Abstract
The role of Echium angustifolium Mill extract for advancing corrosion resistance of carbon steel in 1.0 M H2SO4 solution was discussed in this research. Weight loss (WL), open circuit potential (OCP), potentiodynamic polarization (PP) and electrochemical impedance spectroscopy (EIS) methods were applied to monitor the corrosion behavior of carbon steel in 1.0 M H2SO4 solution. The influence of temperature was studied from 25–45°C and some thermodynamic parameters were computed. The Surface morphology of the carbon steel surface was examined via different techniques. These measurements confirmed that the existence of Echium angustifolium Mill extract reduces the corrosion rate (CR), corrosion current densities (icorr), and double layer capacities (Cdl), simultaneously increase the polarization resistance (Rp) values. Tafel plots observed that the Echium angustifolium Mill extract play as a mixed type inhibitor. Electrochemical impedance confirmed that the existence of the applicable extract reduced the double layer capacitance and enhanced the charge transfer resistance. The extract molecules adsorbed on the metal surface follow Langmuir adsorption isotherm. The gained data confirmed that the inhibition efficiency (η%) increase with the increase of extract concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
Anti-corrosion of carbon steel in various acid media is a field of study which still controls significant attention given the great application of carbon steel for everyday need [1, 2]. Generally, carbon steel has been applied as a construction material for pipe work in the oil and gas manufacture for example, down-hole tubular, flow lines and transportation pipelines. In oil fields sulfuric and hydrochloric acid solutions are suggested because the cheapest system to eliminate calcium carbonate, CaCO3, extent inside the pipelines under several conditions [3, 4]. Therefore, corrosion inhibitors should be inserted with the hydrochloric acid and sulfuric acid solutions to avert the destructive impact of these acids on the pipe line surfaces [5, 6]. Corrosion may purpose hazardous and costly damage to the whole thing from bridges, pipelines and common buildings to vehicles, water and wastewater systems, and even home instruments [7, 8]. Corrosion is one of the largest problem in the oil and gas industries. The employing of organic inhibitors is one of the largest practical methods for resistance of metals and alloys to avoid corrosion process, while adding inhibitors doesn’t cause perturbation of the industrial development. Several researches are published on the plant extract application as potential agents to mitigate corrosion in several industrial solutions [9, 10]. The plant extract inhibitors are readily available, renewable resources, favorable, cheap and eco-friendly. The majority of the efficient inhibitors are substantial with heteroatom’s for example O, N, and S and double bonds in their molecules through which they are adsorbed on the metal surface [11–13]. Finally, It is discovered that adsorption relies essentially on positive physicochemical properties of the inhibitor groups, such as functional groups, electron density at donor atom, π-orbital character, and electronic structure of the molecules. Inhibitor molecules are absorbed by the metal surface immersed in an aqueous state substituting water molecules adsorbed by the metal surface [14–17]. Electrostatic interaction between an inhibitor molecule and a metal are prominent through this action of inhibitors. Electron densities of various functional groups, polarizability and electronegativity are the major factors in this interaction. There are several reviews on the plant extract applications as corrosion inhibitors [18–20].
The present work give an assessment by applying an ethanolic extract, produced from the Echium angustifolium Mill, as eco-friendly corrosion inhibitor to monitor the corrosion performance of carbon steel in 1.0 M H2SO4 solution.
2 MATERIALS AND METHODS
2.1 Carbon Steel Specimens Preparation
Specimens were gained from carbon steel sheets with a composition (wt %) of C = 0.20, Mn = 0.35, P = 0.024, Si = 0.003 and Fe is the rest. Specimens cut into (2.0 × 2.0 × 0.2 cm3) for weight loss method. The tested Specimens were rubbed with varied grades of emery papers, degreased with (CH3)2CO, then washed several times with bi-distilled water, lastly dried between filter papers.
2.2 Solutions
The corrosive solution was 1.0 M H2SO4 which was supplied from El-gomhouria Co., for chemicals, in Egypt. A volume of 100 mL of H2SO4 solution was applied as a test solution and freshly prepared before each experiment. Also, all chemicals were analytical-grade reagents. The experiments were applied under non-stirred and naturally aerated conditions. The adding of the extract didn’t change the pH of the corrosive solution.
2.3 Preparation of the Extract Inhibitor
The inhibitor tested in this work is an Echium angustifolium Mill (Boraginaceae) extract was gained from sandy hills of Abo-Mandor at Rosetta branch of River Nile, Egypt. The dried total plant powder of Echium angustifolium Mill (5 kg) was extracted and used as produced by El-Rokh et al. [21]. After the extraction process, the Echium angustifolium Mill crude extract was filtered by whatman filter papers to eliminate unfavorable solid residues and pollutants. A volume of 10 ml of the crude extract is ejected and desiccated at fixed temperature. The gained desiccated solid residue is weighted to support in estimating the concentration of the tested extract. The plant extract concentration range was as follows: 50–300 ppm. The suggested chemical structures of some phytochemical constituents isolated from the Echium angustifolium Mill extract were listed in Table 1.
2.4 Chemical Measurements (WL Method)
The weight loss method (WL) was performed in a 150 ml beaker put in bath of water thermostat. Rapidly, the specimens were put with dimensions 20 × 20 × 2 mm in the test solution with 1.0 M H2SO4 with and without various Echium angustifolium Mill extract concentrations. After varied immersion time (30 to 150 min) and varied temperatures (25 to 45°C), the specimens were ejected, washed with bi-distilled water, dried, and accurately weighed the tested specimens which exposed to the previous conditions and the mean weight loss was obtained. The weight loss method were applied to adjust the corrosion rate and the inhibition efficiency from the next equation:
Where C.R. is the corrosion rate, ρ is constant; W is mass loss (mg); T is he corrosion period (hr); A is the specimen area cm2; D is the density (g/cm3). The inhibition efficiency (%η) and surface coverage degree (θ) are measured via the next equation:
Where η = Inhibition efficiency; C.R.* and C.R. are the corrosion rates without and with the investigated Echium angustifolium Mill extract doses, respectively.
2.5 Electrochemical Measurements
Electrochemical tests were applied in a three electrode cell in 1.0 M H2SO4 via Versa STAT 4 potentiostat/galvanostat with a frequency response analyzer (FRA) hold in a single unit and associated with laptop. Saturated calomel electrode (SCE), the carbon steel specimen, and platinum wire electrode were selected as reference electrode, working electrode and counter electrode, correspondingly. The carbon steel surface contact with the corrosive solution was a fixed area (10 × 10 mm) and was joined from one side to a copper wire used for electrical connection, the carbon steel electrode was polished sequentially with varied grades of emery papers, cleaned with bi-distilled water and then degreased with acetone. All the experiments were achieved at 25°C in the subsequent order: The open circuit potential vs. time for 0.5 h, the polarization measurements were recorded by sweeping the potential from −5 to +5 V with respect to corrosion potential (Ecorr) at scan rate of 0.5 mV s–1. The data gained were investigated by Tafel plot method. EIS measurements were achieved at open circuit potential. The range of applied frequencies was from 100 kHz to 0.1 Hz using voltage perturbation amplitude of 5 mV peak to peak.
The inhibition efficiency (%η) and the surface coverage (θ) of the tested extract given from the impedance technique were measured from Eq. 3:
Where, \(R_{{{\text{ct}}}}^{^\circ }\) and Rct are the charge transfer resistance in the absence and presence of different dose of extract, respectively.
2.6 Surface Examinations
The carbon steel specimens which were applied for analysis its surface morphology were prepared in 1.0 M H2SO4 (Blank) and with 300 ppm of the tested Echium angustifolium Mill extract at 25°C for 12 h after abraded mechanically by using different emery papers from 400–1200 grit size. Then, after this time, the coin samples were cleaned carefully by bi-distilled water, gently dried and achieved the carbon steel specimens inspected by scanning electron microscope (SEM) via (JEOL JSM-5500, Japan) model associated with energy dispersive X-ray (EDX) for elemental analysis of the tested surfaces and atomic force microscope (AFM) using (Wet–SPM (Scanning Probe microscopy) Shimadzu, Japan).
3 RESULTS AND DISCUSSION
3.1 WL Method
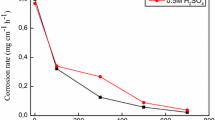
The estimation of weight loss (WL) of carbon steel in mg cm-2 relative to the surface area at different time periods with and without different doses (50, 100, 150, 250, 250 and 300 ppm) of Echium angustifolium Mill extract. The obtained diagram in the existence of various doses of the tested extract below that of free acid as represented in Fig. 1. Generally, the %η increases with increasing the tested extract doses but the corrosion rates are decreased as reported in Table 2. These results indicated that, the Echium angustifolium Mill extract under investigation are a good effective inhibitor for carbon steel dissolution in sulfuric acid medium.
3.1.1. Adsorption isotherm. Various statistical demonstrations were tested to gain the best fit symbolizing the adsorption isotherms. The applied corrosion inhibition system was matches to Langmuir adsorption isotherm derived by the next equation [22]:
Where, θ symbolizes the surface coverage, C is the concentration of the plant extract inhibitor and Kads is the equilibrium constant of adsorption associated to the adsorption free energy \(\Delta G_{{{\text{ads}}}}^{^\circ }\) by the next equation [23]:
Straight lines gained after graphing C/θ vs. C for the adsorption of the extract on the carbon steel surface in H2SO4 solution at 25°C is represented in Fig. 2 and the adsorption thermodynamic parameters were calculated and reported in Table 3. As shown, \(\Delta G_{{{\text{ads}}}}^{^\circ }\) become less negative, in other meaning, raised when rising the temperature. This is recommended that the adsorption process achieved via the electrostatic attraction. The negative values of \(\Delta G_{{{\text{ads}}}}^{^\circ }\) are recommended that the adsorbed layer on the carbon steel surface is fixed and the adsorption process is spontaneous [24]. The examination for the gained data of \(\Delta G_{{{\text{ads}}}}^{^\circ }\) were less negative than –21 kJ mol–1 suggesting that the adsorption mechanism of the tested plant extract on carbon steel in 1.0 M H2SO4 solution is compatible with physisorption process [25, 26]. The heat of adsorption (\(\Delta G_{{{\text{ads}}}}^{^\circ }\)) should be computed via the vanʼt Hoff equation [27]:
So to calculate the heat of adsorption (\(\Delta H_{{{\text{ads}}}}^{^\circ }\)), log Kads was graphed versus 1000/T as represented in Fig. 3. A straight line was gained. The whole values of \(\Delta H_{{{\text{ads}}}}^{^\circ }\) gained in this research was less than 100 kJ mol−1, this signal for physisorption process, and this assist the previous mechanism of adsorption [28]. The negative value of \(\Delta H_{{{\text{ads}}}}^{^\circ }\) (−42.0 kJ mol−1) in the existence of the investigated extract compatible with the exothermic nature of carbon steel dissolution process. So as to the activation enthalpy varied in the same manner as the activation energies, assisting the suggested inhibition mechanism, which are computed via the next equation:
3.1.2. Effect of temperature. The impact of temperature by using Arrhenius equation to estimate the activation energy by the next equation:
where, R is the universal gas constant, Ea is the activation energy, T is the absolute temperature and A is a Arrhenius pre-exponential constant relies upon electrolyte and the metal nature. When plotting the log kcorr versus (1/T) for carbon steel dissolution in 1.0 M H2SO4 with and without Echium angustifolium Mill extract doses are shown graphically in Fig. 4a, provides straight lines that have slope (\( - E_{a}^{*}\)/2.303R) and the values of \(E_{a}^{*}\) were determined and recorded in Table 4. The activation energy (Ea) increases with adding various Echium angustifolium Mill extract doses, this leads to, the improvement of the energy barrier of the corrosion reaction. Since the activation energy over 20 kJ mol–1 [29], the whole process is controlled by surface reaction. The entropy and enthalpy of activation (ΔS*, ΔH*) are determined from the theory of transition state by the following relation [30].
where, N is Avogadro’s number h is Planck’s constant. Draw log(kcorr./T) against (1/T) also provided straight lines as represented in Fig. 4b, for carbon steel dissolution in 1.0 M H2SO4 with and without Echium angustifolium Mill extract doses. The (–ΔH*/2.303R) are the slopes of these lines and the log[RT/Nh] + (ΔS*/2.303R) are the intercepts, that the values of (ΔH*) and (ΔS*) were determined and recorded in Table 4. From these results, it is obvious that the test compound rising the values of activation energy and follow reducing the rate of corrosion of the carbon steel. The ΔH* values be a sign of the strong adsorption of this extract on the carbon steel surface. The ΔS* values in with and without the tested extract is negative and large value; this lead to the rate-determining of activated complex performs an association step, rather than dissociation step, meaning that a decreases in random occurs and foreword from the activated complex of the reactants and placed the activated molecules in more order state than that at the primary state [31].
3.2 Electrochemical Measurements
3.2.1. Open circuit potential (EOCP). The open circuit potential (OCP) values are sketched in Fig. 5. The adding of the extract into the applicable solution caused a positive move in the OCP. A sample without any inhibitor loading shows an initial OCP at –0.45 V and ends at –0.50 V approximately. The move of the OCP in the direction of a negative potential is due to the incidence of general corrosion on the carbon steel surface [32]. The quick shift at 100 s is due to rapid sulfate ions dispersion through the applicable solution. Through this shift, the carbon steel electrode is undergoing the destruction phase. The destruction phase is owing to the damage of the barrier (i.e., F2O3) [33]. With the investigated Echium angustifolium Mill extract, the value of the OCP moves in the direction of the more positive potential section. Firstly, the value of the OCP is scattered for the extract, but the final OCP recorded shows a shift in the direction of the positive section. In this phase, the electrode becomes less active due to the decreased rate of metal dissolution [34]. The scattering pattern is because of the dispersion of sulfate ions inside the applicable solution. For the investigated Echium angustifolium Mill extract, the OCP is regular which indicates that the rate of oxide layer damage are slightly changed due to the creation of adsorbed protective film.
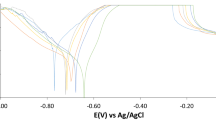
3.2.2. Potentiodynamic polarization (PP). Tafel curves derived from PP measurements were investigated to identify the inhibition effect. Figure 6 illustrates the impact of the Echium angustifolium Mill extract on the polarization curves for carbon steel electrode in 1.0 M H2SO4 solution. Electrochemical parameters and corresponding inhibition efficiencies (%η) which gained from PP measurements are recorded in Table 5. The characterization of both Fig. 6 and Table 5 indicates that the addition of the Echium angustifolium Mill extract reduces both the cathodic and anodic current density. This reduction associated with the increase of the extract doses. The Tafel curves are moved to more positive and more negative potentials in matching with the blank. This behavior leads to that the surfactants act as mixed-type inhibitors [35]. From data gained the rising in the extract dose leads to a reduction in the corrosion current density (icorr), the Tafel slopes (βa‚ βc)‚ are compatible showing that the retardations of the two reactions (anodic metal dissolution and cathodic hydrogen reduction) were affected without changing the dissolution mechanism [36, 37]. The observed inhibitory action of Echium angustifolium Mill extract may be owing to the adsorption of its molecules on the metal surface. The inhibition efficiency reaches 92% at 300 ppm. The % η gained from potentiodynamic polarization method are close to those gained from weight loss method. The surface coverage (θ) and inhibition efficiency (%η) were computed from the next equation (10).
Where, i°, i are the corrosion current density values with and without different Echium angustifolium Mill extract doses, correspondingly.
3.2.3. Electrochemical impedance spectroscopy (EIS). The EIS was applied to achieve the kinetic parameters and significant mechanistic for measured electrochemical method. Nyquist (Fig. 7) and Bode (Fig. 8) impedance plots are derived from the carbon steel electrode in 1.0 M H2SO4 at 25°C with and without different Echium angustifolium Mill extract doses. The plots are represented a similar kind of Nyquist curve for carbon steel with varied doses of the extract. The existence of single semi-circle lead to the single charge transfer process through dissolution which is unchanged with the existence of Echium angustifolium Mill extract. The dissimilar of the Nyquist plots of the extract from ideal semicircles as supposed from the theory of EIS is owing to the frequency diffusion, mass transport resistant and contaminations, electrode surface heterogeneity gained from surface roughness, inhibitor adsorption, disturbances, creation of porous layers and grain boundaries [38–40]. Impedance values are calculated via the parallel circuit shown in Fig. 9, in which Rct = the charge transfer resistance, Rs = the electrolyte resistance and Cdl = double layer capacitance are reported in Table 6. Table 6 demonstrates that the Rct raises and Cdl reduced with the rising of the extract doses. The reduction in Cdl and improvement in Rct data caused by lowering the local dielectric constant (due to the continuous substitution of water molecules by the extract molecules on the carbon steel surface) and/or the raise of the electrical double layer thickness recommended that the extract molecules are adsorbed at the carbon steel surface [41]. The %η recorded from EIS parameters are in great agreement with that given from potentiodynamic polarization measurements. The variation of inhibition efficiency from the two techniques may be adjacent to the surface conditions of the carbon steel electrode in two methods. The %η of the Echium angustifolium Mill extract for the carbon steel dissolution in 1.0 M H2SO4 is calculated via Rct values as follow:
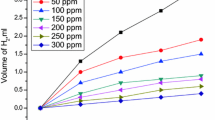
3.2.4. Electrochemical Frequency Modulation (EFM). EFM is a nondestructive corrosion test method which can directly and rapidly investigate the corrosion current density data without previous data of Tafel plots, and associated with a little polarizing signal. These features of EFM method make it a perfect technique for online corrosion monitoring [42, 43]. The powerful of the EFM is the causality factor parameters which support the validity of EFM test. The causality factors CF-2 and CF-3 are derived from the frequency spectrum of the current responses. Figure 10 represents the EFM Inter-modulation graph (current vs. frequency) of carbon steel in 1.0 M H2SO4 with and without different Echium angustifolium Mill extract doses. The harmonic and inter-modulation peaks are visibly clear and are much higher than the background noise. The higher peaks were applied to compute the corrosion current density (icorr), the Tafel slopes (βa and βc) and the causality factors (CF-2 and CF-3). These EFM parameters were recorded in Table 7, which are shown that, the adding of the tested extract at a given dose to the acidic solution reduces the corrosion current density, referring to this extract mitigates the corrosion of carbon steel in 1.0 M H2SO4 via adsorption process. The causality factors gained under various experimental conditions are approximately equal to the theoretical values (2 and 3) suggesting that the measured data are confirmed with high quality. The inhibition efficiency %η derived from EFM measurements raises by the rising of the Echium angustifolium Mill extract doses and was calculated from Eq. (10).
3.3 Surface Examinations
3.3.1. SEM characterization. The micrograph shown for carbon steel specimen with and without addition of 300 ppm of the Echium angustifolium Mill extract after 24 h immersion are represented in Fig. 11. This Figure shows that in 1.0 M H2SO4 the carbon steel surface damages due to corrosion attack, but in the existence of the extract the carbon steel surface became softer, fine and the morphology of metal surface is appear in a good status. Furthermore, the existence of a protective film which is spread in all carbon steel surface was watched, owing to the adsorption of the plant extract on the metal surface for blocking the active sites which exist on the carbon steel surface [44, 45].
3.3.2. Energy dispersive spectroscopy (EDX). The protective film coated on the carbon steel surface was discovered via EDX spectroscopy [46]. Figure 12 illustrates the EDX analyses of the three status of carbon steel (free, with the corrosive medium and the treatment by plant extract). The EDX analysis is shown that iron and oxygen were identified, which confirmed the creation of passive film contained just Fe2O3. Figure 12 represents the EDX analysis of carbon steel in Blank (1.0 M H2SO4) and with 300 ppm of the plant extract. The spectra are shown additional new lines, displaying the occurrence of carbon (related to the carbon atoms represented in the extract molecules). This data demonstrates that the oxygen and carbon atoms covered the carbon steel specimen. This created layer owing to the the extract components, because of the oxygen and carbon signals are disappear on the surface exposed to uninhibited sulfuric acid. Additionally, O, C, and Mn were available in the spectrum. A practically elemental analyses for the three status of carbon steel are reported in Table 8.
3.3.3. Atomic force microscopy (AFM). AFM has been a significant tool in order to estimate the surface morphology investigation that one have been helpful to discuss the Echium angustifolium Mill extract effect on the metal/solution interface [47, 48]. Figure 13a illustrated the AFM graphs of polished carbon steel, Fig. 13b carbon steel in 1.0 M H2SO4, which not including Echium angustifolium Mill extract and carbon steel in 1.0 M H2SO4 treated with 300 ppm of Echium angustifolium Mill extract, respectively. From AFM micrographs, the surface is very obvious for polished carbon steel samples with average roughness 660.44 nm as shown in Fig. 13a. Whereas in the nonexistence of Echium angustifolium Mill extract, the carbon steel surface has been more corroded, with average roughness 2195.88 nm as shown in Fig. 13b. By comparison, the average roughness have been reduced to 812.35 nm in the existence of Echium angustifolium Mill extract at the optimum dose (300 ppm) as shown in Fig. 13c. From the results, I deduced that the lowering in the roughness can be very well via the creation of the adsorbed protective layer of the Echium angustifolium Mill extract on the carbon steel surface.
3.4 The Corrosion Inhibition Mechanism
The experimental results proposed that the corrosion inhibition mechanism is assigned to the adsorption of the tested plant extract on the carbon steel surface. The lone pair of electrons on (O) heteroatom plus π-electrons of aromatic systems and multiple bonds supply practically in the adsorption process. The extract adsorption may be explained via two main processes of interactions: physisorption and chemisorptions. Generally, physisorption needs the existence of both charged species in solution and metal surface which electrically charged. The metal surface charge of the electric field presented in the metal/solution interface. In contrast, a chemisorption process may include charge transfer or charge sharing from the inhibitor particles to the metal surface to form a co-ordination bond. This has been achieved in the case of a positive as a negative charge on the metal surface. The transition metal existence in which involving vacant, low-energy electron orbital’s (Fe+2 and Fe+3) and Echium angustifolium Mill extract molecules which having bound electrons or heteroatom’s involving a lone pair of electrons have been essential for the large inhibiting activity [49]. Commonly, there have been two kinds of inhibition mechanisms have been suggested. One has been the production of polymeric complexes with iron ions (Fe3+) relying on the applied conditions [50], the other one has been the chemical adsorption of Echium angustifolium Mill extract on the carbon steel surface [51]. The inhibition achievement of Echium angustifolium Mill extract doses hasn’t been happened via the simple blocking at the carbon steel surface, chiefly at high temperature. This could be referred to the dissimilar adsorption capacities of the Echium angustifolium Mill extract on the carbon steel surface at different temperatures. We have been discussing that while rising temperature the Echium angustifolium Mill extract adsorption effect on carbon steel surface raised. Most of the hydrophilic groups which have positively charged atoms (O+) adsorbed from the carbon steel surface and influence to allow the H+ became closer to the metal surface. As a result, Echium angustifolium Mill extract has been preferred blocking both anodic and cathodic corrosion processes at higher temperatures as shown in Fig. 14 [52].
4 CONCLUSIONS
The inhibition efficiency IE% raises by rising Echium angustifolium Mill extract doses and increases as temperature increasing. Adsorption of Tetraclinis articulata extract molecules on the carbon steel surface is found to conform Langmuir adsorption isotherm model. Tafel curves referred that Echium angustifolium Mill extract acted as a mixed type inhibitor. SEM, EDX and AFM images confirmed the possibility of protective film creation on the carbon steel surface. Derived from all data of WL, PP, EIS and EFM, Echium angustifolium Mill extract are shown as an efficient inhibitor for carbon steel dissolution in 1.0 M H2SO4 (Fig. 15).
REFERENCES
Deyab, M.A. and Abd El-Rehim, S.S., Corros. Sci., 2012, vol. 65, p. 309.
Deyab, M.A. and Abd El-Rehim, S.S., Int. J. Electrochem. Sci., 2013, vol. 8, p. 12613.
Oddo, J.E. and Tomson, M.B., J. Pet. Technol., 1982, vol. 34, p. 1583.
Ridd, B., Blakset, T.J., and Queen, D., Proc. NACE Int. Conference CORROSION/98, San Diego, CA, 1998, paper no. 78.
Solomon, M.M., Umoren, S.A., Udosoro, I.I., and Udoh, A.P., Corros. Sci., 2010, vol. 52, p. 1317.
Lahhit, N., Bouyanzer, A., Desjobert, J.M., et al., Port. Electrochim. Acta, 2010, vol. 29, p. 127.
Vijayalakshmi, P.R., Rajalakshmi, R., and Subhashini, S., Port. Electrochim. Acta, 2011, vol. 29, p. 9.
Anthony, N., Malarvizhi, E., Maheshwari, P., et al., Indian J. Chem. Technol., 2004, vol. 11, p. 346.
Fouda, A.S., Mostafa, H.A., El-Taib Haekel, F., and Elewady, G.Y., Corros. Sci., 2005, vol. 47, p. 1988.
Ahamd, I. and Quraishi, M.A., Corros. Sci., 2010, vol. 52, p. 651.
Singh, A.K. and Quraishi, M.A., Corros. Sci., 2010, vol. 52, pp. 152–160.
Abdel Hameed, R.S., Al-Shafey, H.I., Abul Magd, A.S., and Shehata, H.A., J. Mater. Environ. Sci., 2012, vol. 3, p. 294.
Li, X., Deng, S., Mu, G., Fu, H., and Yang, F., Corros. Sci., 2008, vol. 50, p. 420.
Abd El Rehim, S.S., Hassan, H.H., and Amin, M.A., Corros. Sci., 2004, vol. 46, p. 5.
Abdel-Gaber, A.M., Khamis, E., Abo-El-Dahab, H., and Adeel, Sh., Mater. Chem. Phys., 2008, vol. 109, p. 297.
Abiola, O.K. and James, A.O., Corros. Sci., 2010, vol. 52, p. 661.
James, A.O. and Akaranta, O., Res. J. Chem. Sci., 2011, vol. 1, p. 31.
Fouda, Abd El-Aziz S., El-Katori, Emad E., and Al-Mhyawi, S., Int. J. Electrochem. Sci., 2017, vol. 12, p. 9104.
Aboia, O.K. and James, A.O., Corros. Sci., 2010, vol. 52, p. 661.
Saratha, R. and Vasudha, V.G., E-J. Chem., 2010, vol. 7, p. 677.
El-Rokh, A.R., Negm, A., El-Shamy, M., El-Gindy, M., and Abdel-Mogib, M., Phytochemistry, 2018, vol. 149, p. 155.
Kamis, E.T., Int. J. Adv. Sci. Tech. Res., 1990, vol. 46, p. 476.
El-Rehim, S.S.A., Ibrahim, M.A.M., and Khaled, K.F., J. Appl. Electrochem., 1999, vol. 29, p. 593.
Fouda, A.S., El-Desoky, A.M., and Ead, D.M., Int. J. Electrochem. Sci., 2013, vol. 8, p. 8823.
Benali, O. and Ouazene, M., Arabian J. Chem., 2011, vol. 4, p. 443.
Larabi, L., Benali, O., and Harek, Y., Mater. Lett., 2007, vol. 61, p. 3287.
Larabi, L., Harek, Y., Benali, O., and Ghalem, S., Prog. Org. Coat., 2005, vol. 54, p. 256.
Hassan, R.M. and Zaafarany, I.A., Materials, 2013, vol. 6, p. 2436.
Khaled, K.F., Samardzija, K.B., and Hackerman, N., J. Appl. Electrochem., 2004, vol. 34, p. 697.
Singh, A.K., Singh, A.K., and Ebenso, E.E., Int. J. Electrochem. Sci., 2014, vol. 9, p. 352.
Benabdellah, M., Aouniti, A., Dafali, A., et al., Appl. Surf. Sci., 2006, vol. 252, p. 8341.
Ruhi, G., Modi, O.P., and Dhawan, S.K., Synth. Met., 2015, vol. 200, p. 24.
Diggle, J.W., Downie, T.C., and Goulding, C.W., J. Electrochem. Soc., 1969, vol. 116, p. 1347.
El Haleem, S.M.A., El Wanees, S.A., El Aal, E.E.A., and Farouk, A., Corros. Sci., 2013, vol. 68, p. 1.
Fouda, A.S., Mahmoud, W.M., and Elawayeb, K.M.A., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, p. 139.
Migahed, M.A., Azzam, E.M.S., and Morsy, S.M.I., Corros. Sci., 2009, vol. 51, p. 1636.
Singh, A.K., Singh, A.K., and Ebenso, E.E., Int. J. Electrochem. Sci., 2014, vol. 9, p. 352.
Bentiss, F., Lebrini, M., and Lagrenee, M., Corros. Sci., 2005, vol. 47, p. 2915.
Silverman, D.C. and Carrico, J.E., Corrosion, 1988, vol. 44, p. 280.
Bayol, E., Kayakirilmaz, K., and Erbil, M., Mater. Chem. Phys., 2007, vol. 104, p. 74.
Benalli, O., Larabi, L., Traisnel, M., Gengembra, L., and Harek, Y., Appl. Surf. Sci., 2007, vol. 253, p. 6130.
Ramachandran, S., Tsai, M., Blanco, M., Chen, H., and Tang, W.A., Electrochim. Acta, 2008, vol. 53, p. 3484.
Fouda, A.S. and Ibrahim, A.R., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, p. 1194.
Anupama, K.K., Ramya, K., Shainy, K.M., and Joseph, A., Mater. Chem. Phys., 2015, vol. 167, p. 28.
Yuli Yetri, Emriadi, Novesar Jamarun, and Gunawarman, Proc. Int. Conference on Biological, Chemical and Environmental Sciences (BCES-2014), Penang, 2014, vol. 14, p. 15.
Shalabi, K. and Abdel Nazeer, A.,Prot. Met. Phys. Chem. Surf., 2015, vol. 51, p. 908.
Hazazi, O.A., Fawzy, A., and Awad, M., Int. J. Electrochem. Sci., 2014, vol. 9, p. 4086.
Jevremović, I., Singer, M., Nešić, S., and Mišković-Stanković, V., Corros. Sci., 2013, vol. 77, p. 265.
Ehsani, A., Mahjani, M.G., Hosseini, M., Safari, R., Moshrefi, R., and Shiri, H.M., J. Colloid Interface Sci., 2017, vol. 490, p. 444.
Li, X., Deng, S., and Fu, H., Corros. Sci., 2009, vol. 51, p. 1344.
Saliyan, R. and Adhikari, A.V., Bull. Mater. Sci., 2008, vol. 31, p. 699.
Sin, H.L.Y., Rahim, A.A., Gan, C.Y., Saad, B., Salleh, M.I., and Umeda, M., Measurement, 2017, vol. 109, p. 334.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emad E. El-Katori The Role of Echium Angustifolium Mill Extract in The Corrosion Mitigation of Carbon Steel in Sulfuric Acid Solution. Prot Met Phys Chem Surf 56, 1081–1095 (2020). https://doi.org/10.1134/S207020512005010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207020512005010X