Abstract
Corrosion inhibition effects of water-soluble peripheral substituted cobalt, copper and zinc metallophthalocyanines (CoPc (1), CuPc (2) and ZnPc (3)) on the copper metal in 0.1 N HCl were investigated by using gravimetric, electrochemical, SEM-EDS analysis and quantum chemical calculations. On the electrochemical investigations and gravimetric analysis the highest inhibitor efficiency was obtained with CoPc (1) at 1 × 10–2 mol/L concentration. SEM-EDS results indicated parallel results and oxygen atom content increased in the order of ZnPc (3), CuPc (2) and CoPc (1). According to the quantum chemical calculations, following corrosion inhibition efficiency ranking was obtained: CoPc (1) > CuPc (2) > ZnPc (3).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
Copper metal has been used in the industry for wide range of applications such as metal alloys, production of tube, wires and sheets. The metal has many advantages of usage in the industry because of its soft structure, but it is susceptible to corrosion in aggressive media [1]. Thus, many corrosion inhibitors have been identified by researchers for copper metal as being some of them inorganic [2] and big parts of them organic inhibitors such as azoles [3, 4], amines [5] and many others.
Presence of nitrogen, sulfur and phosphorous atoms in the organic compounds improves their corrosion inhibition activity and this is explained by the presence of vacant d orbitals in copper atom which forms coordinative bonds with atoms able to donate electrons [1]. Phthalocyanines (Pcs), on the other hand, are organic molecules with eight N atoms in their structure and their unique redox chemistry of their metal complexes has brought about their incorporation into a large number of donor–acceptor systems [6]. The molecules are useful for wide range of applications such as photodynamic therapy [7], chemical sensors [8] and antibacterial activity [9, 10].
In the studies, corrosion inhibition properties of PC molecules were investigated but this was the subject of few studies. Copper containing phthalocyanine was found an effective corrosion inhibitor on high strength low alloy (HSLA) steel in 16% HCl [11], zinc phthalocyanine was found an excellent inhibitor for mild steel in 1 mol/L H2SO4 [12]. Adsorption behavior of organic inhibitors at the aluminum-HCl solution interface and their corrosion inhibition performance were investigated and benzothiazole-substituted gallium phthalocyanine showed inhibition activity [13].
In the present study, the corrosion inhibition properties of previously synthesized water-soluble peripheral substituted cobalt, copper and zinc metallophthalocyanines (CoPc (1), CuPc (2) and ZnPc (3)) bearing 8-hydroxyquinoline-5-sulfonic acid groups on copper metal in 0.1 N HCl were evaluated. Gravimetric, electrochemical impedance, polarization, SEM-EDS techniques were used and quantum chemical calculations were done.
2 EXPERIMENTAL
2.1 Chemicals and Materials
The aluminum copper electrode used in electrochemical measurements was supplied cylindrical (10 × 1 cm) and it was sealed by epoxy resin with exposure surface 0.785 cm2 as the working electrodes. The copper plates were mechanically cut in to 1 × 1 cm dimensions for weight loss measurements. The working surfaces of the electrodes and plates were polished with emery papers 400, 800 and 1200 grit, then, washed with distilled water and acetone respectively.
CoPc (1), CuPc (2) and ZnPc (3) molecules was previously synthesized and published by A. Günsel et al., [14]. The chemical structures of the molecules were given in Scheme 1. 0.5 N HCl solution was purchased from Merck and appropriate dilutions were made by using distilled water. Acetone and ethanol were purchased from Sigma. The solutions used for the electrochemical measurements were prepared with 0.1 N HCl by addition of different concentration of Pc molecules (1 × 10–2, 1 × 10–4, 1 × 10–6 mol/L). The acid solution in the absence of Pc molecules was taken as blank solution.

Scheme 1. The structure of water-soluble peripheral substituted cobalt, copper and zinc metallophthalocyanines (CoPc (1), CuPc (2) and ZnPc (3)) bearing 8-hydroxyquinoline-5-sulfonic acid groups.
2.2 Weight Loss Measurements
Weight loss corrosion measurements were performed according to the method previously described [15] and 1 cm2 metal specimens were used in the experiments. Before the experiments, metal plates were polished with thick and thin emery papers and washed with acetone, rinsed in distilled water, dried and weighed accurately. The prepared metal plates were immersed in the 250 mL 0.1 N HCl solution in the presence and absence of Pc molecules at different concentrations so that all surfaces were in contact with the corrosive medium. After 7 days of immersion time, the plates were taken out, washed with acetone and distilled water, air dried and weighed accurately.
The measured weight loss and the inhibition efficiency (IE, %) were calculated using the formula:
where Wo and Wi are the charge weight loss with and without the inhibitor, respectively.
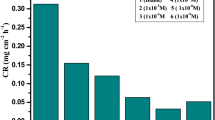
The corrosion rate (CR) was calculated using the following formula:
Where Δm is weight loss in mg, A is the area of exposure in cm2, and t is the time in hours.
2.3 Electrochemical Measurements
All electrochemical measurements were carried out by using Gamry Interface 1000 E Potentiostat in a conventional three-electrode cell; saturated calomel electrode (SCE) and graphite were used as the reference and counter electrodes. Copper electrode with 0.785 cm2 exposure area was used as working electrode and it was immersed in the test solution for 30 min and the electrode potential was stabilized. All experiments were done at room temperature.
Frequency range of 1 kHz to 100 mHz with amplitude of 10 mV open circuit potential (OPC) was used for the Electrochemical Impedance Spectroscopy (EIS) measurements. The charge transfer values were obtained from the diameter of the semicircles of the Nyquist plots and the inhibition efficiency was calculated using the following equation:
where R°t and Rt are the charge transfer resistances in the absence and presence of the inhibitors, respectively.
The potentiodynamic polarization curves were obtained from –250 mV versus OCP to +250 mV versus OCP with a scan rate of 0.5 mV/s. Electrochemical parameters such as Ecorr, Icorr and cathodic tafel slopes (βa, βc) were obtained by the Tafel extrapolation method and the inhibition of efficiency was calculated by following equation [16]:
where Icorr and \(I_{{{\text{corr}}}}^{^\circ }\) signify the corrosion current density in the absence and presence of the inhibitors, respectively.
2.4 Quantum Chemical Method
All calculations made in this study were performed using Gaussian package program and studied complexes were optimized in the gas phase on the Hartree–Fock (HF) [17] method with 3–21 g basis set. Quantum chemical parameters like HOMO, LUMO and ΔE (HOMO–LUMO energy gap) obtained from quantum chemical calculations were used to compare the activities of inhibitor molecules.
2.5 SEM and EDS Analysis
Metal plates (1 × 1 cm) were immersed in the 250 mL 0.1 N HCl solution in the presence and absence of Pc molecules at 1 × 10–2 mol/L concentration for 7 days. Then, they were removed, rinsed with acetone and distilled water and dried at room temperature. Surface morphologies were examined by scanning electron microscopy (SEM) and energy dispersive X‑Ray spectroscopy (EDS) (JEOL JSM-6060LV).
3 RESULTS AND DISCUSSION
3.1 Weight Loss Measurements
In the presence and absence of Pc molecules, weight loss measurements of copper in 0.1 N HCl solution were studied. Inhibition efficiencies (%) and metal surface measurements are given in Table 1.
When the results of the study are examined, it is seen that the molecule with the highest inhibitory efficiency is CoPc (1) at 1 × 10–2 mol/L concentration (76.88%), followed by CuPc (2) (67.06%) and ZnPc (3) (64.04%). The inhibition efficiency increased with increasing concentration in all tested Pc molecules. At the highest concentration of 1 × 10–2 mol/L, all inhibitors showed the efficiency more than 60%. Because Pc molecules could be protonated in the acidic solutions, Cl ions create an excess negative charge towards to metal surface and through electrostatic interactions inhibitor molecules may adsorb to the metal surface [18].
In a previous study, Chen et al. investigated four hydrosoluble macroazacyclic compounds, phthalocyanine with phosphorus and substituent phosphorus triaza tetrabenzo corrole carrying amino or sulfonate groups respectively as corrosion inhibitors for mild steel in 1 mol/L HCl. The weight loss measurements showed that the inhibition efficiency of phosphorus phthalocyanine was higher than that of the phosphorus triaza tetrabenzo corrole while the amino substituent phosphorus phthalocyanine showed the strongest inhibition ability among the molecules [19].
3.2 Electrochemical Measurements
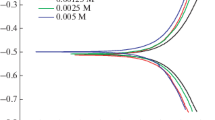
3.2.1. Polarization measurements. Polarization curves of copper in 0.1 N HCl in the presence and absence of Pc molecules are given in Figs. 1–3 while the calculated inhibition efficiency and other parameters are listed in Table 2. CoPc (1), CuPc (2) and ZnPc (3) acted as effective inhibitors in 0.1 N HCl for copper at the inhibitor concentration 1 × 10–2 mol/L. The potentiodynamic curves in all three figures showed a clear reduction of anodic and cathodic currents and the Tafel plots indicated the cathodic (H+ evolution) and anodic reductions (metal dissolution). The inhibition efficiency decreased in the order of CoPc (1) > CuPc (2) > ZnPc (3) as 94.06, 88.6 and 61.21% respectively at this concentration. The Icorr values (corrosion current density), on the other hand, increased in the order of ZnPc (3) > CuPc (2) > CoPc (1).
Effect of metal ion in the core of Pc molecules has an inhibition effect when compared to the non-metallo Pc molecules. When Zhao et al. investigated the H2Pc and CuPc molecules’ inhibitory activities on mild steel in 1 M HCl, the results of polarization curves indicated that metallo-Pc molecule had higher inhibition efficiency than non-metallo Pc molecule [20].
3.2.2. EIS measurements. Effect of metal phthalocyanines corrosion inhibition properties on copper was studied at different concentrations by using EIS measurements. Impedance activities of Pc molecules on the copper metal in 0.1 N HCl were given in Figs. 4–6 and semicircles diameters increased with the addition of inhibitor. Rt value increased from 1.493 to 9.202 for CoPc (1), to 5.2 for CuPc (2) and 3.711 for ZnPc (3) by increasing of inhibitor concentration. On the other hand, Cdl value decreased as the concentration of inhibitor molecules increased (Table 3), and it can be explained with an increase in adsorption of inhibitor molecules to metal surface [21, 22]. The equivalent Randles circuit model is shown in Fig. 7, where RΩ represents the solution and corrosion product resistance, and Rt and Cdl represent corroding interfaces. The double-layer capacitance (Cdl) was calculated as follows:
When the inhibitory activities of metallo Pc molecules on carbon steel/epoxy coating in 3.5% NaCl were investigated, Nyquist plots showed that ZnPc (3) molecule as the inhibitor was not highly effective to protect the metal for a long time period and this was consistent with our results [23].
3.2.3. Quantum chemical study. As a result of the calculations made; optimized structures, HOMO and LUMO shapes of inhibitor molecules were drawn. The optimized structures of the inhibitor molecules are shown in the first illustration in Figs. 8–10. When the drawn figures are examined, it is seen that the optimized structures of inhibitor molecules are planar. As the structures are planar, the surface of the contact between the Pc molecule and the copper metal surface will be the highest when it approaches parallel to the copper metal surface and this increases the inhibition efficiency [24].
Quantum chemical parameters are calculated from quantum chemical calculation by gaussian software. The obtained results of the studied Pc complexes were given in Table 4. In the explanation of the activity of the inhibitory molecules, two important parameters are used and these are HOMO and LUMO which are the energy values of highest occupied and lowest unoccupied molecular orbital respectively [25–27]. In Figs. 8–10, it is observed that HOMO and LUMO orbital of inhibitor molecules are generally concentrated on central atoms and because of their great contribution to HOMO orbital, atoms where HOMO orbital are concentrated focus on these atoms. This represents that interaction with metal surfaces will be through these atoms [28–31].
As a result of the calculations, graphs were drawn against the HOMO–LUMO energy gap. In the graphs drawn, it is seen that the inhibition efficiency declines with the energy value difference between HOMO-LUMO of inhibitor molecules [32, 33] and the inhibition efficiency was found to decrease with LUMO energy values of inhibitor molecules indicating the ability of that molecule to accept electrons.
If the LUMO value of the inhibitor molecules decreases, electrons of Fe atoms in 4s orbitals increases the ability to accept and this increases the value of the % IE. Previous studies show that there is a linear relationship between HOMO energy values and the inhibition efficiency values [34, 35]. On the other hand, the HOMO energy value of the inhibitory molecule tends to give electrons indicating a tendency to give electrons to metal atoms with low energy empty molecular orbital but the correlation of this EHOMO value against % IE cannot be plotted [28, 35–38]. It should be noted that the Pc derivatives possess the cyclic conjugated system having a greater electron density and the HOMO properties of the compounds provide important information about the interaction between copper metal and these molecules. The molecular orbital density distributions of these derivatives can be studied in detail in Figs. 8–10. In these pictures, it is seen that the central atoms in the Zn complexes have HOMO balloons larger than the other complexes and this affects the inhibition efficiency values. In Figs. 11–13, it should be noted that in experimental studies, it can be seen that as the molar concentration of solutions increases, weight loss increases. The most important factor affecting the value of weight loss is the HOMO–LUMO energy gap value of the complexes. As this value increases, it is known that the value of the inhibition efficiency of the complex is increased and in the light of this information, the following corrosion inhibition efficiency ranking was obtained: CoPc (1) > CuPc (2) > ZnPc (3).
3.2.4. SEM and EDS analysis. After immersion of copper metal specimens in 0.1 N HCl for 7 days, SEM and EDS analysis were performed and the results were shown in Fig. 14 and Table 5. Figure 14a shows the corrosion of copper in HCl solution without any inhibitor and the oxygen atom ratio of 0.88% indicates a poor protective oxide layer formed on the metal surface. When CoPc (1) was added to the HCl solution, the amount of oxygen atom increased to the 15.74% representing the formation of highly protective oxide layer on the metal surface (Fig. 14b). After the addition of CuPc (2) and ZnPc (3), oxygen atom content is found 1.009 and 2.891% respectively. When the results obtained from the SEM and EDS surface analyzes were examined, the protection sequences of the molecules were determined as CoPc (1) > CuPc (2) > ZnPc (3). It is quite clear that these results support the results of the electrochemical and gravimetric analysis.
4 CONCLUSION
Water-soluble CoPc (1), CuPc (2) and ZnPc (3) were evaluated for their corrosion inhibition properties on copper metal in 0.1 N HCl. Gravimetric, electrochemical impedance, polarization, SEM-EDS techniques were used and quantum chemical calculations were done. The highest inhibition capacity was found with CoPc (1), CuPc (2), ZnPc (3) respectively on the basis of electrochemical measurement and SEM-EDS analysis. The highest IE% value was obtained with CoPc (1) (76.88%) at 1 × 10–2 mol/L concentration in gravimetric analysis and CoPc (1) exhibited the highest %IE value for EIS and polarization measurements at the same concentration. The experimental and theoretical studies have shown that the HOMO and LUMO values of the complexes are important parameters for corrosion and the difference between these HOMO and LUMO energy values called the ∆E energy gap (HOMO–LUMO) value has a large effect on the value of inhibition efficiency. As a result, following corrosion inhibition efficiency ranking was obtained: CoPc (1) > CuPc (2) > ZnPc (3).
REFERENCES
Antonijevic, M.M. and Petrovic, M.B., Int. J. Electrochem. Sci., 2008, vol. 3, p. 1.
Muñoz, A.I., Antón, J.G., Guiñón, J.L., and Herranz, V.P., Electrochim. Acta, 2004, vol. 50, no. 4, p. 957.
Selatnia, K.A., Sid, A., and Mosset, P., Chem. Phys. Lett., 2018, vol. 707, p. 117.
Tiana, H., Cheng, Y.F., Li, W., and Hou, B., Corros. Sci., 2015, vol. 100, p. 341.
Peña, L.F., Veyan, J.-F., Todd, M.A., Derecskei-Kovacs, A., and Chabal, Y.J., ACS Appl. Mater. Interfaces, 2010, vol. 10, no. 44, p. 38610.
Dibetsoe, M., Olasunkanmi, L.O., Fayemi, O.E., Yesudass, S., Ramaganthan, B., Bahadur, I., Adekunle, A.S., Kabanda, M.M., and Ebenso, E.E., Molecules, 2015, vol. 20, p. 15701.
Güzel, E., Atsay, A., Nalbantoglu, S., Şaki, N., Dogan, A.L., Gül, A., and Koçak, M.B., Dyes Pigm., 2013, vol. 97, p. 238.
Sukhikh, A.S., Polyakov, M.S., Klyamer, D.D., Gromilov, S.A., and Basova. T.V., J. Struct. Chem., 2017, vol. 58, p. 1039.
Güzel, E., Şaki, N., Akin, M., Nebioğlu, M., Şişman, I., Erdoğmuş, A., and Koçak, M.B., Synth. Met., 2018, vol. 245, p. 127.
Alici, E.H., Günsel, A., Akin, M., Bilgiçli, A.T., Arabaci, G., and Yarasir, M.N., J. Coord. Chem., 2018, vol. 71, no. 19, p. 3077.
Aoki, I.V., Guedes, I.C., and Maranhão, S.L.A., J. Appl. Electrochem., 2002, vol. 32, p. 915.
Li, Y., Zhao, P., and Liang, Q., Proc. Material Corrosion and Control Conference of Shandong, Qingdao, 2004, p. 196.
Nnaji, N., Nwaji, N., Mack, J., and Nyokong, T., Molecules, 2019, vol. 24, p. 207.
Günsel, A., Kocabaş, S., Bilgiçli, A.T., Güney, S., and Kandaz, M., J. Lumin., 2016, vol. 76, p. 387.
Akin, M., Nalbantoglu, S., Cuhadar, O., Uzun, D., and Saki, N., Res. Chem. Intermed., 2015, vol. 41, p. 899.
Tao, Z., Zhang, S., Li, W., and Hou, B., Ind. Eng. Chem. Res., 2011, vol. 50, p. 6082.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, no.7, p. 5648.
Valle-Quitana, J.C., Dominguez-Patiño, G.F., and Gonzalez-Rodriguez, J.G., ISRN Corros., 2014, https://doi.org/10.1155/2014/945645
Chen, J. and Zhang, X., Trans. Indian Inst. Met., 2018, vol. 71, p. 1113.
Zhao, P., Liang, Q., and Li, Y., Appl. Surf. Sci., 2005, vol. 252, p. 1596.
Singh, A.K., Shukla, S.K., and Ebenso, E.E., Int. J. Electrochem. Sci., 2011, vol. 6, p. 5689.
Popova, A., Sokolova, E., Raicheva, S., Christov, M., Corros. Sci., 2003, vol. 45, p. 33.
Deyab, M.A., De Riccardis, A., and Mele, G., J. Mol. Liq., 2016, vol. 220, p. 513.
Bastidas, J.M., Pinilla, P., Cano, E., Polo, J.L., and Miguel, S., Corros. Sci., 2003, vol. 45, no. 2, p. 427.
Kaya, S., Tüzün, B., Kaya, C., Obot, I.B., J. Taiwan Inst. Chem. Eng., 2016, vol. 58, p. 528.
Kaya, S., Guo, L., Kaya, C., Tüzün, B., Obot, I.B., Touir, R., and Islam, N., J. Taiwan Inst. Chem. Eng., 2016, vol. 65, p. 522.
Obot, I.B., Kaya, S., Kaya, C., and Tüzün, B., Phys. E(Amsterdam,Neth.), 2016, vol. 80, p. 82.
Tüzün, B. and Kaya, C., J. Bio-Tribo-Corros., 2018, vol. 4, no. 4, p. 69.
Tüzün, B., and Sayin, K., Spectrochim. Acta, Part A, 2019, vol. 1141, p. 48.
Güzel, E., Günsel, A., Tüzün, B., Atmaca, G.Y., Bilgiçli, A.T., Erdoğmuş, A., and Yarasir, M.N., Polyhedron, 2019, vol. 158, p. 316.
Günsel, A., Kırbaç, E., Tüzün, B., Erdoğmuş, A., Bilgiçli, A.T., and Yaraşır, M.N., J. Mol. Struct., 2019, vol. 1180, p. 127.
Zhao, P., Liang, Q., and Li, Y., Appl. Surf. Sci., 2005, vol. 225, no. 5, p. 1596.
Lai, T.Y., Guo, J.D., Fettinger, J.C., Nagase, S., and Power, P.P., Chem. Commun., 2019, vol. 55, no. 3, p. 405.
Bentiss, F., Traisnel, M., Vezin, H., and Lagrenée, M., Corros. Sci., 2003, vol. 45, no.2, p. 371.
Khaled, K.F., Electrochim. Acta, 2003, vol. 48, no. 17, p. 2493.
Law, K.Y., Chem. Rev., 1993, vol. 93, no. 1, p. 449.
Guo, L., Safi, Z., Kaya, S., Shi, W., Tüzün, B., Altunay, N., and Kaya, C., Front. Chem., 2018, vol. 6, p. 155.
Alaoui, K., Touir, R., Galai, M., Serrar, H., Ouakki, M., Kaya, S., Tüzün, B., Boukhris, S., Ebn Touhami, M., and El Kacimi, Y., J. Bio-Tribo-Corros., 2018, vol. 4, no. 3, p. 37.
ACKNOWLEDGMENTS
This work was supported by The Research Fund of Sakarya University (Project Number: Hızdep-2019-5-19-56). This research was made possible by TUBITAK ULAKBIM, High Performance and Grid Computing Center (TR-Grid e-Infrastructure).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Akin, M., Günsel, A., Bilgiçli, A.T. et al. The Water-Soluble Peripheral Substituted Phthalocyanines as Corrosion Inhibitors for Copper in 0.1 N HCl: Gravimetric, Electrochemical, SEM-EDS, and Quantum Chemical Calculations. Prot Met Phys Chem Surf 56, 609–618 (2020). https://doi.org/10.1134/S207020512003003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207020512003003X