Abstract
A method is proposed for increasing the resistance of passivated metals (in particular, stainless steels) to corrosion that is based on the use of the principle of cathodic alloying by electrolytic deposition of local palladium nanocoatings. It was found that the corrosion potential of AISI 321 steel samples in a 0.1 M NaCl solution electrochemically treated at a current density of 1 μA/cm2 and a frequency of 0.06 Hz in chloride-containing solutions with 0.1% Pd2+ shifts towards more positive values by almost 150 mV. It was also shown by scanning electron microscopy and Auger spectroscopy that, in the process of electrochemical polarization, subindividuals of a new phase (Pd) with sizes of the order of 5–80 nm are deposited on the surface of AISI 321 steel, the formation of which causes the potential improvement of the samples under study. It was found that the largest accumulation of subindividuals of the new phase (Pd) is observed in areas with artificially created surface defects (scratches).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
When protecting passivated metals from corrosion in aggressive environments, the method of cathodic alloying of stainless steels, titanium and alloys thereof, and other passivated metals is used, which was confirmed and developed in the works of Tomashov, Stern, Fischer, Zwicker, Green, Schleicher, Rudiger, etc. The essence of cathodic alloying is to increase the efficiency of cathodic processes in passivated systems, as a result of which the potential of the system shifts towards positive values, and it goes into a passive state. Small amounts (0.1–0.5%) of palladium, platinum, ruthenium, etc., are used as cathode dopants [1].
The main condition for achieving a positive effect during cathodic alloying of an alloy is the need to shift its potential under given corrosion conditions to the region of stable passivity, i.e., into the region between full passivation potentials Epp and transpassivity ET or pitting Ept.
The effectiveness of different metals used as cathode additives is different. The more positive the stationary potential of the metal of the cathode additive and the lower its cathodic polarizability, the more effective the passivating action. A lower cathodic polarizability will correspond to a lower overvoltage of the cathodic depolarizing process (for example, overvoltage of hydrogen evolution under corrosion conditions and a lower tendency to increase overvoltage over time, due to, for example, hydrogenation) [2].
Modification of passivated alloys with additions of a cathode-active electropositive metal (Pd, Pt, Ru) has a strong effect on increasing passivability and corrosion resistance even with a low content of the cathode component in the alloy (fractions of a percent). This effect of cathode additives is explained by the electrochemical mechanism of action of the additives and the accumulation of an electropositive alloying element in the initial period of active corrosion on the alloy surface.
An increase in the concentration of the cathode component on the surface of the alloy in the initial period of corrosion can be traced by the nature of the change in the corrosion rate of these alloys over time. As a rule, in the initial period of corrosion, cathode-alloyed alloys have a higher corrosion rate. However, after enrichment of the surface with a noble metal in the amount required for passivation under the conditions under study, a sharp decrease in the corrosion rate occurs. It was found by the method of electron microscopy that the accumulation of the alloying component (Pd) on the surface of the alloy occurs not in the form of a continuous layer, but in the form of separate small particles [2].
In the patent literature—for example, [3]—other methods of protecting passivated metals from corrosion are described by alloying a small surface area (no more than 1%) with metals of the platinum family. Surface alloying is carried out in different ways [4], including irradiation of the surface with high-energy metal ions [5], by welding, or by applying local coatings from the gas phase. All these methods are energy-intensive and involve the use of specialized unique equipment, which prevents the widespread use of such approaches.
In this regard, the objective of this study is to develop a simple and reliable method of surface alloying, which consists in the deposition of nanoscale point coatings from noble metals—for example, palladium. The function of palladium is to reduce the overvoltage of hydrogen evolution to values comparable to those on pure palladium and, thereby, shift the potential of the entire system to the region of steel passivation [6]. According to a number of authors [7], a layer of passivating oxide forms on the protected metal, which spontaneously regenerates when it is damaged. In addition, palladium atoms are capable of performing the function of an electron donor in a semiconductor passivated oxide formed on a metal surface in a corrosive environment.
EXPERIMENTAL
High-alloyed chromium–nickel steel of the austenitic type AISI 321 was used as a passivated metal.
Steel samples 50 × 20 mm in size and 1 mm thick were degreased in a standard chemical degreasing solution and washed with distilled water and, then, with bidistilled water.
Electrochemical the studies were carried out in a standard YSE-2 electrochemical cell at a temperature of 20 ± 1°С. A 0.1 M sodium chloride solution was used as a working medium with the introduction of palladium chloride into it in amounts not exceeding hundredths of a percent. The solution was prepared from reagents of chemically pure grade on bidistilled water. The reference electrode was an EVL-IMZ silver-chloride electrode; the auxiliary electrode was a platinum wire electrode. The experimental setup consisted of an IPC-Pro potentiostat–galvanostat, G6-27 signal generator of special form, and a personal computer. The samples under study were polarized with an anodic current with a density of 1 μA/cm2 with superposition of a sinusoidal component with a frequency multiple of 0.01 Hz. To control the dynamics of the process, the change in the potential of the electrode under study was recorded over time. After processing in the specified mode, the samples were tested to assess the corrosion properties by means of polarization measurements in a solution of 0.1 M NaCl [8]. All electrochemical potential values measured in this study are given relative to a silver-chloride reference electrode.
The surface morphology of the studied samples, the elemental composition of the surface and near-surface layers, and the distribution of elements in depth were determined by scanning electron microscopy and Auger electron spectroscopy using a JAMP-9500F Auger microprobe (JEOL) in accordance with the ASTM E 827 08 method. The research regime was an accelerating voltage of up to 10 kV, a primary electron-beam current of 5 × 10–10 C, a spatial resolution (electron-beam diameter) of no worse than 0.01 μm, and a pressure in the analytical chamber of no less than 1 × 109 Torr (ultrahigh vacuum). Rectangular specimens 17 × 27 mm in size (AISI 321 steel sample no. 1, control, not subjected to electrochemical treatment) and 18 × 23 mm in size (specimen no. 2) were cut out of the objects under study; Before carrying out the studies, the samples were washed in an ultrasonic bath in acetone (extra pure 9-5) and, then, in isopropyl alcohol (“reagent grade”), after which they were dried in a stream of dry air.
RESULTS AND DISCUSSION
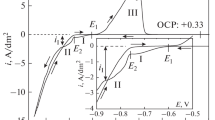
Polarization with weak currents is applied based on the facts of local processes on the surface of the passivated metal in chloride-containing media, leading to the appearance of local submicron foci of dissolution [9], the use of the low-frequency component is due to the assumption of the appearance in the “cathodic” half-period (the maximum of the current values of which is characterized by the most negative values) in solutions containing noble metal ions, and zero-dimensional phases, which are groups of metal atoms concentrated around microscopic defects (kinks, surface dislocations, etc.). The latter, according to the literature data, cause a potential shift to the region of more positive values [10].
Chronopotentiograms obtained during the polarization of AISI 321 steel samples in the working solution are shown in Fig. 1. Obviously, if the above conditions are met, an oscillatory change in potential is observed and there is a shift in the average trend of potential–time dependence E-t towards more positive values. This indirectly indicates a change in the surface layer of steel and, in particular, the formation of surface structures that increase the resistance of the metal surface to corrosion. The superposition of the alternating-current component, in our opinion, promotes the etching of surface impurities or the leveling of the surface potential in the half-period, the maximum of the current values of which is the most positive, and the local deposition of the noble metal (Pd) in the opposite half-period.
After electrochemical polarization of steel, characterized by the indicated parameters, the corrosion resistance of the modified samples was assessed by measuring the corrosion potential of these samples in a 0.1 M NaCl solution. The results of the experiment are presented in Table 1.
It was found that the samples polarized at a current density of 1 μA/cm2 and a frequency of 0.06 Hz have potential for free corrosion Ecor (measurements were carried out in accordance with GOST (State Standard) 9.912–89) in a chloride-containing solution equal to +15 mV, which is almost 150 mV more positive than the value recorded in the case of control samples of AISI 321 steel that were not subjected to electrochemical treatment.
The samples under study were checked by scanning electron microscopy and Auger spectrometry. Figure 2 shows microimages of the surface of the objects under study: AISI 321 steel (sample no. 1 is the control sample, and sample no. 2 is the sample subjected to electrochemical treatment), indicating the zones (points) of analysis.
Microimage of the test samples. (a) Sample no. 1. Analysis zones (points): (1–3) diameter of analysis zones (points): (1) 1, (2) 30 (defocused beam), and (3) ≤ 0.01 μm (focused beam). Secondary electron image. Instrument magnification 2000×. (b) Sample no. 2. Analysis zones (points) 1–3. Diameter of analysis zones: (1, 2) 1 and (3) 60 μm (defocused beam). Secondary electron image of the surface. Instrument magnification 1000×.
The results of investigations of sample no. 1 demonstrate the presence on the surface in analysis zones 1–3 before ion etching of the elements (in order of decreasing amplitude of Auger peaks) O, C, Fe, Cr, Ni, S, P, Ti, and N. Moreover, the sources of O, C, and N are adsorbed molecules of atmospheric gases and oxide/carbide/nitride inclusions and films on the sample surface. Fe, Cr, Ni, and Ti are the elements that form the basis of AISI 321 stainless steel.
A different picture is observed when analyzing a sample subjected to electrochemical treatment. There is an “increase” in the Pd concentration (even after 5 s of ion etching). One probable reason for this is surface diffusion (migration) of Pd atoms, which equalizes the concentration (density) of atoms in the upper surface layer. For example, analysis zones 1–3 on a smooth steel surface area before ion etching (in decreasing order of Auger-peak amplitude) contain O, C, Fe, Cr, Ni, S, Pd, and, possibly, N and P. As mentioned above, O, C, and N are part of the adsorbed molecules of atmospheric gases and oxide/carbide/nitride surface formations (Fig. 3a).
Auger electron spectra of the surface of sample no. 2: (a) in zones of analysis 1–3 before ion etching, (b) in analysis zone 2 before and after Ar+ ion etching (1 and 3 keV), and (c) in analysis zone 3 before and after Ar+ ion etching (1 and 3 keV). The locations of the analysis zones are shown in accordance with the image in Fig. 2b.
The elemental composition of the surface in smooth and defective areas (artificially applied scratch) in analysis zone 2 after Ar+ ion etching for 100 s at an energy of 3 keV (in decreasing order of the amplitudes of Auger peaks) is Fe, Cr, Ni, Ar, and C, where C are the adsorbed gas molecules of the residual atmosphere in the vacuum chamber of the spectrometer (CO2, CH4, and others), considering the ability of carbon to accumulate when exposed to an electron beam on the surface of most materials (the sources being residual atmosphere, surface diffusion, internal pores, and grain boundaries), and Ar are argon atoms implanted into the surface when exposed to an ion beam during ion etching of the sample. The change in the concentration of elements with depth in accordance with the time of ion etching can be estimated from the spectra (the approximate rate of ion etching of Ar+ stainless steel is about 20–25 nm/min, and that for Pd is about 100–130 nm/min) (Fig. 3b).
Similarly, it is can be shown that the elemental composition of the surface integrally (including defective and smooth areas) in analysis zone 3 after ion etching with Ar+ for 100 s at an energy of 3 keV is (in decreasing order of the amplitudes of Auger peaks) Fe, Cr, Ni, O, Ar, Cu, and Ti (Fig. 3c).
The results of the distribution of the concentration of Fe and Pd over depth (the dependence of the concentration of elements on the etching time) in zones 1 and 2 of the surface of the modified steel sample are shown in Fig. 4. It is obvious that the atoms of the deposited metal (Pd) are concentrated mainly in the surface and near-surface layers of the steel sample. It can be seen that the concentration of palladium within the artificially created defect (scratch) is significantly higher.
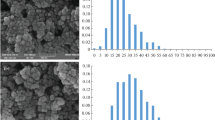
Figure 5 shows a microimage of the surface of a sample subjected to electrochemical treatment with a weak current of infralow frequency. It is obvious that subindividuals of the new phase (Pd) are formed on the surface with sizes on the order of 5–80 nm, which have a predominantly smoothed shape. Their greatest accumulation was found in areas with artificially created surface defects (scratches).
Microimage of the surface of a sample of AISI 321 steel exposed in a solution of 0.1 M NaCl + 0.01% PdCl2 with electrochemical polarization with a current characterized by a density of 1 μA/cm2 and infralow frequency of 0.06 Hz: (a) on a defect-free area of the surface; (b) in the area of an artificial defect.
CONCLUSIONS
Thus, based on the results of the experimental study, it can be stated:
1. A method for surface alloying of stainless steels has been developed, based on the use of modes characterized by a current with a density of the order of 1 μA/cm2 and a frequency multiple NS × 10–2 Hz (NS = 0.1–10), realized in chloride-containing media in the presence of a noble metal ion (Pd2+).
2. It was found that AISI 321 steel samples polarized at a current density of 1 μA/cm2 and a frequency of 0.06 Hz have a corrosion potential in a chloride-containing solution (0.1 M NaCl) equal to +15 mV, which is almost 150 mV more positive than the value obtained for the control samples that were not subjected to electrochemical treatment.
3. It is shown that the enhancement of the potential of the samples under study is due to the presence on the surface of AISI 321 steel of subindividuals of a new phase (Pd) with sizes of about 5–80 nm that have a smoothed shape, with their greatest accumulation being observed in areas with artificially created surface defects (scratches).
REFERENCES
Rachev, H.D. and Stefanova, S.T., Spravochnik po korozia, Sofia: Tehnika, 1977. Rachev, Kh. and Stefanova, S., Spravochnik po korrozii (Handbook on Corrosion), Moscow: Mir, 1982.
Tomashov, N.D. and Chernova, G.P., Korroziya i korrozionnostoikie splavy. Seriya “Uspekhi sovremennogo metallovedeniya” (Corrosion and Corrosion-Resistant Alloys. Series “Advances in Modern Metal Science”), Moscow: Metallurgiya, 1973.
Grauman, J.S., Miller, J.J., and Adams, R.I., RF Patent 2336366, Byull. Izobret., 2005, no. 29.
Budovskikh, E.A., Karpij, S.V., and Gromov, V.E., Bull. Rus. Acad. Sci.: Phys., 2009, vol. 73, no. 9, pp. 1253–1256.
Bratushka, S.N. and Malikov, L.V., Vopr. At. Nauki Tekh., Ser.: Vak., Chist. Mater., Sverkhprovodn., 2011, no. 6 (19), pp. 126–140.
Tomashov, N.D., Tashlykov, I.S., Zhil’tsova, O.A., et al., Zashch. Met., 1987, vol. 23, no. 5, pp. 791–795.
Tanga, J., Zhanga, Zh., Wanga, Y., et al., Corros. Sci., 2018, vol. 135, pp. 222–232.
Anantha, K.H., Ornek, C., Ejnermark, S., et al., J. Electrochem. Soc., 2017, vol. 164, no. 4, pp. 85–93.
Rozenfel'd, I.L., Korroziya i zashchita metallov (lokal’nye korrozionnye protsessy) (Corrosion and Protection of Metals (Local Corrosion Processes)), Moscow: Metallurgiya, 1970.
Budevski, E., Staikov, G., and Lorenz, W.J., Electrochim. Acta, 2000, vol. 45, pp. 2559–2574.
ACKNOWLEDGMENTS
The work was carried out on the equipment of the Center for Collective Use “Nanomaterials and Nanotechnologies” of Kazan National Research Technological University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dresvyannikov, A.F., Akhmetova, A.N. & Denisov, A.E. Increasing Corrosion Resistance of Passivating Metals by Electrochemical Application of Local Nanosized Coatings. Prot Met Phys Chem Surf 57, 1165–1171 (2021). https://doi.org/10.1134/S2070205121060071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121060071