity” and “larger number of individuals” hypotheses satisfactorily explain the relationship between these parameters both for individual sites and in general for all biomass samples with the predomination of alien or native species. Exceptions are two fragments of communities predominated by native species (Melilotus officinalis and Rubus caesius).
In addition, the total number of species identified in combined groups of biomass samples with high shares of both native and alien species turned out to be approximately the same as in groups of samples similar in total biomass but with low dominance levels of native and alien species. With regard to fragments of communities predominated by certain species, only five of them in groups of samples with relatively high shares of dominants (in two cases with the predominance of alien species and in three cases with the predominance of native ones) feature significantly smaller numbers of species in comparison with groups of samples with similar biomass reserves of associated species and low shares of dominants. It must also be noted that Asclepias syriaca and Solidago сanadensis were studied on two sites each, and the results obtained on these sites turned out to be different. This may be due to random processes or due to the fact that the impact of the same dominants may have different consequences in different communities (Hejda et al., 2017; Vítková et al., 2017).
Finally, our data indicate that the occurrence frequency of most associated species decreases as shares of both native and alien dominants go up. Furthermore, in both cases, occurrence frequencies of synanthropic and nonsynanthropic species decrease to the same extent. Therefore, the proportion of synanthropic species in communities with high shares of both alien and native dominants is not higher than in communities with their low shares. In addition, the species composition similarity between groups of samples with high and low shares of alien dominants turns out to be approximately the same as between groups of samples with high and low shares of native dominants.
Overall, the results obtained by applying different methods of analysis to the field data are generally in good agreement with each other and indicate a similar and predominantly nonselective nature of impacts exercised by alien and native dominants on associated species in plant communities. Accordingly, we found no evidence that alien dominants pose a greater threat to the species richness of plant communities than native ones. According to our data, the only species exercising a selective effect on other species is Rubus caesius that predominates the edge community. This is a native low-growing perennial shrub whose shoots crawl and take roots forming a dense canopy. Apparently, an increase in its canopy density leads to the disappearance of species sensitive to low-light conditions, including Convolvulus arvensis, Achillea millefolium, Vicia pannonica, V. hirsuta, Plantago lanceolata, Inula germanica, and Torilis arvensis. Poa pratensis, Elytrigia repens, Calystegia silvatica, Stellaria holostea, and some other species persist in areas with high proportions of dewberry biomass and, accordingly, with high canopy density levels. Of them, Calystegia silvatica and Stellaria holostea occur in forests and shrub thickets, while Elytrigia repens often predominates in edge phytocoenoses in the area under investigation. There are three possible reasons why this shrub exercises a more selective effect on associated plant species compared to herbaceous species: (1) there is a predominantly horizontal arrangement of its leaves; (2) the dense canopy formed by Rubus caesius above the grass stand can mechanically prevent the upward growth of grasses; and (3) compared to wasteland and fallow communities, edge communities include species that are more differentiated in terms of shade tolerance.
According to Hejda et al. (2009), species forming tall and dense thickets unsuitable for the growth of shade-sensitive plants have the strongest effect on accompanying species in herbaceous cenoses. Overviews included in Black Book of the Flora of Central Russia (Vinogradova et al., 2009) also indicate this. Gioria and Osborne (2014), as well as Czarniecka-Wiera et al. (2019), note that the shading effect exercised by alien species owing to their significant biomass is one of the main mechanisms of impact affecting recipient communities. Therefore, taking that biomass of alien dominants on the studied sites is, on average, greater than biomass of native dominants, one could expect the consequences of their impacts on synanthropic communities to be different. In this regard, it must be noted that our results do not necessarily indicate the complete absence of differences between impacts exercised by alien and native dominants. But it follows from our data that the role of these differences in the formation of the studied communities is, apparently, relatively small compared to other environmental processes (fluctuations in the abiotic environment, disturbances, inflow of propagules, etc.).
Our findings are not unexpected: similar conclusions were made by other authors as well. For instance, Houlahan and Findlay (2004) showed that the share of alien dominants exercising significant adverse impacts on local plant communities in 58 inland wetlands in Ontario (North America) is the same as the share of native dominants exercising similar effects. Hejda et al. (2021) examined the impacts of several native and alien dominants on plant communities in Central Europe and did not find significant differences between them in this respect. Powell et al. (2013) compared sites occupied by forest biomes of different types (Hawaii, Missouri, and Florida, USA) dominated by alien tree species with forest sites featuring similar growing conditions but dominated by native plants present in smaller proportions. The results indicate that communities dominated by alien species feature lower species richness than similar communities with native dominants on small sites; however, on large sites, communities dominated by species of different origins feature similar species richness values. Similar results were obtained for plant communities on abandoned pastures in Poland (Czarniecka-Wiera et al., 2019). A number of overviews also note that the increasing number and abundance of alien plant species do not cause significant consequences for the phytodiversity of recipient regions (Gaertner et al., 2009; Powell et al., 2011; Rejmánek et al., 2013).
However, it must be noted again that other data indicate a stronger impact of alien species on communities compared to native ones and/or the selective nature of this impact (Richardson et al., 1989; Standish et al., 2001; Hejda et al., 2009; Akatov et al., 2012; Gusev, 2016, 2017; Rijal et al., 2017; Veselkin et al., 2020; etc.). In addition, two circumstances must be taken into account; in our opinion, they may be the reasons behind some underestimation of consequences caused by the impact of dominants (both alien and native ones) on other species. The first circumstance applies both to our findings and to results obtained by other authors: in most cases, the impact of dominant species on the species richness of plant communities is estimated on different spatial scales based on data collected on their relatively large fragments (Richardson et al., 1989; Hejda et al., 2009; Powell et al., 2011, 2013; Stohlgren and Rejmánek, 2014; Rejmánek and Stohlgren, 2015). However, within such relatively large fragments of communities with a high (on average) projective cover of dominants, microsites with a relatively low projective cover of dominants (“openings”) can be distinguished, which can affect the results of the dominance effect assessment (Hejda and Pyšek, 2006). This is why this study uses data collected on series of homogeneous microplots (0.25 m2) established using both the regular and typical methods within larger fragments of phytocoenoses dominated by certain species. In addition, purposeful sampling was used to enhance the contrast between samples in terms of shares of dominants. But even in such cases, the formed groups of biomass samples with high (on average) shares of dominant species often include samples with moderate degrees of dominance that could include species sensitive to the impact of this factor.
The second circumstance pertains to the mechanism that, according to the “larger number of individuals” hypothesis, determines the relationship between the productivity and species richness in communities: productivity limits the density of individuals, while the density of individuals limits the number of species. A key link in this causal chain is the density of individuals. However, the number of individuals on small sites depends not only on the aboveground biomass but also on their size, which presumably can decrease along the growth gradient of the share of the dominant. Therefore, the number of individuals and, accordingly, species in biomass samples with significant shares of dominants may in some cases be somewhat higher than one could expect according to the biomass weight.
CONCLUSIONS
Overall, our results support the assumption that, on average, alien and native dominants exercise similar impacts on synanthropic plant communities, and an increase in their share leads to a nonselective (i.e., random) elimination of associated species from communities. Such impacts cannot significantly affect the species pool size in phytocoenoses and, accordingly, the species richness of large fragments of vegetation cover. Nevertheless, in communities with high shares of dominants, the occurrence frequency of most associated species is lower than in communities with low shares of dominants. This makes such communities more vulnerable to impacts of other factors (e.g., phytophagous organisms, environmental fluctuations, fragmentation, etc.). But since the nature of such impacts weakly depends on the origin of dominants, the replacement of native dominants in the vegetation cover by alien ones should not cause significant consequences for accompanying species, at least, on large sites.
It must be noted though that, for a number of reasons, the ongoing increase in the total area of fragments of vegetation cover dominated by herbaceous alien species cannot be considered absolutely harmless for the phytodiversity of recipient regions. First, in some cases, alien dominants can reach significantly higher abundance levels than local dominants. Second, it is known that grass communities, including synanthropic ones, often do not have well-defined dominants. For instance, within the area under investigation, such communities occupy some 40% of lands covered by ruderal vegetation. The penetration of alien species into communities having no well-defined dominants and the subsequent growth of their shares are likely to cause more significant consequences for the species richness of recipient communities than strengthening of the positions of alien dominants in initially monodominant cenoses. Third, the available data indicate that alien dominants can stop restorative successions at the stage of monodominant communities poor in species for a long period of time (Gusev, 2016, 2017). Finally, most researchers assess the consequences of impacts exercised by alien dominants on communities in relation to associated plant species. But local dominants may be more vulnerable to such impacts. The ideas of Mirkin and Naumova (2012) on the organization of herbaceous phytocoenoses support this supposition. In their opinion, herbaceous phytocoenoses are structured by different mechanisms: competition between dominants (C-strategists) and random processes in groups of less abundant species (S- and R-strategists). However, we are not aware of studies examining the relationships between dominants of different origins in herbaceous communities on a large spatial scale.
REFERENCES
Akatov, V.V., Akatova, T.V., and Shadzhe, A.E., Species richness of tree and shrub layers in riparian forests of the Western Caucasus dominated by alien species, Russ. J. Ecol., 2012, vol. 43, no. 4, pp. 294–301.
Akatov, V.V., Akatova, T.V., and Chefranov, S.G., The relationship of dominance and evenness with productivity and species richness in plant communities with different organization models, Russ. J. Ecol., 2018, vol. 49, no. 4, pp. 296–305.
Akatov, V.V., Akatova, T.V., Afanas’ev, D.F., Sazonets, N.M., Sushkova, E.G., and Chefranov, S.G., The nature of correlation between the degree of dominance and species richness in plant communities of different types: Are the processes biological or stochastic?, Russ. J. Ecol., 2019, vol. 50, no. 5, pp. 422–430. https://doi.org/10.1134/S1067413619040039
Bartha, S., Szentes, Sz., Horváth, A., Házi, J., Zimmermann, Z., Molnár, Cs., Dancza, I., Margóczi, K., Pál, R., Purger, D., Schmidt, D., Óvári, M., Komoly, C., Sutyinszki, Zs., Szabó, G., Csathó, A.I., Juhász, M., Penksza, K., and Molnár, Zs., Impact of mid-successional dominant species on the diversity and progress of succession in regenerating temperate grasslands, Appl. Veg. Sci., 2014, vol. 17, no. 2, pp. 201–213.
Blackburn, T.M., Bellard, C., and Ricciardi, A., Alien versus native species as drivers of recent extinctions, Front. Ecol. Environ., 2019, vol. 17, no. 4, pp. 203–207. https://doi.org/10.1002/fee.2020
Callaway, R.M. and Ridenour, W.M., Novel weapons: A biochemically based hypothesis for invasive success and the evolution of increased competitive ability, Front. Ecol. Environ., 2004, no. 2, pp. 433–436.
Chase, J.M., Powell, K.I., and Knight, T.M., ‘Bigger data’ on scale-dependent effects of invasive species on biodiversity cannot overcome confounded analyses: A comment on Stohlgren and Rejmánek (2014), Biol. Lett., 2015, vol. 11, p. 20150103. https://doi.org/10.1098/rsbl.2015.0103
Csergő, A.M., Demeter, L., and Turkington, R., Declining diversity in abandoned grasslands of the Carpathian Mountains: Do dominant species matter?, PLoS One, 2013, vol. 8, no. 8, e73533.https://doi.org/10.1371/journal.pone.0073533
Czarniecka-Wiera, M., Kacki, Z., Chytry, M., and Palpurina, S., Diversity loss in grasslands due to the increasing dominance of alien and native competitive herbs, Biodiversity Conserv., 2019, vol. 28, pp. 2781–2796.
Davis, M.A., Chew, M.K., Hobbs, R.J., et al., Don’t judge species on their origins, Nature, 2011, vol. 474, pp. 153–154.
Gaertner, M., Breeyen, A.D., Hui, C., and Richardson, D.M., Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A meta-analysis, Prog. Phys. Geogr., 2009, vol. 33, pp. 319–338.
Garsía L.V., Marañόn T., Moreno, F., and Clemente, L., Above-ground biomass and species richness in a Mediterranean salt march, J. Veg. Sci., 1993, vol. 4, pp. 417–424.
Gioria, M. and Osborne, B.A., Resource competition in plant invasions: Emerging patterns and research needs, Front. Plant Sci., 2014, vol. 5, 501. https://doi.org/10.3389/fpls.2014.00501
Grime, J.P., Competitive exclusion in herbaceous vegetation, Nature, 1973, vol. 242, pp. 344–347.
Gusev, A.P., Alien species-transformers as the reason of regenerative processes blocking (on an example of the southeast of Belarus), Ross. Zh. Prikl. Ekol., 2016, no. 3, pp. 10–14.
Gusev, A.P., Inhibition of restorative succession by invasive plant species: Examples from southeastern Belarus, Russ. J. Ecol., 2017, vol. 48, no. 4, pp. 321–325.
Gusev, A.P., The invasion of Canadian goldenrod (Solidago canadensis L.) into anthropogenic landscapes of Belarus, Russ. J. Biol. Invasions, 2018, no. 9, pp. 22–28.
Hejda, M. and Pyšek, P., What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation?, Biol. Conserv., 2006, vol. 132, pp. 143–152.
Hejda, M., Pyšek, P., and Jarošik, V., Impact of invasive plants on the species richness, diversity, and composition of invaded communities, J. Ecol., 2009, vol. 97, pp. 3393–3403.
Hejda, M., Štajerová, K., and Pyšek P., Dominance has a biogeographical component: Do plants tend to exert stronger impacts in their invaded rather than native range?, J. Biogeogr., 2017, vol. 44, pp. 18–27.
Hejda, M., Sádlo, J., Kutlvašr, J., Petřík, P., Vítková, M., Vojík, M., Pyšek, P., and Pergl, J., Do invasive alien plants impact the diversity of vegetation more compared to native expansive dominants?, in Invasion of Alien Species in Holarctic. Borok-VI: Abstr. Sixth Int. Symp., Dgebuadze, Yu.Yu., Krylov, A.V., Petrosyan, V.G., and Karabanov, B.P., Eds., Kazan: Buk, 2021, pp. 88–89.
Houlahan, J.E. and Findlay, C.S., Effect of invasive plant species on temperate wetland plant diversity, Conserv. Biol., 2004, vol. 18, no. 4, pp. 1132–1138.
Ivanov, A.L., Konspekt flory Rossiiskogo Kavkaza (sosudistye rasteniya) (Conspectus Florae Caucasi Rossicae (Plantae Vasculares)), Stavropol’: Severo-Kavkaz. Fed. Univ., 2019.
Lanta, V., Hyvonen, T., and Norrdahl, K., Non-native and native shrubs have differing impacts on species diversity and composition of associated plant communities, Plant Ecol., 2013, vol. 214, no. 12, pp. 1517–1528. https://doi.org/10.1007/s11258-013-0272-0
Levine, J.M., Vila, M., D’Antonio, C.M., et al., Mechanisms underlying the impacts of exotic plant invasions, Proc. R. Soc. London, Ser. B, 2003, vol. 270, pp. 775–781. https://doi.org/10.1098/rspb.2003.2327
Meiners, S.J., Pickett, S.T.A., and Cadenasso, M.L., Effects of plant invasions on the species richness of abandoned agricultural land, Ecography, 2001, vol. 24, pp. 633–644.
Mirkin, B.M., Which plant communities do exist?, J. Veg. Sci., 1994, vol. 5, no. 2, pp. 283–284.
Mirkin, B.M. and Naumova, L.G., The problem of plant communities species richness (current state), Usp. Sovrem. Biol., 2012, vol. 132, no. 3, pp. 227–238.
Mirkin, B.M., Yamalov, S.M., and Naumova, L.G., Synanthropic plant communities: Models of organization and features of classification, Zh. Obshch. Biol., 2007, vol. 68, no. 6, pp. 435–443.
Oksanen, J., Is the humped relationship between species richness and biomass an artefact due to plot size?, J. Ecol., 1996, vol. 84, pp. 293–295.
Powell, K.I., Chase, J.M., and Knight, T.M., A synthesis of plant invasion effects on biodiversity across spatial scales, Am. J. Bot., 2011, vol. 98, no. 3, pp. 539–548.
Powell, K.I., Chase, J.M., and Knight, T.M., Invasive plants have scale-dependent effects on diversity by altering species-area relationships, Science, 2013, vol. 339, pp. 316–318.
Rabotnov, T.A., Fitotsenologiya (Phytocenology), Moscow: Mos. Gos. Univ., 1983.
Reinhart, K.O., Greene, E., and Callaway, R.M., Effects of Acer platanoides invasion on understory plant communities and tree regeneration in the Rocky Mountains, Ecography, 2005, vol. 28, pp. 573–582.
Rejmánek, M. and Simberloff, D., Origin matters, Environ. Conserv., 2017, vol. 44, no. 2, pp. 97–99.
Rejmánek, M. and Stohlgren, T.J., Scale-dependent impacts of invasive species: A reply to Chase et al., Biol. Lett., 2015, vol. 11, 20150402. https://doi.org/10.1098/rsbl.2015.0402
Rejmánek, M., Richardson, D.M., and Pyšek, P., Plant invasions and invasibility of plant communities, in Vegetation Ecology, van der Maarel, E. and Franklin, J., Eds., Chichester, New York: Wiley, 2013, 2nd ed., pp. 387–424.
Richardson, D.M., Macdonald, I.A.W., and Forsyth, G.G., Reductions in plant species richness under stands of alien trees and shrubs in the fynbos biome, S. Afr. For. J., 1989, vol. 149, pp. 1–8.
Rijal, D.P., Alm, T., Inger, L.N., and Alsos, G., Giant invasive Heracleum persicum: Friend or foe of plant diversity?, Ecol. Evol., 2017, vol. 7, pp. 4936–4950.
Sagoff, M., Do non-native species threaten the natural environment?, J. Agric. Environ. Ethics, 2005, vol. 18, pp. 215–236.
Seabloom, E.W., Borer, E.T., Buckley, Y.M., et al., Plant species’ origin predicts dominance and response to nutrient enrichment and herbivores in global grasslands, Nat. Commun., 2015, vol. 6, pp. 1–8.
Silliman, B.R. and Bertness, M.D., Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes, Conserv. Biol., 2004, vol. 18, pp. 1424–1434.
Šímová, I., Li, Y.M., and Storch, D., Relationship between species richness and productivity in plants: The role of sampling effect, heterogeneity and species pool, J. Ecol., 2013, vol. 101, pp. 161–170.
Smith, D.M., Finch, D.M., Gunning, C., et al., Post-wildfire recovery of riparian vegetation during a period of water scarcity in the Southwestern USA, Fire Ecology Special Issue, 2009, vol. 5, no. 1, pp. 38–55.
Srivastava, D.S. and Lawton, J.H., Why more productive sites have more species: An experimental test of theory using tree-hole communities, Am. Nat., 1998, vol. 152, pp. 510–529.
Standish, R.J., Robertson, A.W., and Williams, P.A., The impact of an invasive weed tradescantia fluminensis on native forest regeneration, J. Appl. Ecol., 2001, vol. 38, pp. 1253–1263.
Stohlgren, T.J. and Rejmánek, M., No universal scale-dependent impacts of invasive species on native plant species richness, Biol. Lett., 2014, vol. 10, 20130939. https://doi.org/10.1098/rsbl.2013.0939
Veselkin, D.V. and Dubrovin, D.I., Diversity of the grass layer of urbanized communities dominated by invasive Acer negundo, Russ. J. Ecol., 2019, vol. 50, no. 5, pp. 413–421.
Veselkin, D.V., Zolotareva, N.V., Lipikhina, Yu.A., Podgaevskaya, E.N., and Kiseleva O.A., Diversity of plants in thickets of invasive Sorbaria sorbifolia: Differences in the effect on aboveground vegetation and seed bank, Russ. J. Ecol., 2020, vol. 51, pp. 518–527.
Vinogradova, Yu.K., Maiorov, S.R., and Khorun, L.V., Chernaya kniga flory Srednei Rossii (Chuzherodnye vidy rastenii v ekosistemakh Srednei Rossii) (Black Book of Flora of Central Russia (Alien Species of Plants in Ecosystems of Central Russia)), Moscow: GEOS, 2009.
Vítková, M., Müllerová, J., Sádlo, J., Pergl, J., and Pyšek, P., Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe, For. Ecol. Manage., 2017, vol. 384, pp. 287–302.
Wright, D.H., Species-energy theory: An extension of species-area theory, Oikos, 1983, vol. 41, pp. 496–506.
Zernov, A.S. Flora Severo-Zapadnogo Kavkaza (Flora of the Northwestern Caucasus), Moscow: KMK, 2006.
Zobel, M. and Partel, M., What determines the relationship between plant diversity and habitat productivity?, Global Ecol. Biogeogr., 2008, vol. 17, pp. 679–684.
Funding
This study was supported by the Russian Foundation for Basic Research, projects no. 16-04-00228 and no. 20-04-00364.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement of the welfare of animals. The article does not contain any studies involving animals in experiments performed by any of the authors.
Additional information
Translated by L. Emeliyanov
Rights and permissions
About this article
Cite this article
Akatov, V.V., Akatova, T.V., Eskina, T.G. et al. Alien and Native Dominants Exercise Similar Effects on the Species Richness in Synanthropic Plant Communities of the Western Caucasus. Russ J Biol Invasions 13, 271–283 (2022). https://doi.org/10.1134/S207511172203002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207511172203002X