Abstract
The process of remineralization of hard dental tissues using electrophoresis with a BV preparation that provides the formation of a precipitate of calcium phosphate (brushite) upon the interaction of its components is studied by physicochemical methods including electrochemical impedance spectroscopy and optical and scanning probe microscopy with microanalysis. It is shown in the in vitro experiments that the electrical resistance of a tooth can serve as a criterion of the efficiency of blocking of the dentin canaliculi by the formed precipitate of brushite. The depth of the zone of remineralization is assessed in the case of the treatment with a BV preparation by the method of applications and using electrophoresis. It is found that the latter method possesses a higher efficiency with respect to both the degree of blocking of the canaliculi and depth of penetration into the hard dental tissues. A method of therapy of teeth with the vital pulp is developed based on these studies that shows a high efficiency in the patients from the control group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Increased tooth sensitivity of hard dental tissues is one of the most common dental diseases, and its prevalence is steadily on the rise according to the World Health Organization [1]. It seems unlikely that there is anyone alive who is unfamiliar with the discomfort that appears upon the contact of teeth with hot, cold, or sour food. In cases of increased tooth sensitivity (hyperesthesia), these phenomena constitute a serious problem, which can be called “noncarious lesions of hard dental tissues.” The reason for hyperesthesia is changes in the composition and/or structure of dentin, which makes up the main part of the hard tissue of a tooth. The hydrodynamic theory that associates this process with changes in the circulation of the liquid phase in dentin canaliculi penetrating the bulk of the tissue is the most widespread of several theories explaining the reasons for increased tooth sensitivity. They can induce the displacement of odontoblast cells and irritation of nerve endings [2].
Hyperesthesia of dentin can be induced by various reasons including enamel defects and chippings, erosion as a result of action of mechanical or chemical factors, periodontosis events, and tooth bleachings, as well as tooth grinding procedures during dental prosthetic rehabilitation. The exposure of dentin canaliculi occurs as a result of these processes.
One treatment method of hyperesthesia is the use of preparations inducing the formation of low-soluble precipitates on the surface and inside the naked dentin [3, 4]. Various gels, lacquers, and pastes that produce dense, rather stable films on the surface and block the contact of the “environment” with the canaliculi are generally used. Also, chemical agents (of both organic and inorganic nature) that form low-soluble compounds directly with dentin tissues are used. They include propolis [5], fluorides [6], preparations of strontium and calcium, and phosphates [7–10]. The efficiency of a BV preparation [11] for the treatment of the sites of demineralization of hard dental tissues in the case of noncarious lesions was studied in this work. It is a two-component system that, upon the interaction of 30% Ca(NO3)2 (solution no. 1) and 30% (NH4)2HPO4 (solution no. 2), forms brushite (CaHPO4) crystals on the surface of dentin which block the movement of liquid in the dentin tubules. This precipitate is similar in nature to hydroxyapatite, of which the mineral part of dentin mainly consists, and, because of this, well remineralizes the damaged areas of dental tissue.
The choice of the treatment methods of hyperesthesia is generally based on the statistical processing of the results of the use of the chosen preparation for different patient groups with its manifestations. However, in the specific case of the BV preparation under study that forms blocking precipitates of brushite in the entrances of dentin canaliculi, there is a possibility of applying physicochemical procedures for studying the structure and properties of microporous objects, to which dentin penetrated with canaliculi with different permeabilities can be assigned. Measurements of electrical impedance and scanning electron microscopy (EMPA) with the possibility of in vitro determination of the local composition of the chosen areas of the study object were chosen from among these methods.
It appears that the impedance (complex electrical resistance) of a porous object formed by a poorly conducting tooth base (predominantly consisting of hydroxyapatite) and an aqueous electrolyte filling its porous structure will depend on the degree of permeability of the canaliculi and can serve as a quantitative macrocriterion during the assessment of the efficiency of blocking of the micropores with a low-soluble precipitate. Conversely, scanning microscopy can give precise local information on the spatial distribution of the applied precipitate and its structure and elemental composition. In addition, the structural features of the object both in the surface layer and along the depth can be studied by it.
MATERIALS AND METHODS
The objects of the laboratory in vitro study were samples of teeth extracted due to periodontal indications. To prevent the leaching of hydroxyapatite from the dental tissues and to standardize their states, the prepared teeth were preliminarily soaked for 30 min in mineral water (Novoterskaya tselebnaya, mineralization of 3.2–5.8 g/L) prior to the investigations. Whole teeth were used in experiments on the measurement of the impedance. Sections and chippings of tooth fragments were used in the study by EMPA.
The efficiency of application of a BV preparation in the case of its use in combination with electroionophoresis was studied in this work. To perform electrophoresis, a prototype instrument—a bipolar galvanostat with an adjustable current level (±10 μA) and a built-in timer—was developed and fabricated [12].
A temporary plastic dental crown equipped with a built-in electrode and a cavity, into which a pad impregnated with the active component was placed, was used for the local supply of electric current to the areas of the tooth under treatment [13]. The active electrode of the galvanostat in the form of an angular probe was brought into contact with the metal rod of the temporary dental crown. At the clinic, the passive electrode in the form of a plate was held in the right hand palm or fixed on the lip of the patient, while it was brought into contact with the electrically conducting medium of the model cell in the laboratory experiments. A direct electric current of from 2 to 5 μA was supplied for 2 min.
A laboratory cell for the modeling of the processes of electroionophoresis and impedancemetry on an extracted tooth had to provide reliable fixation of the object at the level of the root neck (like in the oral cavity) and point of contact with the passive (auxiliary) electrode via an ion conductor. A rectangular neutral soap bar was used as the latter. Both firm adherence of the auxiliary electrode and strong attachment of the sample of a tooth with a dental crown placed on it under study were provided at a certain humidity of the soap.
During the laboratory experiment, an IPC-Pro–FRA electrochemical complex (Russia) was used for carrying out the polarization of impedancemetry [14, 15]. The instrument was commuted by a two-electrode scheme: the working electrode contacted with the temporary dental crown, and the auxiliary electrode together with the reference electrode, with the “passive electrode.” Electrodes made of VT1-0 titanium were used, which was determined by its high chemical inertness under the experimental conditions at any polarity of the supplied current. A cotton pad placed into the cavity of the temporary dental crown was saturated with the components of a BV preparation under study.
During modeling of the process of electroionophoresis, the electrochemical complex operated in a galvanostat mode. A potentiostat mode was applied to study the impedance of the system. The frequency dependence of the impedance was recorded in a range of from 10 kHz to 10 Hz upon applying a harmonic signal of 100 mV.
To study the depth of mineralization with the solutions of a BV preparation, sections of the teeth were prepared. For this purpose, the teeth were longitudinally cut by a diamond disk, and the halves were fixed in a protacrylic autopolymer resin with the section plane upwards. The sections were prepared for microscopic studies by mechanical treatment with abrasive wheels in several passages and hand polishing using a fine diamond paste. The studies of the surface of the sections were performed on a Neophot 2 optical microscope (Germany) at a 30× magnification and on a CAMEBAX scanning electron microscope (France). As opposed to optical microscopy, a much higher spatial resolution of the object and possibility of acquiring information about the local chemical composition of the focusing area of the electron beam or along its scanning path from a depth of about 1 μm are provided in the case of using EMPA. Since the objects under study did not possess metallic conductivity, surface sputtering with molybdenum was performed.
RESULTS AND DISCUSSION
Impedancemetry
The frequency dependences of the impedance obtained on an electrochemical cell with a fixed tooth sample and without it when the working titanium electrode directly contacted with soap were compared in the preliminary experiments. In both cases, the hodographs are the fragments of semicircles and correspond to the equivalent circuit of R0(C1/R1) type, where R0 models the ohmic resistance of the electrolyte (soap), C1 is the characteristic of the capacity of this system of electrodes, and R1 is the capacitance self-discharge resistance. The calculations performed by the specially developed DCSFootnote 1 program [14] made it possible to determine the ratings of these elements (Table 1).
It is seen from the results that resistance of the “soap electrolyte” R0 somewhat varies from measurement to measurement because it depends on the conditions of the contact of the electrodes with the electrically conducting medium but differs no more than twofold in this series. No substantial difference is seen between the “blank sample” and measurements with teeth. On the opposite, the self-discharge resistance R1 in the “blank sample” turned out to be five times higher than in the experiments with the tooth samples. An even stronger difference—almost 400-fold!—is observed for the interelectrode capacitance. It can be concluded that the “blank sample” predominantly possesses a reactive resistance determined by a relatively high capacity (3.9 μF) and a low leak (276 kΩ). The inclusion of tooth samples in the interelectrode circuit drastically changes the situation; thus, the capacitance of the system decreases down to 0.01 μF, and the insulating properties of the interelectrode space simultaneously deteriorate (leak resistance R2 decreases down to 32–40 kΩ). The hodographs of such systems acquire the shape of perfect semicircles.
The sharp decrease in the capacitance in comparison with the “blank sample” is due to the increase in the distance between the electrodes of the system and change in the dielectric characteristics of the cell due to its inclusion into the interelectrode space of the tooth. The variations of the capacitance can also be strongly affected by the mutual position of the electrodes (as well as the shape and size of the tooth), which prevents the use of this parameter as a treatment efficiency criterion.
It should be taken assumed during the analysis of the character of the change in R2 that the intrinsic electrical resistance of hard dental tissues is quite high, with current mainly passing through the system of capillaries of the dentin canaliculi filled with a liquid. Because of this, the increase in the rating of R2 can serve as the criterion of the efficiency of blocking of the dentin canaliculi with the precipitate of applied brushite.
For the objective comparison of the efficiency of the remineralizing action of a BV preparation depending on the method of its application, we calculated the ratings of the equivalent circuit for the frequency dependences of the impedance obtained on the samples of teeth preliminarily ground for dental crowns in the case of application of the preparation by the method of applications (tooth no. 1) and in the case of the use of electrophoresis (tooth no. 2).
In the case of the method of applications, the components of the preparation (solutions nos. 1, 2) were successively applied onto the surface of a tooth with an exposure of 10 min each to provide impregnation and interaction. In the case of the use of electrophoresis, the time of treatment with each solution also was 10 min at a current of 5 μA. Here, anodic polarity of the working electrode was applied for solution no. 1 (calcium nitrate), and cathodic, for solution no. 2 (ammonium hydrophosphate), to provide electromigration of Ca2+ cations and \({\text{HPO}}_{4}^{{2 - }}\) anions, respectively, into the depth of the porous structure of dentin. The results of these experiments are presented in Table 2.
It is seen from the obtained data that the resistance insignificantly changes in the case of chemical application of the remineralizing preparation. Its certain decrease (by 15%) is even observed, although it turned out to be within the scatter of the initial value. Here, the value of the scatter of the measurements after the chemical treatment substantially decreases. After applying the components of the preparation in the mode of electrophoresis, the resistance almost doubled, and the scatter of the values proportionately increased.
It is likely that these results can be explained by the fact that, in the case of chemical application, the layer of the precipitate of brushite is only formed on the surface and no efficient blocking of the canaliculi is achieved, while the ammonium and nitrate ions saturating the pores increase the electrical conductivity of the intradentinal electrolyte.
During electroionophoresis taking into account the current polarity for each component of the preparation, the penetration of the active substances into the depth of the pores is stimulated and brushite more efficiently blocks the canaliculi and obstructions for the penetration of \({\text{NH}}_{4}^{ + }\) and \({\text{NO}}_{3}^{ - }\) ions into the depth are simultaneously generated.
Impedance hodographs of the measuring cell in a frequency range of 10 kHz–15 Hz: (1) without a sample and (2) with the sample of a tooth in the initial state. (solid line) Result of calculation by the equivalent circuit in the specified frequency range and (dashed line) calculation by the equivalent circuit for a blank sample in a range of 10 kHz–1 Hz.
To optimize the duration of electrophoresis of a tooth with a BV preparation, a series of measurements were performed on samples of extracted single rooted teeth with the preliminarily removed enamel. An IPC-FRA instrument complex makes it possible to carry out measurements at a certain frequency (in this case, 1 kHz) and to perform continuous recording of the results not in the form of the components of the impedance vector, but as the ratings of the chosen equivalent circuit. This mode was used to trace the changes in the rating of resistance during the entire experiment including the stages of electrophoresis as well. An example of such a temporal dependence of the rating of resistance R1 for a parallel equivalent circuit (at R0 = 170 Ω) is shown in Fig. 2.
During preliminary exposure in mineral water (section 1), the resistance increases from 75 to 120 kΩ, probably, due to rinsing. It then sharply decreases during the electrophoresis with solution no. 1 (section 2) because its electrical conductivity is substantially higher when compared to water. Section 3 refers to the stage of electrophoresis with solution no. 2. It is seen that a noticeable increase in both the rating of resistance and rate of its change during electrophoresis occurs. This evidences that, despite the intake of ammonium ions that decrease the resistance into the system, the process of blocking of the canaliculi with the precipitate of brushite formed in them, which increases the resistance predominates.
During section 4, electrophoresis was switched off and rinsing of the treated tooth (with mineral water) was performed. The resistance first decreased, probably due to the effect of the accumulated in the system ammonium and nitrate ions (which were “repressed” during the electrophoresis), but the resistance increased more and more with rinsing (up to 140 kΩ) and stabilized at a level of 150–160 kΩ, i.e., by 30% higher than before the treatment, at the final stage (section 5).
The effect of the duration of the stages of electrophoresis can be judged from the data of Table 3.
It is seen from Table 3 that the teeth had a relatively high resistance in the initial state. Note that the sample subjected to electrophoresis for 2 min had a higher resistance due to the smaller cross section of the tooth in comparison with the tooth sample used for the 5‑min treatment. After 1-day exposure in mineral water, the resistance of the samples decreased 2- and 3.5-fold, respectively, and these values were stable for 10 min prior to electrophoresis. Upon the completion of electrophoresis with a BV preparation, the resistance increased in all the experiments; however, the rate of the increase decreased with the growth in the duration of action, although the absolute efficiency of blocking of the canaliculi increased.
Therefore, the optimum and sufficient for the efficient treatment with a BV preparation duration of electrophoresis of 2 × 2 min was chosen based on these results instead of the “conventional” duration of 2 × 10 min in the case of the method of chemical application.
Scanning Microscopy
Impedancometric studies confirmed the efficiency of the blocking action during the remineralizing treatment of dentin with a BV preparation, and it is higher with the use of electrophoresis of the components of the preparation. However, these studies do not provide information on the structure and local distribution of the applied precipitate over the surface and depth of the treated tooth. The study of the surface and sections using an optical microscope did not find visible changes as a result of the remineralizing treatments, probably due to the low-contrast (white) color of the thin layers of brushite against the background of the dental tissues.
Scanning electron microscopy possesses a high spatial resolution in comparison with an optical microscope and makes it possible to acquire data on the concentration of individual elements for selected local areas. Figure 3 compares the images of approximately the same area of a tooth section without a preliminary treatment obtained on electron and optical microscopes. The dark regions to the left refer to the reinforcing resin poured over the sample. A vertical boundary separates different dental tissues: enamel (bright on an optical microscope) and dentin. The analysis of the surface layer of the section to a depth of about 1 μm was performed in points A and B. Peaks related to individual elements are seen in the obtained spectra, by the amplitude of which it can be qualitatively judged about the concentration of the element in the surface layer. The main peaks in the spectra correspond to carbon, oxygen, calcium, phosphorus, and molybdenum.Footnote 2 The quantitative determination of the elemental composition requires a set of reference standards. However, it is possible to assess the relative change in the composition by specific elements, having calculated the ratio of the signals of these elements.
The main mineral of hard dental tissues is hydroxyapatite, which consists of calcium phosphate, because of which the ratio of the intensities of the Ca/P signals of the spectrum was studied. This ratio is different in hard dental tissues. Having traced the changes in the composition depending on the treatment, it is possible to assess the enrichment or depletion of the composition of the tissues with respect to these elements. For the untreated tooth, the ratio of calcium to phosphorus in point A (enamel) is 1.449, while in point B (dentin) it is 1.283. For a sample of calcium phosphate, this is a stoichiometric ratio of 1.5. Therefore, it is seen that the enamel is enriched with calcium in comparison with the dentin.
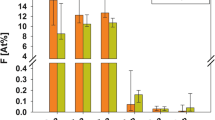
Different modes of application of a BV remineralizing composition were studied on the sections of the in vitro treated tooth samples (Table 3). The measurement of the signals of the spectrum with respect to calcium and phosphorus along the line from the surface into the depth of the sample was performed on these sections. For the areas corresponding to enamel, dentin, and loose layer of the precipitate of brushite,Footnote 3 the averaged signal by each component was determined and their ratio was calculated. These results are presented in Table 4, and the EMPA images are presented in Fig. 4.
Table 4
Sample | Mode of in vitro treatment | Ca/P relative signal | ||
|---|---|---|---|---|
layer of brushite | enamel | dentin | ||
0 | Without a treatment | 1.449 | 1.283 | |
I no. 3 | Application five times 2 × 3 min | 1.127 | – | 1.198 |
II no. 1 | Application seven times 2 × 10 min | 1.272 | ||
III no. 2 | Electrophoresis 5 mA 2 × 10 min with consideration of polarity with respect to components | 1.430 | 1.348, 1.319 | |
IV no. 4 | Electrophoresis 5 mA 2 × 5 min with consideration of polarity with respect to components | 1.432 | 1.394, 1.391 | |
V no. 5 | Electrophoresis 5 mA 2 × 2 min with consideration of polarity with respect to components | 1.360 | ||
EMPA images of the sections of the samples of teeth with different procedures of remineralizing treatment and profile of the signals of Ca (white (green in the low-contrast region of the precipitate of brushite)) and P (orange) along the scanning line (blue) from the surface (to the left) into the depth of the sample (to the right).
The use of a BV preparation by the method of applications (impregnation) leads to the formation of a loose precipitate of brushite on the surface of the areas of ground enamel (Fig. 4b). This layer with a thickness of from 100 to 200 μm poorly adheres to the surface of the tooth. The ratio of the averaged Ca/P signal for this area together with the enamel is 1.127, while Ca/P = 1.198 for dentin. Since, for brushite, the theoretical ratio Ca/P = 1, the external layer is very close to expected. A “dip” in the level of signals is observed at the boundary with dentin, which is explained by the loose adherence of the precipitate with the base and its detachment.
For sample II no. 1 with the ground enamel also prepared by the method of applications, areas with the loose layer of brushite did not get into the section zone, its individual fragments are seen. It is seen by the results for the layer of dentin that the ratio Ca/P = 1.272 and almost does not differ from the ratio for both previous and untreated samples. The character of distribution of the signals along the scanning line does not find any areas of changed mineralization, which evidences a uniform composition of dentin without visible enrichment of the surface. In other words, the method of applications does not provide a change in the mineralization of dentin even upon long-term and repeated impregnation.
Sample III no. 2 was treated according to the procedure of electrophoresis with a BV preparation. A layer of enamel with through microcracks is seen in the photographic image in Fig. 4d. There are no traces of the precipitate of brushite on its external surface like in the case of the treatment by the method of applications. For the area of the enamel, the ratio Ca/P = 1.430, i.e., like for the initial state. On the opposite, a higher relative concentration of calcium has been recorded in the external layer of dentin in comparison with the untreated sample and samples after the applications of a BV preparation (by 6.6% on average), while the ratio of the elements is close to the untreated sample in the deeper layer.
Therefore, during electrophoresis, the reaction between the components of a BV preparation occurs not on the surface of the hard tissues but predominantly in the deep layers of the porous structure of dentin. The mineralization of the saturated with calcium enamel remains unchanged, while the external layer of dentin is enriched with this element. This effect is also preserved in the case of reducing the electrophoresis time (samples IV no. 4 and IV no. 5), and remineralization is noticeable in the surface layer with a thickness of at least 300 μm.
Figure 5a shows the section of the tooth sample with the preserved enamel that had through defects that appeared upon grinding. As a result of the treatment with electrophoresis, predominant remineralization occurred in the layer of dentin adjacent to these enamel damage lesions, which is seen as brighter areas in the photographic image from the optical microscope.
Dentin canaliculi are seen on the brittle fracture of sample IV no. 4, the lumen of which is blocked by the precipitate of brushite (the bright sediments) formed in the depth of the layer of dentin under the action of electrophoresis.
CONCLUSIONS
Therefore, the use of electrophoresis as a method of remineralization of dentin with a BV preparation provides an advantage over the method of applications with respect to both the increase in the electrical conductivity (decrease in the porosity) and depth of action on the layers of dentin. The comparative tests of these methods under clinical conditions fully confirmed the results of the laboratory physicochemical in vitro studies.
The patients from the experimental group (42 subjects and 68 teeth) and control group (20 subjects and 45 teeth) underwent the preparation of vital teeth for the fabrication of dental prostheses.Footnote 4 After the preparation, electrophoresis with each solution of a BV material for 2 min acted on the teeth with vital pulp in the experimental group with the use of a new procedure [13]. For the period of fabrication of porcelain fused metal prostheses, the abutment teeth in the experimental and control groups were covered with temporary composite dental crowns and fixed onto temporary Temp-Bond.
The advantage and efficiency of the developed method of remineralization with the use of electrophoresis is apparent from Table 4. This method is not only more efficient and provides a longer-term effect of hyperesthesia reduction but also requires a significantly shorter time for the treatment (2 × 2 min) as opposed to the method of applications (2 × 10 min). The patients for whom the protection of the vital teeth by the method of electrophoresis with the use of a BV material was performed did not present problems with temperature, tactile, and chemical irritants. No pathological changes in the periodontium of the abutment teeth with vital pulp were detected during the examination of the X-rays.
Therefore, a combination of laboratory in vitro and clinical in vivo methods for studying the processes of remineralization of dentin made it possible to find out the mechanisms of the processes and reduce the time of development of an efficient procedure for reducing the hyperesthesia of vital teeth [16].
Notes
Dummy Circuits Solver©. Program developed by V.E. Kasatkin.
As a result of metallization.
On the surface of sample I.
Both in the experimental and control groups, the odontopreparation was performed under infiltration or conduction anesthesia. The vital teeth were prepared with the consideration of the zones of safety and adherence to all the generally valid safety precautions for the grinding of hard dental tissues.
REFERENCES
Mehta, D. and Venkata, S., http://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/eos.12067/abstract. Accessed Sep-tember 20, 2015. Mehta, D. and Venkata, S., Eur. J. Oral. Sci., 2013, vol. 121, no. 35, p. 477. 10.1111/eos.12067
West, N.X., Periodontol, 2008, vol. 2000, no. 48, p. 31.
Trowbridge, H.O. and Silver, D.R., Dent. Clin. North Am., 1990, vol. 34, no. 3, p. 561.
Yadav, B.K., Jain, A., Rai, A., and Jain, M., J. Int. Oral Health, 2015, vol. 7, no. 10, p. 137.
Tal, M., Oron, M., Gedalia, I., and Ehrlich, J., Arch. Oral Biol., 1976, vol. 21, no. 5, p. 285.
Levin, M.P., Yearwood, L.L., and Corpenter, W.N., Oral Surg., Oral Med., Oral Pathol., 1973, vol. 35, p. 741.
Belenova, I.A., Sushchenko, A.V., Kudryavtsev, O.A., Koretskaya, I.V., and Rozhkova, E.N., Vestnik Novykh Meditsinskikh Tekhnologii, 2018, no. 6.
Hiatt, W.H. and Johansen, E., J. Periodontol., 1972, vol. 43, no. 6, p. 373.
Vishnyakova, M.A., PROTECO, 2018.
Samarina, Ya.P., Nauchnoe Obozrenie. Meditsinskie Nauki, 2017, no. 4, p. 88.
Borovskii, E.V. and Volkov, E.A., Registratsionnoe udostoverenie na meditsinskoe izdelie (Certificate of Registration for Medical Product) no. 29/13111201/6011-03, 2003.
Adzhieva, A.K., Adzhiev, K.S., Makeeva, I.M., et al., RF Minor Patent 58041 U1, 2006.
Adzhieva, A.K., Ter-Asaturov, G.P., Abakarov, S.I., and Abakarova, D.S., RF Patent 2236828 A 61, 2003.
Shcherbakov, A.I., Korosteleva, I.G., Kasatkina, I.V., Kasatkin, V.E., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 4, p. 689.
Shcherbakov, A.I., Korosteleva, I.G., Kasatkina, I.V., Kasatkin, V.E., et al., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, no. 2, p. 288.
Gazhva, S.I., Kasumov, S.A., and Shurova, N.N., Sovremennye Problemy Nauki i Obrazovaniya, 2017, no. 4, p. 82; Gazhva, S.I., Kasumov, S.A., and Shurova, N.N., Sovremennye Problemy Nauki i Obrazovaniya, 2017, vol. 19, no. 11, p. 222.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Kasatkin, V.E., Adzhieva, A.K., Kasatkina, I.V. et al. The Application of Physicochemical Methods to Studying the Processes of Remineralization of Hard Dental Tissues in Dentistry. Prot Met Phys Chem Surf 58, 1090–1099 (2022). https://doi.org/10.1134/S2070205122050094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205122050094