Abstract
The paper presents descriptions, original drawings and digital photos, and also the key for identifying naupliar stages of recent invader species in the Black Sea Oithona davisae (Copepoda: Cyclopoida). Nauplii were taken from a laboratory culture originating from the offspring of O. davisae females selected from living zooplankton sampled in Sevastopol Bay. Copepods were reared under natural illumination, temperature of 21 ± 2°C, and feeding with cryptophyte culture IBSS-CrPr54 (ESD = 10.9 ± 1.4 μm) (mean concentration constituted 6 × 103 cells mL–1; 0.7 μg C mL–1). The duration of the development from the first naupliar stage (N1) to the first copepodite (C1) stage was 5.3 ± 0.7 days (1 day for N1–N3, 1 day for N3–N4, and 3.3 days for N4–C1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Oithona davisae Ferrari and Orsi, 1984 was recorded for the first time as new species from the Sacramento–San Joaquin Estuary (California) (Ferrari and Orsi, 1984). This species is a typical representative of neritic fauna (Uchima, 1988) and occurs, occasionally in large aggregations, in river mouths and coastal areas (Nishida, 1985; Hirota, 1990). Owing to its abundance and small size, O. davisae is an essential part of ecosystem processes and a source of nutrition for larvae of many fish species.
A number of works (Uye and Sano, 1995; Takahashi and Uchiyama, 2007) mention naupliar stages of O. davisae with reference to M. Uchima (1979) as the author of its first record. In 1979, when Uchima pursued the research, O. davisae had not been described. Uchima identified his object from Tokyo Bay as Oithona brevicornis f. minor Nishida et al. (1977) (the small form is specified in the text and abstract of the article; it is missing from the title).
Multiple novel species of small coastal oithonids were described in the 1980s, including those with morphological similarity to O. brevicornis Giesbrecht, 1981 s. str., but distinguished from it by a smaller size and the presence of long distal seta on first inner lobe of maxillula (Ferrari, 1981; Nishida and Ferrari, 1983; Ferrari and Orsi, 1984). Analogous seta in O. brevicornis s. str. is much shorter.
The authors of one of the above-mentioned works (Nishida and Ferrari, 1983) supplemented the description of O. brevicornis s. str. and re-described the O. brevicornisaruensis Fruchtl, 1923, raising its taxonomic status to a species. At the end of the article, they noted that specimens used by Uchima (1979) to obtain nauplii were reconsidered and proved to be representative of O.aruensis Früchtl, 1923: “The specimens described as O. brevicornis f. minor Nishida et al. (1977) and as O. brevicornis by Uchima (1979) were reexamined and proved to be O. aruensis.”
O. aruensis is particularly close morphologically and in size to O. davisae (Temnykh and Nishida, 2012). Accordingly, its nauplii should be expected to closely resemble nauplii of the latter. The grounds on which the authors of the articles (Uye and Sano, 1995; Takahashi and Uchiyama, 2007) assigned the nauplii described by Uchima (1979) to O. davisae are unclear; adequate explanations are not included in the text.
At the same time, the following record exists in the NEMESIS database (National Exotic Marine and Estuarine Species Information System, Smithsonian Environmental Research Center) in California Non-native Estuarine and Marine Organisms (Cal-NEMO) section on the webpage devoted to O. davisae: “The copepodite and naupliar stages of this copepod have not been described. Morphology and development should be similar to that described for Oithona brevicornis by Uchima (1979)” (Fofonoff et al., 2018). Consequently, it can be inferred that naupliar stages of O. davisae have never been described, though they should bear resemblance to nauplii of the small form O. brevicornis (=O.aruensis (Nishida and Ferrari, 1983)) first described by Uchima (1979).
O. davisae was reported as a new species from the east of the Pacific Ocean off the coast of California. However, in the opinion of some researchers (Uchima, 1988; Uye and Sano, 1995), the species originated from the nearshore waters in the western part of the Pacific Ocean, including its high abundance off the coast of Japan, and was endemic to nearshore waters of East Asia. As a result of its further expansion, apparently after having been transported in ballast waters, it was discovered in many coastal areas of the World Oceans: along the western coast of the United States (Ferrari and Orsi, 1984; Ambler et al., 1985); along the southern coast of Chile (Hirakawa, 1988); in the Mediterranean Sea (Saiz et al., 2003) and North Sea (Cornils and Wend-Heckmann, 2015); in the estuary of Bilbao that empties into the Bay of Biscay (Uriarte et al., 2016); and in the Black Sea and the Sea of Azov.
Alien cyclopoid copepod of genus Oithona, misidentified as O. brevicornis (Zagorodnyaya, 2002; Altukhov and Gubanova, 2006; Gubanova and Altukhov, 2007; Selifonova, 2009, 2011), was first recorded from Sevastopol Bay (Black Sea) in December 2001 (Zagorodnyaya, 2002). It was identified as O. davisae later (Temnykh and Nishida, 2012). From 2008 onward, О. davisae numbers periodically reached 99% of the total population of copepods in Sevastopol Bay (Altukhov et al., 2014; Svetlichny et al., 2016), as in the case of О. davisae in Tokyo Bay, where it was similarly dominant among other copepod species with 99% of their total population during warm periods of the 1980s (Tsuda and Nemoto, 1988). To date О. davisae has been reported off the Black Sea coast of Romania (Timofte and Tabarcea, 2012), Bulgaria (Mihneva and Stefanova, 2013), and, recently, Turkey (Üstün and Kurt, 2016; Yildiz et al., 2016), as well as the Sea of Marmara (Isinibilir et al., 2016).
It appears problematic to assess the contribution of naupliar stages of the introduced small-sized cyclopoid copepod to the total population of copepods not only in the absence of plankton net equipped with fine mesh sieve but also in the absence of a current description of the naupliar stages. The recent considerable increase in fish larvae in the Black Sea ichthyoplankton appears to be due to both an increase in total copepod population and a rising number of small-sized О. davisae in the plankton (Gubanova et al., 2015; Khanaychenko et al., 2015), which has occupied the ecological niche of О. nana (which disappeared in 1989) (Altukhov et al., 2014; Svetlichny et al., 2016). Owing to their large quantities in zooplankton, the probability of occurrence of this introduced copepod and their nauplii, in particular, during the early developmental stages in the diet of larvae of summer-spawning fish species has been very high in recent years (Vdodovich et al., 2017a). However, the early growth stages of О. davisae are often difficult to identify in guts of fish larvae (Vdodovich et al., 2017b).
The goal of the present work was to provide a detailed description of morphological characteristics of naupliar stages in the introduced Black Sea species О. davisae, as well as acquire data on duration of naupliar development of this crustacean in the laboratory culture environment.
MATERIALS AND METHODS
Nauplii of O. davisae used in the research were selected from their laboratory culture, originating from progeny of fertilized overwintering females sorted from zooplankton samples shortly after they had been collected using a plankton net with mesh size of 100 µm from the shore of Sevastopol Bay. Active females were sorted intact under a binocular in Bogorov chamber. Fertilized overwintering females O. davisae can store viable sperm under low temperature conditions (6–8°C) over a long period of time and produce viable offspring after the conditions return to favorable (Svetlichny et al., 2016).
The copepod monoculture was kept at room temperature of 21 ± 2°C, under natural light conditions, and fed cryptophyte microalgae (at mean concentration of 6 × 103 cells mL–1; 0.7 μg C mL–1) of IBSS-CrPr54 (ESD = 10.9 ± 1.4 µm) strain from culture in the exponential growth phase. Multiple generations of the copepods were produced in laboratory culture.
To obtain various successive naupliar stages of O. davisae, after hatching of the egg ovisacs, nauplii of the first stage were transferred to 10-mL containers and maintained under the same temperature and trophic conditions. As the nauplii were going through ecdysis (molts), preceding the first copepodite stage, they were recorded daily. Then, a necessary number of individuals at the corresponding stage were selected for further examinations.
Individuals at different naupliar stages (as well as their exuviae) were collected from the culture in February 2017 and fixed in 4% formaldehyde in seawater (18‰ salinity). Then we placed them into aqueous glycerin (1 : 1) on microscope slides using a LOMO MBR-10 binocular microscope and took measurements under Leica DM LS2 microscope at 400× magnification. All drawings were made with a drawing tube on a Leica DM LS2 (magnification of 400×) in ventral and lateral views. A Canon PowerShot A520 camera was used to capture images of the nauplii.
For the specimen length, we used the maximum distance from the anterior tip to the posterior end of the body, excluding the lengths of caudal spines and setae; the maximum distance between lateral margins of nauplius in ventral or dorsal view was measured for the width.
Intact individuals and exuviae in different projections, as well as the limb fragments, were used to draw limbs. The final limb drawing reflects information about its structure acquired on the basis of examination of appendages of several individuals in the corresponding developmental stage.
To compare dimensions of laboratory specimens with the specimens from the sea, we selected and measured O. davisae nauplii from a zooplankton sample collected in Kryglaya Bay (Sevastopol) on September 27, 2015, using a sieve with a mesh size of 64 µm. At the time of sampling (10 a.m.), the bay water temperature was at 23°C. The sample was fixed in 4% formaldehyde in seawater.
The following symbolic representation is employed with description of nauplii in the paper: (N1–N6) first to sixth naupliar stages; (C1) first copepodite stage; (Lb) labrum; (An1) first antenna (antennule); (An2) second antenna (antenna); (Md) mandible; (Mx1) first maxilla (maxillula); (Mx2) second maxilla (maxilla); (Mxp) maxilliped; (Р1 and Р2) first and second thoracic limbs; (Cxp) coxopodite; (Bsp) basipodite; (Enp) endopodite ((Enp1,2) endopodite segments); (Exp) exopodite ((Exp1–5) exopodite segments); (L and Lav) length and average body length; (Wav) average body width; and (n) number of examined individuals.
RESULTS AND DISCUSSION
Description of Naupliar Stages of Oithona davisae
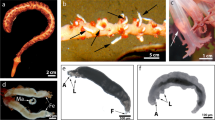
General characterization of the nauplii. The body is egg-shaped (first three stages, N1–N3) or teardrop-shaped (N4–N6). The body is dorsiventrally flattened with a wider anterior part (Photo, Fig. 1). Table 1 shows length of individuals in different naupliar stages, as well as average values of length and its ratio to average width of the individuals.
The dorsal surface of nauplii is covered with cephalothoracic shield (Sazhina, 1985). The shield covers the entire surface in the first two stages (orthonauplii), with its height exceeding the ventral surface length. The body is more elongated in nauplii of subsequent stages (metanauplii). In the third stage, the cephalothoracic shield and posterior end of the abdomen are on approximately the same level. In the fourth, fifth, and sixth stages, the posterior part of the body elongates, extending beyond the cephalothoracic shield margin.
The upper lip (Lb) is found in the anterior ventral part. It is rounded in ventral view; in lateral view, it strongly projects above the ventral surface, overhanging the mouth opening, and has four groups of thin short hairs on its inferior border. An X-shaped “naupliar eye” is superior to Lb; the first three pairs of mouth appendages (An1, An2, and Md) are present on the labrum bilaterally. Living nauplii have a red-colored eye (Photo a).
The posterior part of the body is equipped with paired caudal setae and spines. Their quantity, length, and structural features are among the main characteristics for distinguishing between different naupliar stages.
Below is a more detailed description of the outgrowths corresponding to different naupliar stages. We referred to stiff long or short outgrowths, which are thicker at the base, as spines and long or short slender outgrowths as setae (Sazhina, 1985). The word hair was occasionally used to denote very fine outgrowths.
The First Stage, N1 (Photo a; Figs. 1A and 1B)
Аn1 is uniramous with three segments (Fig. 2 A1). The first segment is unarmed; the second bears three setae on the ventral side (Fig. 2 A1–А6 above). One of the setae, close to a joint between the second and third segments, is about twice as long as the third segment. The latter is compressed laterally. In N1, it is provided with three apical setae, one of which is shorter and much thinner than the other two. It is inconspicuous owing to a close proximity to the next seta. Two short hair-like setae are positioned on the dorsal (Fig. 2 A1–А6 lower) side of the third segment. Three longest setae from the second and third segments are plumose.
An2 is biramous (Fig. 2 В1). On Cxp closer to a point of junction with Вsp, there is a protrusion with two spine-like setae attached, with one of them being thinner and bearing long hairs (often perceived as a group of several thin setae). The spines are directed toward the mouth opening. Bsp is rather short (its length only slightly exceeds the width) with three thin setae. Enp is unsegmented with two long apical plumose setae and two lateral setae, with one of them being markedly longer. Exp consists of five segments; Exp1 is the largest, elongated, with long distal seta. The next three segments are short; each bears one long seta. The length of the last segment is subequal to the width; two long apical setae are present. On Exp, all long setae are plumose.
Md is biramous (Fig. 2 С1). Сxp has one seta. Bsp has two spine-like setae, one on each side of the segment. Enp is two-segmented. Enp1 with two thick spines, which are armed with rare coarse setae almost perpendicular to the axis of spines; Enp2 bears two long plumose apical and two short lateral setae. Exp is four-segmented; each segment features one long plumose seta on its distal end; the final segment, bearing an apical seta, is tiny.
Caudal armature: one pair of long setae.
The Second Stage, N2 (Photos b and c; Figs. 1C and 1D)
Аn1 is three-segmented (Fig. 2 A2). Armature of the first two segments is unchanged; the third segment bears four long apical setae (with one being shorter than the others, thin, inconspicuous, and non-plumose) and three relatively short hair-like setae (one on ventral and two on dorsal sides).
An2 (Fig. 2 В2): Сxp and Bsp is unchanged. Enp has three apical and two lateral setae. Exp1 has one relatively short lateral seta and one long distal seta; the remaining four segments are unchanged.
Md (Fig. 2 С2): Сxp, Bsp, and Exp are unchanged. Enp1 is armed with three thick spines covered by rare coarse setae. Enp2 has three apical and two short lateral setae.
Primordia of Mx1 resemble a pair of small bumps, each bearing one thin seta; they are positioned between mandibles and caudal setae (Figs. 1C and 1D).
Caudal armature: one pair of long setae.
The Third Stage, N3 (Photos d and e; Figs. 1E and 1F)
Аn1 (Fig. 2 А3) consists of four segments, but division between the second and third segments is not clearly defined. The first segment is unarmed. The second has one relatively small seta. The third has one short and one long distal seta. The fourth bears four apical setae, with one being thin and inconspicuous, and five to six short hair-like setae, with one on the ventral and four to five on dorsal side.
An2 (Fig. 2 В3): Cxp with two spines and one relatively short thin seta located more distally. Bsp and Enp unchanged. Exp is five-segmented; on Exp1, there are two lateral setae and one long distal seta; the final fifth segment bears three apical setae with one of them less than half the length of the other two; three intermediate short segments (Exp2–4) have one long seta each, similar to other naupliar stages.
Md (Fig. 2 С3) is unchanged.
Mx 1 primordia (Figs. 1E and 1F) are represented by a pair of protrusions, each bearing one or, occasionally, two setae.
Caudal armature consists of two pairs of long and one pair of short spines equipped with secondary spinelets and located intermediate to long setae close to the central axis of the body.
The Fourth Stage, N4 (Photos f and g; Figs. 1G and 1H)
Аn1 (Fig. 2 А4) is four-segmented. The setation of segments is unchanged.
An2 (Fig. 2 B4) is similar to that of N3, but with three lateral setae on Exp1.
Md (Fig. 2 C4) is unchanged.
Mx1 (Fig. 2 D1) is biramous; segmentation is not clearly defined. Each branch has three terminal setae, with one being very long (approximately three times the length of the branch proper). Three lateral setae are found on the inner lateral surface of the limb.
Caudal armature: the number of caudal outgrowths is unchanged; however, the spines are noticeably stronger and longer than those in N3 and extend slightly beyond middle of the next long setae.
The Fifth Stage, N5 (Photos h and i; Figs. 1I and 1J)
Аn1 (Fig. 2 A5) is four-segmented. Setation of the first three segments is unchanged. The fourth segment bears four long apical setae (with one of them being short and non-plumose) and two ventral and eight dorsal setae.
An2 (Fig. 2 B5): Сxp, Bsp, and Exp are unchanged. Enp bears three apical and four lateral setae; one of the lateral setae is relatively long and covered by setules.
Md (Fig. 2 C5): Enp2 has three setae on lateral surface; other segments are unchanged.
Mx1 (Fig. 2 D2) is biramous; segmentation is unremarkable. Similar to N4, each branch ends in three apical setae, one of which is very long (approximately three times the length of the branch proper), extending nearly to the tips of caudal setae and spines (Figs. 1I and 1J). On the inner lateral surface of the limb, there are five lateral setae with three of them on the limb basis. In addition to three apical, Exp has one lateral subterminal seta on the outer side of the branch.
Primordia of Мх2 and Мхp are noticeable. Mx2 rudiments place inferior to maxillulae, Mxp rudiments are intermediate to them (Fig. 1I).
Caudal armature consists of two pairs of long setae; one pair of long thick spines, extending to the tips of the long setae; and one pair of very short lateral setae. Additionally, another pair of very short thin setae can be seen between two long spines in many specimens of N5.
The Sixth Stage, N6 (Photo j–m; Figs. 1K and 1L)
Аn1 consists of four segments (Fig. 2 A6); the final segment is partially segmented. Armature of the first three segments is unchanged; the fourth carries four apical, five ventral, and eight dorsal setae.
An2 (Fig. 2 B6) and Md (Fig. 2 C6) are unchanged.
Мx1 (Fig. 2 D3): segmentation is not well defined; setae are as many as in N5; Enp is somewhat longer and appears two-segmented (second segment is very short).
In addition to Mx2 and Mxp, primordia of thoracic limbs P1 and P2 are present.
Caudal armature: two thin crossed central setae extend approximately to the middle of the spines; the remaining setae and spines are unchanged.
Naupliar stages of O. davisae in ventral (all stages left) and lateral (right) views: (a) for N1; (b and c) for N2; (d and e) for N3; (f and g) for N4; (h and i) for N5; and (j and k) for N6. (l) is N6 ventrally; medial part of the body shows primordia of Мх2 and Мхр; (m) is posterior part of N6; segmentation of nauplius body is clearly seen. Scale is common for all images except m.
Below is a key compiled on the basis of features sufficient to identify nauplii of the Black Sea O. davisae to one of the stages. Note that the more mature the naupliar stage, the more problematic it is to rely on its size for identification, inasmuch as it largely depends on the temperature and feeding conditions during naupliar development.
Key for identification of naupliar stages of O. davisae
1. L standard <100 µm. Cephalodorsal shield extends beyond the length of ventral surface. An2 Eхp5 with two apical setae. Caudal armature: one pair of long setae………………………………………………………...........2
—L standard >100 µm. Cephalodorsal shield is shorter or subequal to the length of ventral surface. An2 Eхp5 with three apical setae; one of them extends to less than the middle of the others. Caudal armature: three or more pairs of setae and spines.………………………………………………………………………………………….......3
2. Md Enp1 with two thick spines covered by coarse setae positioned almost perpendicular to the axis of spines. An1 with three apical setae, one being shorter and thinner, positioned close to the next seta, and inconspicuous. An2 Enp and Md Enp2 with two apical setae. Mx1 is missing.…………………………….….N1
—Md Enp1 with three thick spines. An1 with four apical setae, one being shorter, thinner, and inconspicuous. An2 Enp and Md Enp2 bear three apical setae. Mx1 is in the form of tubercle with one thin seta…………………………………………………………………………………………………………………………………..N2
3. Cephalodorsal shield is subequal to the length of ventral surface. Mx1 is in the form of tubercle with one to two thin setae. Caudal armature: two pairs of long setae + one pair of short spines ……………………………N3
—Cephalodorsal shield is shorter than the length of ventral surface. Mx1 is biramous; one of three apical setae on each branch is very long (more than three times the length of the branch proper)………………….…………4
4. Body with four pairs of limbs. Inner lateral surface of Mx1 with three lateral setae. Caudal armature: two pairs of long setae + one pair of mid-length spines (approximately half the length of the long setae)………………N4
—Body with primordia of the fifth to eightn pairs of limbs. Inner lateral surface of Mx1 bears five lateral setae. Caudal armature: five pairs of setae and spines…………………………………………………………………………….5
5. Body with primordia of Mx2 and Mxp. Caudal armature: two pairs of long setae + one pair of thick long spines (extending nearly to the tips of the long setae) + one pair of short lateral setae + one pair of very short thin central setae…………………………………………………………………………………………………………………..N5
—Body with primordia of P1 and P2. Caudal armature: thin crossed central setae approximately half the length of spines …………………………………………………………………………………………………………………..N6
Nauplii of the Black Sea O. davisae are very similar to specimens previously described (Uchima, 1979) as nauplii of small form O. brevicornis (=O. aruensis (Nishida and Ferrari, 1983)). However, some distinctions exist, specifically, four-segmented (rather than three-segmented) An1 in nauplii of the fourth to sixth stages; presence of primordia of Mx2 (no mention of them is made at all in the Uchima (1979) description), Mxp, and thin central setae in posterior end of the body in N5; and some discrepancies in the quantity of fine lateral setae. These distinctions may entirely be attributed to the difficulties in working with nauplii associated with their small sizes.
No descriptions of naupliar stages for other species having morphological similarities to O. davisae (females less than 1 mm in size with pointed rostrum that curves ventrally) exist, except for a not particularly detailed description of nauplii of O. brevicornis (Goswami, 1975). However, when comparing his own data with those of Goswami and analyzing his descriptions of copepodite stages of the studied species, Uchima (1979) inferred that specimens described by Goswami appeared to belong to a species other than O. brevicornis.
Duration of Naupliar Development of O. davisae in Laboratory Culture
Previous research of feeding selectivity of O. davisae (Khanaychenko et al., 2018) demonstrated that these copepods prefer cryptophytes to other microalgae and can reproduce in culture through multiple generations feeding exclusively on cryptophytes strain IBSS-CrPr54 (ESD = 10.9 ± 1.4 µm) at concentrations about 6 × 103 cells mL–1; 0.7 µg C mL–1. Therefore, in this paper, only laboratory specimens that were fed exclusively cryptophytes IBSS-CrPr54 were examined for their morphology and duration of development of the naupliar stages.
Development of O. davisae from N1 to C1 lasted for 5.3 ± 0.7 days (1 day for N1–N3, 1 day for N3–N4, and 3.3 days for N4–C1). This data supports somewhat more rapid development of naupliar stages in the conditions of our laboratory culture compared to laboratory culture of other researchers wherein nauplii were reared under similar temperature conditions but fed on different food, i.e., heterotrophic dinoflagellates Oxyrrhismarina, 6.3–7.03 days (Almeda et al., 2010), or Dunaliella tertiolecta and Platymonas sp., 7–9 days (Uchima, 1979). In addition, in the final two naupliar stages (N5 and N6), the nauplii from our culture were larger in comparison with those fed Oxyrrhis (Almeda et al., 2010) (Table 1).
The sizes of different naupliar stages of O. davisae vary considerably depending on the combination of temperature and trophic conditions (Almeda et al., 2010: Figs. 5a and 5b). The small sizes of nauplii collected in the Kruglaya Bay (Table 1) during a time when the water temperature was close to the experimental one could be a result of a diet inadequate for their development.
According to unpublished material of Vdodovich et al. (Vdodovich, personal communication), nauplii of O. davisae significantly contribute to the diet of the earliest larvae of Mullus barbatus and Trachurus trachurus. Their abundance in plankton apparently ensure high survival rates of the larvae of summer-spawning fish. Further research into the biology and cultivation of O. davisae may support the development of methods for their large-scale cultivation with a view to increase survival rates of larvae in commercially important marine fish species in an aquaculture environment.
CONCLUSIONS
This work is of importance for further both fundamental and applied research.
The detailed descriptions, original digital photos and drawings of nauplii of the Black Sea invader O. davisae made it possible to define major features to distinguish between each of its six naupliar stages. These findings may facilitate their identification in zooplankton samples, hat is important for study of contribution of this species to the changes in structure of the planktonic community and productivity of the Black Sea region, as well as other water bodies, where this invasive species contributes significantly to the copepod yield crop. In addition, specifying the morphological features of different naupliar stages will facilitate their identification among the remnants found in fish larvae guts and assessment of selectivity by fish larvae of different age.
These preliminary findings on duration of naupliar development, and the knowledge of importance of small-sized O. davisae nauplii for feeding of the smallest larvae of the Black Sea fish essential for their survival make it possible to consider this copepod species as a potential live food object of marine larviculture, for the development of methods for its large-scale cultivation and use in the diet of commercially important marine fish larvae to increase their survival in aquaculture environment.
REFERENCES
Almeda, R., Calbet, A., Alcaraz, M., Yebra, L., and Saiz, E., Effects of temperature and food concentration on the survival, development and growth rates of naupliar stages of Oithona davisae (Copepoda, Cyclopoida), Mar. Ecol. Prog. Ser., 2010, vol. 410, pp. 97–109.
Altukhov, D.A. and Gubanova, A.D., Oithona brevicornis Giesbrecht in the Sevastopol Bay from October, 2005 to March, 2006, Mar. Ekol. J., 2006, vol. 5, no. 2: 32.
Altukhov, D.A., Gubanova, A.D., and Mukhanov, V.S., New invasive copepod Oithona davisae Ferrari and Orsi, 1984: seasonal dynamics in Sevastopol Bay and expansion along the Black Sea coasts, Mar. Ecol., 2014, vol. 35, pp. 28–34.
Ambler, J.W., Cloern, J.E., and Hutchinson, A., Seasonal cycles of zooplankton from San Francisco Bay, Hydrobiologia, 1985, vol. 129, pp. 177–197.
Cornils, A. and Wend-Heckmann, B., First report of the planktonic copepod Oithona davisae in the northern Wadden Sea (North Sea): evidence for recent invasion?, Helgol. Mar. Res., 2015, vol. 69, pp. 243–248.
Ferrari, F.D., Oithona wellershausi, new species, and O. spinulosa Lindberg, 1950 (Copepoda: Cyclopoidae: Oithonidae) from the mouth of the Pearl river, China, Proc. Biol. Soc. Washington, 1981, vol. 94, no. 4, pp. 1244–1257.
Ferrari, F.D. and Orsi, J., Oithona davisae, new species, and Limnoithona sinensis (Burckhardt, 1912) (Copepoda: Oithonidae) from the Sacramento-San Joaquin Estuary, California, J. Crustacean Biol., 1984, vol. 4, no. 1, pp. 106–126.
Fofonoff, P.W., Ruiz, G.M., Steves, B., Simkanin, C., and Carlton, J.T., National exotic marine and estuarine species information system, 2018. http://invasions.si. edu/nemesis/. Accessed August 6, 2018.
Goswami, S.C., Metamorphosis of two species of genus Oithona Baird (Copepoda), Ind. J. Mar. Sci., 1975, vol. 4, pp. 60–67.
Gubanova, A.D. and Altukhov, D.A., Establishment of Oithona brevicornis Giesbrecht, 1892 (Copepoda: Cyclopoida) in the Black Sea, Aquat. Invasions, 2007, vol. 2, no. 4, pp. 407–410.
Gubanova, A., Khanaychenko, A., Tokarev, Y., Altukhov, D., Vdodovich, I., Popova, E., Klimova, T., and Garbazey, O., Small cyclopoid copepod Oithona davisae invasion into the Black Sea as a factor of the changes in zooplankton and ichthyoplankton in Crimean coastal waters, Proc. Perseus Final Sci. Conf. “Integrated Marine Research in the Mediterranean and the Black Sea,” Brussels, December 7–9, 2015, Brussels, 2015, pp. 271–272.
Hirakawa, K., New records of the North Pacific coastal planktonic copepods, Acartia omorii (Acartiidae) and Oithona davisae (Oithonidae) from southern Chile, Bull. Mar. Sci., 1988, vol. 42, pp. 337–339.
Hirota, R., Microdistribution of the marine copepod Oithona davisae in the shallow waters of Ariake-kai mud flats, Japan, Mar. Biol., 1990, vol. 105, pp. 307–312.
Isinibilir, M., Svetlichny, L., and Hubareva, E., Competitive advantage of the invasive copepod Oithona davisae over the indigenous copepod Oithona nana in the Marmara Sea and Golden Horn Estuary, Mar. Freshwater Behav. Physiol., 2016, vol. 49, no. 6, pp. 392–405.
Khanaychenko, A., Gubanova, A., Svetlichny, L., Vdodovich, I., Gavrilova, N., Mukhanov, V., Aganesova, L., and Giragosov, V., When the invaded community profit from the invader (the case of Asian cyclopoid in the Black Sea), Proc. VI Int. Symp. of Ecologists of Montenegro, Montenegro, Ulcinj, October 15–18, 2015, Abstracts of Papers, Podgorica, 2015, p. 63.
Khanaychenko, A., Mukhanov, V., Aganesova, L., Besiktepe, S., and Gavrilova, N., Grazing and feeding selectivity of Oithona davisae in the Black Sea: importance of cryptophytes, Turk. J. Fish. Aquat. Sci., 2018, vol. 18, no. 8, pp. 937–949.
Mihneva, V. and Stefanova, K., The non-native copepod Oithona davisae (Ferrari F.D. and Orsi, 1984) in the Western Black Sea: seasonal and annual abundance variability, BioInvasions Rec., 2013, vol. 2, no. 2, pp. 119–124.
Nishida, S., Taxonomy and distribution of the family Oithonidae (Copepoda, Cyclopoida) in the Pacific and Indian Oceans, Bull. Ocean Res. Inst., Univ. Tokyo, 1985, vol 20, pp. 1–167.
Nishida, S. and Ferrari, F.D., Redescription of Oithona brevicornis Gisbrecht, and O. aruensis Fruchtl, new rank, with notes on the status of O. spinulosa Lindberg, Bull. Plankton Soc. Japan, 1983, vol. 30, no. 1, pp. 71–80.
Nishida, S., Tanaka, O., and Omori, M., Cyclopoid copepods of the family Oithonidae in the Suruga Bay and adjacent waters, Bull. Plankton Soc. Jpn., 1977, vol. 24, pp. 119–158.
Saiz, E., Calbet, A., Broglio, E., and Mari, P., Effects of small-scale turbulence on copepods: the case of Oithona davisae, Limnol. Oceanogr., 2003, vol. 48, no. 3, pp. 1304–1311.
Sazhina, L.I., Naupliusy massovykh vidov pelagicheskikh kopepod Mirovogo okeana: opredelitel’ (Nauplii of the Most Common Species of Pelagic Copepods of the World Ocean: Guide), Kiev: Naukova Dumka, 1985.
Selifonova, Zh.P., Oithona brevicornis Giesbrecht (Copepoda, Cyclopoida) in harborages of the northeastern part of the Black Sea shelf, Inland Water Biol., 2009, vol. 2, no. 1, pp. 30–32.
Selifonova, Zh.P., Oithona brevicornis Giesbrecht (Copepoda: Cyclopoida), invader into the Black Sea and in the Sea of Azov, Russ. J. Biol. Invasions, 2011, vol. 2, nos. 2–3, p. 227.
Svetlichny, L., Hubareva, E., Khanaychenko, A., Gubanova, A., Altukhov, D., and Besiktepe, S., Adaptive strategy of thermophilic Oithona davisae in the cold Black Sea environment, Turk. J. Fish. Aquat. Sci., 2016, vol. 16, pp. 77–90.
Takahashi, T. and Uchiyama, I., Morphology of the naupliar stages of some Oithona species (Copepoda: Cyclopoida) occurring in Toyama Bay, southern Japan Sea, Plankton Benthos Res., 2007, vol. 2, no. 1, pp. 12–27.
Temnykh, A. and Nishida, S., New record of the planktonic copepod Oithona davisae Ferrari and Orsi in the Black Sea with notes on the identity of “Oithona brevicornis,” Aquat. Invasions, 2012, vol. 7, no. 3, pp. 425–431.
Timofte, F. and Tabarcea, C., Oithona brevicornis Giesbrecht, 1892 (Copepoda: Cyclopoida)—first record in the Romanian Black Sea waters, J. Environ. Prot. Ecol., 2012, vol. 13, no. 3A, pp. 1683–1687.
Tsuda, A. and Nemoto, T., Feeding of copepods on natural suspended particles in Tokyo Bay, Japan, J. Oceanogr. Soc. Japan, 1988, vol. 44, pp. 217–227.
Uchima, M., Morphological observation of developmental stages in Oithona brevicornis (Copepoda, Cyclopoida), Bull. Plankton Soc. Japan, 1979, vol. 26, no. 2, pp. 59–76.
Uchima, M., Gut content analysis of neritic copepods Acartia omorii and Oithona davisae by a new method, Mar. Ecol. Prog. Ser., 1988, vol. 48, pp. 93–97.
Uriarte, I., Villate, F., and Iriarte, A., Zooplankton recolonization of the inner estuary of Bilbao: influence of pollution abatement, climate and non-indigenous species, J. Plankton Res., 2016, vol. 38, no. 3, pp. 718–731.
Üstün, F. and Kurt, T.T., First report of the occurrence of Oithona davisae Ferrari F.D. and Orsi, 1984 (Copepoda: Oithonidae) in the Southern Black Sea, Turkey, Turk. J. Fish. Aquat. Sci., 2016, vol. 16, pp. 413–420.
Uye, S. and Sano, K., Seasonal reproductive biology of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet, Mar. Ecol. Prog. Ser., 1995, vol. 118, pp. 121–128.
Vdodovich, I.V., Khanaychenko, A.N., and Giragosov, V.E., Nutrition of the juveniles of the Mugilidae family in the coastal and open waters of the Black Sea near Sevastopol, and copepod-invader Oithona davisae as a component of their food supply, Zh. Sib. Fed. Univ., Biol., 2017a, vol. 10, no. 1, pp. 106–113.
Vdodovich, I.V., Khanaychenko, A.N., Gubanova, A.D., Kolesnikova, E.A., and Aganesova, L.O., Identification of some common food items in the guts of fish larvae and juveniles in the Black Sea, Mar. Biol. J., 2017b, vol. 2, no. 1, pp. 3–10.
Yildiz, I., Feyzioglu, A.M., and Besiktepe, S., First observation and seasonal dynamics of the new invasive planktonic copepod Oithona davisae Ferrari and Orsi, 1984 along the southern Black Sea (Anatolian Coast), J. Nat. Hist., 2016, vol. 51, nos. 3–4, pp. 1–13.
Zagorodnyaya, Yu.A., Oithona brevicornis in the Sevastopol Bay: is it a single event or a new invader in the Black Sea fauna?, Ekol. Morya, 2002, no. 61, p. 43.
ACKNOWLEDGMENTS
This work was carried out in accordance with topics of state assignments to the Institute for Marine Biological Research: “General Trends of Formation and Anthropogenic Transformation of Biodiversity and Bioresources of the Azov-Black Sea Basin and Other Areas of the World Ocean,” state reg. no. АААА-A18-118020890074-2, and “Research into Mechanisms for Controlling Production Processes in Biotechnological Complexes Intended to Develop Scientific Bases for Deriving Biologically Active Substances and Technological Products of Marine Origin,” state reg. no. АААА-A18-118021350003-6.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by E. Kuznetsova
Rights and permissions
About this article
Cite this article
Drapun, I.E., Khanaychenko, A.N. Morphology of the Nauplii and Duration of Naupliar Development of the Black Sea Alien Species Oithona davisae Ferrari and Orsi, 1984 (Copepoda: Cyclopoida) in Laboratory Culture. Russ J Biol Invasions 10, 12–21 (2019). https://doi.org/10.1134/S2075111719010053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075111719010053